Bhushan B. Nanotribology and Nanomechanics: An Introduction
Подождите немного. Документ загружается.

970 Bharat Bhushan and Huiwen Liu
The schematic (bottom) in Fig. 18.8 shows relative size and sources of menis-
cus. The native oxide layer (SiO
2
) on the top of Si(100) wafer exhibits hydrophilic
properties, and some water molecules can be adsorbed on this surface. The con-
densed water will form a meniscus as the tip approaches the sample surface. In the
case of a sphere (such as a single-asperity AFM tip) in contact with a flat surface,
the attractive Laplace force (F
L
) caused by capillary is:
F
L
= 2πRγ
la
(cosθ
1
+ cosθ
2
) , (18.2)
where R is the radius of the sphere, γ
la
is the surface tension of the liquid against
air, θ
1
and θ
2
are the contact angles between liquid and flat and spherical surfaces,
respectively [2,3,40]. As the surface tension value of Z-15 (24dyn/cm) is smaller
than that of water (72 dyn/cm), the larger adhesive force in Z-15 cannot only be
caused by the Z-15 meniscus. The nonpolarized Z-15 liquid does not have complete
coverage and strong bonding with Si(100). In the ambient environment, the con-
densed water molecules will permeate through the liquid Z-15 lubricant film and
compete with the lubricant molecules present on the substrate. The interaction of
the liquid lubricant with the substrate is weakened, and a boundary layer of the liq-
uid lubricant forms puddles [20,34]. This dewetting allows water molecules to be
adsorbed on the Si(100) surface as aggregates along with Z-15 molecules. And both
of them can form meniscus while the tip approaches the surface. In addition, as the
Z-15 film is pretty soft compared to the solid Si(100) surface, penetration of the tip
in the film occurs while pushing the tip down. This leads to a large area of the tip
involved to form the meniscus at the tip–liquid (water aggregates along with Z-15)
interface. These two factors of the liquid-like Z-15 film result in higher adhesive
force. It should also be noted that Z-15 has a higher viscosity compared to that of
water. Therefore, Z-15 film provides higher resistance to sliding motion and results
in a larger coefficient of friction. In the case of Z-DOL(BW) film, both of the active
groups of Z-DOL molecules are strongly bonded on Si(100) substrate through the
thermal and washing treatment. Thus, the Z-DOL(BW) film has relatively low free
surface energy and cannot be displaced readily by water molecules or readily ad-
sorb water molecules. Thus, the use of Z-DOL(BW) can reduce the adhesive force.
We further believe that the bonded Z-DOL molecules can be orientated under stress
(behaving as a soft polymer solid), which facilitates sliding and reduces coefficient
of friction.
Thesestudies suggest that, if the lubricant films exist as liquid-like,such as Z-15
films, they easily form meniscus (by themselves and the adsorbed water molecules),
and thus have higher adhesive force and higher friction force. Whereas, if the lubri-
cant film exists in solid-likephase, such as Z-DOL(BW) films,they are hydrophobic
with low adhesion and friction.
In order to study the uniformity of lubricant film and its influence on friction
and adhesion, friction force mapping and adhesive force mapping of PFPE have
been carried out by Koinkar and Bhushan [34] and Bhushan and Dandavate [36],
respectively. Figure 18.9 shows gray scale plots of surface topography and friction
force images obtained simultaneously for unbonded Demnum-type PFPE lubricant

18 Nanoscale Boundary Lubrication Studies 971
Friction force
5.00
2.50
0
0 2.50 5.00 0 2.50 5.00
Surface topography
0 1.3 2.5 nm 0 4.0 8.0 nm
Fig. 18.9. Gray scale plots
of the surface topography
and friction force obtained
simultaneously for unbonded
2.3-nm-thick Demnum-type
PFPE lubricant film on sili-
con [20]
film on silicon [34].The friction force plot shows well-distinguished low- and high-
friction regions corresponding roughly to high- and low-surface-height regions in
the topography image (thick- and thin-lubricant regions). A uniformly lubricated
sample does not show such a variation in friction. Figure18.10 shows the gray scale
plots of the adhesive force distribution for silicon samples coated uniformly and
nonuniformlywith Z-DOL lubricant. It can be clearly seen that there exists a region
that has an adhesive force distinctly different from the other region for the nonuni-
formly coated sample. This implies that the liquid film thickness is nonuniform,
giving rise to a difference in the meniscus forces.
