Bhushan B. Nanotribology and Nanomechanics: An Introduction
Подождите немного. Документ загружается.

1002 B. Bhushan et al.
Equation (19.5) provides with the value of the static contact angle for given surface
energies. Note that although we use the term “air”, the analysis does not change in
the case of another gas, such as water vapor.
19.2.2 Heterogeneous Interfaces and the Wenzel
and Cassie–Baxter Equations
In this section, we will discuss the so-called heterogeneous interface and intro-
duce the equations that govern the contact angle for the heterogeneous inter-
face.
Contact Angle with a Rough and HeterogeneousSurfaces
The Wenzel [133] equation, which was derived using the surface force balance and
empirical considerations, relates contact angle of a water droplet upon a rough
solid surface, θ, with that upon a smooth surface, θ
0
(Fig. 19.1b), through the non-
dimensionalsurface roughness factor, R
f
, equal to the ratio of the surface area to its
flat projected area
cosθ =
dA
LA
dA
F
=
dA
SL
dA
F
dA
LA
dA
SL
= R
f
cosθ
0
(19.6)
R
f
=
A
SL
A
F
. (19.7)
The dependence of the contact angle on the roughness factor is presented in
Fig. 19.2 for different values of θ
o
. The Wenzel model predicts that a hydropho-
bic surface (θ
o
> 90
◦
) becomes more hydrophobic with an increase in R
f
, while
a hydrophilic surface (θ
o
< 90
◦
) becomes more hydrophilic with an increase in
R
f
[67,97].
1
R
f
1.25 1.50 1.75 2
180
θ
0
= 120°
θ
0
= 90°
θ
0
= 60°
θ
0
= 30°
150
120
90
60
30
0
θ
0
= 150°
Fig. 19.2. Contact angle for
rough surface (θ) as a func-
tion of the roughness factor
(R
f
) for various contact
angles for smooth surface
(θ
o
) [97]

19 Lotus Effect: Roughness-Induced Superhydrophobic Surfaces 1003
In a similar manner, for a surface composed of two fractions, one with the frac-
tional area f
1
and the contact angle θ
1
and the other with f
2
and θ
2
respectively (so
that f
1
+ f
2
= 1), the contact angle is given by the Cassie equation
cosθ = f
1
cosθ
1
+ f
2
cosθ
2
. (19.8)
For the case of a composite interface (Fig. 19.1c), consisting of the solid-liquid
fraction (f
1
= f
SL
, θ
1
= θ
0
) and liquid-air fraction (f
2
= f
LA
= 1− f
SL
,cosθ
2
= −1),
combining (19.7) and (19.8) yields the Cassie–Baxter equation
cosθ = R
f
f
SL
cosθ
0
−1+ f
SL
or
cosθ = R
f
cosθ
0
− f
LA
(R
f
cosθ
0
+ 1) . (19.9)
The opposite limiting case of cosθ
2
= 1(θ
2
= 0
◦
corresponds to the water-on-water
contact) yields
cosθ = 1+ f
SL
(cosθ
0
−1) . (19.10)
Equation (19.10) is used sometimes [41] for the homogeneous interface instead
of (19.6), if the rough surface is covered by holes filled with water (Fig. 19.1d).
The Cassie–Baxter Equation
Two situations in wetting of a rough surface should be distinguished: the homo-
geneous interface without any air pockets (sometimes called the Wenzel interface,
since the contact angle is given by the Wenzel equation or (19.6)) and the composite
interface with air pockets trapped between the rough details as shown in Fig. 19.3a
(sometimes called the Cassie or Cassie–Baxter interface, since the contact angle is
given by (19.9)).
While (19.9) for the composite interface was derived using (19.6) and (19.8), it
could also be obtained independently. For this purpose, two sets of interfaces are
considered: a liquid-air interface with the ambient and a flat composite interface un-
der the droplet, which itself involves solid-liquid,liquid-air,and solid-air interfaces.
For fractional flat geometrical areas of the solid-liquid and liquid-air interfaces un-
der the droplet, f
SL
and f
LA
, the flat area of the composite interface is
A
C
= f
SL
A
C
+ f
LA
A
C
= R
f
A
SL
+ f
LA
A
C
. (19.11)
In order to calculate the contact angle in a manner similar to the derivationof (19.6),
the differential area of the liquid-air interface under the droplet, f
LA
dA
C
, should be
subtracted from the differential of the total liquid-air area dA
LA
, which yields
cosθ =
dA
LA
− f
LA
dA
C
dA
C
=
dA
SL
dA
F
dA
F
dA
C
dA
LA
dA
SL
− f
LA
= R
f
f
SL
cosθ
0
− f
LA
= R
f
cosθ
0
− f
LA
(R
f
cos
0
+1). (19.12)