Adhesive force
3.5 nm uniform Z-DOL/ Si(100)
30μm
0
20 nN
2 – 10 nm nonuniform Z-DOL/ Si(100)
30μm
60
030μm
Adhesive force
30μm
Fig. 18.10. Gray scale plots of the adhesive force distribution of a uniformly coated,
3.5-nm-thick unbonded Z-DOL film on silicon and 3–10 nm-thick unbonded Z-DOL film
on silicon that was deliberately coated nonuniformly by vibrating the sample during the coat-
ing process [36]
972 Bharat Bhushan and Huiwen Liu
18.4.2 Rest Time Effect
It is well known that, in the computer rigid disk drive, the stiction force increases
rapidly with an increase in rest time between the head and magnetic-medium
disk [10,11]. Considering that the stiction and friction are two of the major issues
that lead to the failure of computer rigid disk drives and MEMS, it is very important
to find out if the rest time effect also exists on the nanoscale. First, the rest time
effect on the friction force, adhesive force, and coefficient of Si(100) sliding against
70 nN, 2μm/s, 22°C, RH 45–55%
Friction force (nN)
Time (s)
Adhesive force (nN)
Coefficient of friction
From friction force plot
Si(100)
a)
0.15
0.10
0.05
0
1,000
100
10
1,000
100
10
1
1
10,0001,000100
10 100,000
1
10,0001,000100
10
100,000
1
10,0001,000100
10
100,000
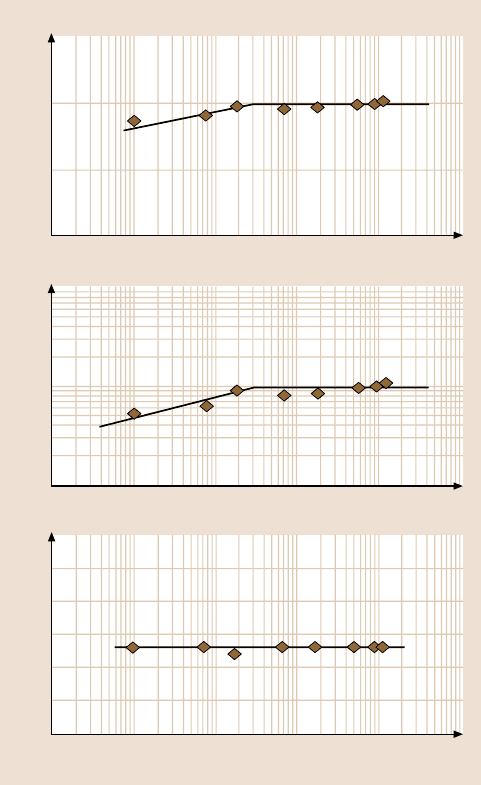
Fig. 18.11. (a) Rest time effect on friction force, adhesive force, and coefficient of friction of
Si(100).
18 Nanoscale Boundary Lubrication Studies 973
Si
3
N
4
tip was studied, Fig. 18.11a [21]. It was found that the friction and adhesive
forces logarithmically increase up to a certain equilibrium time after which they
remain constant. Figure 18.11a also shows that the rest time does not affect the co-
efficient of friction. These results suggest that the rest time can result in the growth
of the meniscus, which causes a higher adhesive force, and in turn, a higher friction
force. But in the whole testing range the friction mechanisms do not change with
70 nN, 2μm/s,
22°C, RH 45 –55%
Friction force (nN)
Adhesive force (nN)
Coefficient of friction
From friction force plot
t =0s
t = 180 s
Z-DOL(BW)Z-15Si(100)
b)
0.15
0.10
0.05
0
200
150
100
50
0
25
20
15
10
5
0
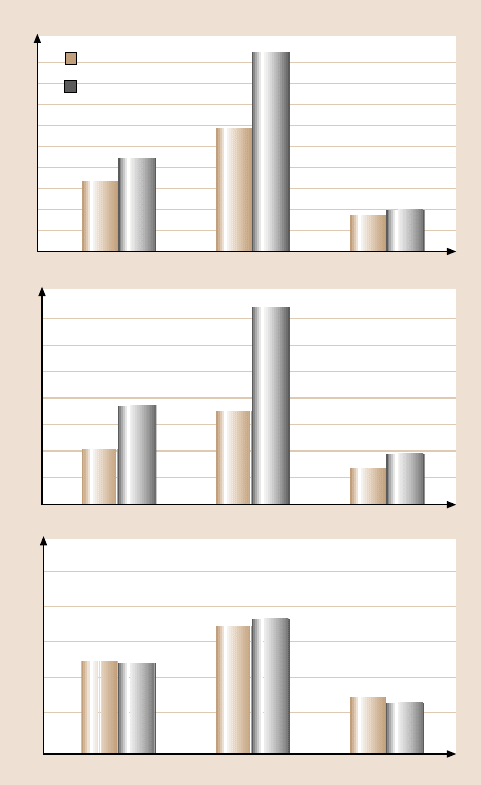
Fig. 18.11. (b) Summary of the rest time effect on friction force, adhesive force, and coeffi-
cient of friction of Si(100), 2.8-nm-thick Z-15 film, and 2.3-nm-thick Z-DOL(BW) film. All
of the measurements were carried out at 70 nN, 2 µm/s, and in ambient air [21]
974 Bharat Bhushan and Huiwen Liu
the rest time. Similar studies were also performed on Z-15 and Z-DOL(BW) films.
The results are summarized in Fig. 18.11b [21]. It is seen that a similar time effect
has been observed on Z-15 film, but not on Z-DOL(BW) film.
AnAFMtipincontactwithaflatsamplesurfacecanbetreatedasasingle-asperity
contact. Therefore, a Si
3
N
4
tip in contact with Si(100) or Z-15/Si(100)can be mod-
eled as a sphere in contact with a flat surface covered by a layer of liquid (adsorbed
water and/orliquidlubricant),Fig.18.12a.Meniscusformsaroundthecontactingas-
perity and growswith time until equilibrium occurs [41]. The meniscus force, which
is the product of meniscus pressure and meniscus area, depends on the flow of li-
quid phase toward the contact zone. The flow of the liquid toward the contact zone
is governed by the capillary pressure P
c
, which draws liquid into the meniscus, and
the disjoining pressure Π, which tends to draw the liquid away from the meniscus.
Based on the Young and Laplace equation, the capillary pressure, P
c
,is:
P
c
= 2Kγ, (18.3)
where 2K is the mean meniscus curvature (= K
1
+ K
2
,whereK
1
and K
2
are the cur-
vatures of the meniscus in the contact plane and perpendicular to the contact plane)
and γ is the surface tension of the liquid. Mate and Novotny [6] have shown that the
disjoining pressure decreases rapidly with increasing liquid film thickness in a man-
ner consistent with a strong van der Waals attraction. The disjoining pressure, Π,
for these liquid films can be expressed as:
Π =
A
6πh
3
, (18.4)
where A is the Hamaker constant and h is the liquid film thickness. The driving
forces that cause the lubricant flow that results in an increase in the meniscus force
are the disjoining pressure gradient, due to a gradient in film thickness, and the
capillary pressure gradient, due to the curved liquid–air interface. The driving pres-
sure, P, can then be written as:
P = −2Kγ −Π. (18.5)
a)
Asperity
x
0
Sample surface
A
h
h
0
R
1
θ
D
x
R
α
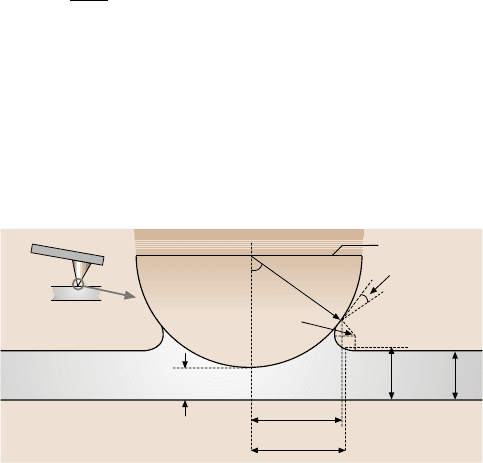
Fig. 18.12. (a) Schematic of a single asperity in contact with a smooth flat surface in the
presence of a continuous liquid film when φ is large
18 Nanoscale Boundary Lubrication Studies 975
10
0
78
77
76
10
2
10
4
10
6
10
8
10
0
78
77
76
10
2
10
4
10
6
10
8
10
0
15.7
15.6
15.5
15.4
15.3
15.2
10
2
10
4
10
6
10
8
Meniscus force (μN)
Rest time (s)
R
= 250 μm
η = 0.25 Pa s
h
0
= 3 nm
R = 250 μm
h
0
= 1 nm
η = 0.001 Pa s
0.05
0.25
R =50μm
h
0
= 1 nm
η = 0.25 Pa s
Meniscus force (μN)
Rest time (s)
Meniscus force (
μN)
Rest time (s)
1
b)
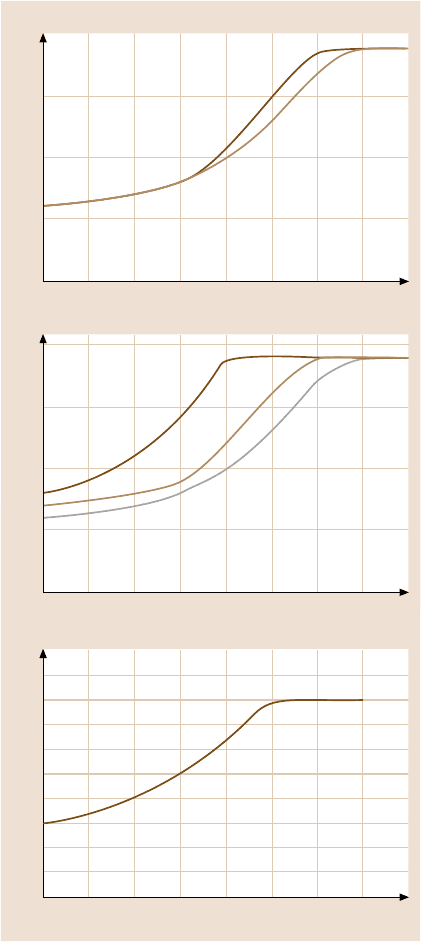
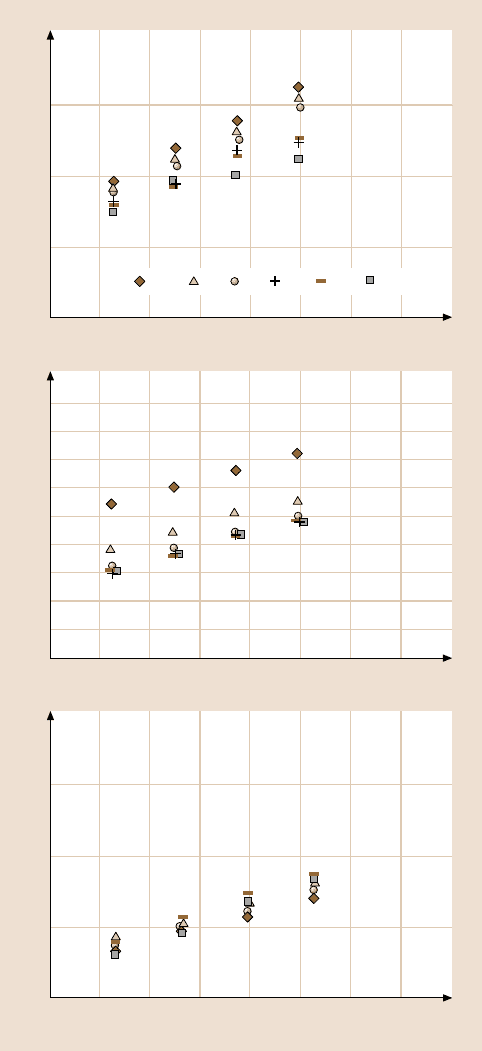
Fig. 18.12. (b) Results of the
single-asperity model. Effect
of viscosity of the liquid,
radius of the asperity, and
film thickness is studied with
respect to the time-dependent
meniscus force [41]
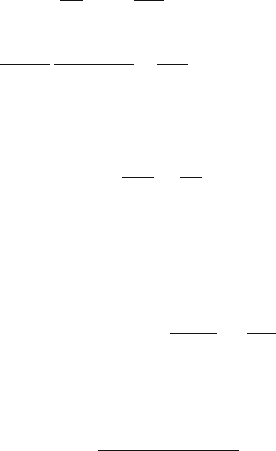
976 Bharat Bhushan and Huiwen Liu
Based on these three basic relationships, the following differential equation has
been derived by Chilamakuri and Bhushan [41], which can describe the meniscus at
time t:
2πx
0
⎛
⎜
⎜
⎜
⎜
⎜
⎝
D+
x
2
0
2R
−h
0
⎞
⎟
⎟
⎟
⎟
⎟
⎠
dx
0
dt
=
2πh
3
0
γ
3η
(1+ cosθ)
D+ a−h
0
−
Ax
0
3ηh
cotα,
(18.6)
where η is the viscosity of the liquid and a is given as
a = R(1−cosφ) ∼
Rφ
2
2
∼
x
2
0
2R
. (18.7)
The differential equation (18.4) was solved numerically using Newton’s iteration
method. The meniscus force at any time t less than the equilibrium time is propor-
tional to the meniscus area and meniscus pressure (2Kγ), and it is given by
f
m
(t) = 2πRγ(1+ cosθ)
x
0
(x
0
)
eq
2
K
K
eq
, (18.8)
where (x
0
)
eq
is the value of x
0
at the equilibrium time
(x
0
)
eq
2
= 2R
⎡
⎢
⎢
⎢
⎢
⎢
⎣
−6πh
3
0
γ(1+ cosθ)
A
+ (h
0
−D)
⎤
⎥
⎥
⎥
⎥
⎥
⎦
. (18.9)
This modeling work (at the microscale) showed that the meniscus force initially
increases logarithmically with the rest time up to a certain equilibrium time, after
which it remains constant. Equilibrium time decreases with an increase in liquid
film thickness, a decrease in viscosity, and a decrease in the tip radius, Fig. 18.12b.
This early numerical modeling work and the data at the nanoscale in Fig. 18.11aare
in good agreement.
18.4.3 Velocity Effect
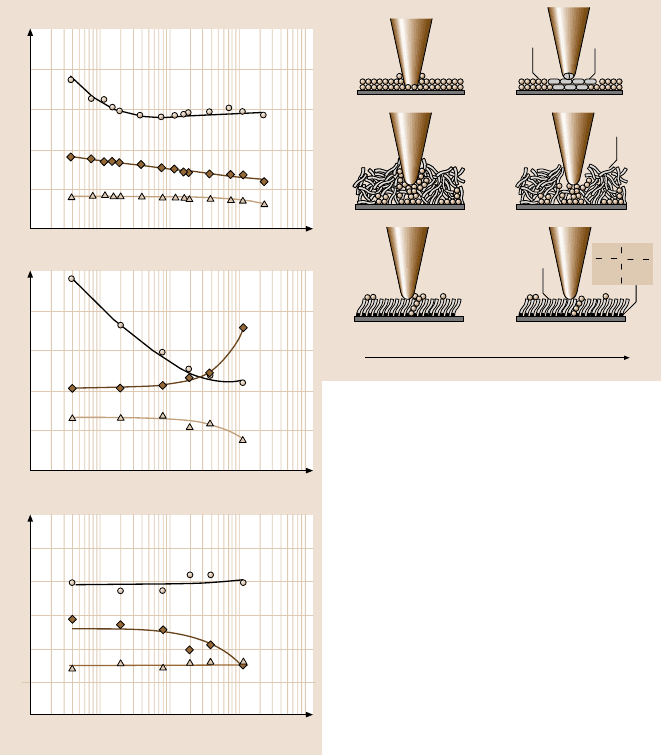
To investigate the velocity effect on friction and adhesion, the relationshipsbetween
friction force and normal load for Si(100), Z-15, and Z-DOL(BW) at different ve-
locities were measured, Fig. 18.13 [21]. Based on these data, the adhesive force and
coefficient of friction values can be calculated by (18.1). The variation of friction
force, adhesive force, and coefficient of friction of Si(100), Z-15, and Z-DOL(BW)
as a function of velocity are summarized in Fig. 18.14. This indicates that, for sili-
con wafer, the friction force decreases logarithmically with increasing velocity. For
Z-15,the frictionforce decreaseswith increasingvelocity up to 10µm/s, afterwhich
it remains almost constant. The velocity has a much smaller effect on the friction
force of Z-DOL(BW); it reduced slightly only at very high velocity. Figure 18.14
18 Nanoscale Boundary Lubrication Studies 977
10
5
0
25
20
15
10
5
0
10
5
0
0 100755025
120 μm/s0.4 2 8 20 40
0 100755025
0 100755025
22°C, RH 45 – 55%
Friction force (nN)
Z-15
Si(100)
Z-DOL(BW)
Normal load (nN)
Normal load (nN)
Normal load (nN)
Fig. 18.13. Friction forces versus normal load data of Si(100), 2.8-nm-thick Z-15 film, and
2.3-nm-thick Z-DOL(BW) film at various velocities in ambient air [21]
978 Bharat Bhushan and Huiwen Liu
25
20
15
10
5
0
125
100
75
50
25
0
0.15
0.10
0.05
0
1 1,000100100.1
70 nN, 22°C, RH 45 – 55%
Friction force (nN)
Si(100)
Z-DOL(BW)
Z-15
Velocity (μm/s)
From friction force plot
Adhesive force (nN)
Si(100)
Z-DOL(BW)
Z-15
Coefficient of friction
Si(100)
Z-DOL(BW)
Z-15
Z-DOL(BW)
Si(100)
H
2
O
Increasing velocity
0.4
μm/s
240 μm/s
Si(OH)
4
Z-15
Z-15
Z-DOL
O
Si
O
O
Fig. 18.14. The influence of velocity
on the friction force, adhesive force,
and coefficient of friction of Si(100),
2.8-nm-thick Z-15 film, and 2.3-nm-thick
Z-DOL(BW) film at 70 nN, in ambi-
ent air. The schematic (right)showsthe
change of surface composition (by tribo-
chemical reaction) and change of menis-
cus with increasing velocity [21]
also indicates that the adhesive force of Si(100) is increased when the velocity is
higher than 10µm/s. The adhesive force of Z-15 is reduced dramatically with a ve-
locity increase up to 20 µm/s, after which it is reduced slightly; the adhesive force
of Z-DOL(BW) also decreases at high velocity. In the testing range of velocity, only
the coefficient of friction of Si(100) decreases with velocity, but the coefficients
of friction of Z-15 and Z-DOL(BW) almost remain constant. This implies that the
friction mechanisms of Z-15 and Z-DOL(BW) do not change with the variation of
velocity.
The mechanisms of the effect of velocity on the adhesion and friction are ex-
plained based on the schematics shown in Fig. 18.14 (right). For Si(100), tribo-
chemical reaction plays a major role. Although at high velocity the meniscus is
18 Nanoscale Boundary Lubrication Studies 979
broken and does not have enough time to rebuild, the contact stresses and high ve-
locity lead to tribochemicalreactions of the Si(100)wafer andSi
3
N
4
tip, whichhave
native oxide (SiO
2
) layers with water molecules. The following reactions occur:
SiO
2
+ 2H
2
O → Si(OH)
4
(18.10)
Si
3
N
4
+ 16H
2
O →3Si(OH)
4
+ 4NH
4
OH . (18.11)
The Si(OH)
4
is removed and continuously replenished during sliding. The Si(OH)
4
layer between the tip and Si(100) surface is known to be of low shear strength and
causes a decrease in friction force and coefficient of friction in the lateral direc-
tion [42–46]. The chemical bonds of Si
−
OH between the tip and Si(100) surface
induce large adhesive force in the normal direction. For Z-15 film, at high velocity
the meniscus formed by condensed water and Z-15 molecules is broken and does
not have enough time to rebuild. Therefore, the adhesive force and, consequently,
friction force is reduced. For Z-DOL(BW) film, the surface can adsorb few water
molecules in ambient condition, and at high velocity these molecules are displaced,
which is responsible for a slight decrease in friction force and adhesive force. Even
in the high-velocity range, the friction mechanisms for Z-15 and Z-DOL(BW) films
are still shearing of the viscous liquid and molecular orientation, respectively. Thus
the coefficients of friction of Z-15 and Z-DOL(BW) do not change with velocity.
Koinkar and Bhushan [20,34] have suggested that, in the case of samples with
mobilefilms,such ascondensedwaterand Z-15films,alignmentof liquidmolecules
(shear thinning) is responsible for the drop in friction force with an increase in
scanning velocity. This could be another reason for the decrease in friction force
with velocity for Si(100) and Z-15 film in this study.
18.4.4 Relative Humidity and Temperature Effect
The influence of relative humidity on friction and adhesion was studied in an envi-
ronmentally controlled chamber. The friction force was measured by making meas-
urements at increasing relative humidity, the results are presented in Fig. 18.15 [21].
These shows that, for Si(100) and Z-15 film, the friction force increases with a rel-
ative humidity increase up to RH 45%, and then it shows a slight decrease with
a further increase in relative humidity. Z-DOL(BW) has a smaller friction force than
Si(100) and Z-15 in the whole testing range, and its friction force shows a relatively
apparent increase when the relative humidity is higher than RH 45%. For Si(100),
Z-15, and Z-DOL(BW), adhesive forces increase with relative humidity. And their
coefficients of friction increase with relative humidity up to RH 45%, after which
they decrease with further increases of the relative humidity. It is also observed that
the humidity effect on Si(100) really depends on the history of the Si(100) sample.
As the surface of Si(100) wafer readily adsorbs water in air, without any pretreat-
ment the Si(100) used in our study almost reaches its saturated stage of adsorbing
water and is responsible for a smaller effect with increasing relative humidity. How-
ever, once the Si(100) wafer was thermally treated by baking at 150
◦
Cfor1h,
a bigger effect was observed.
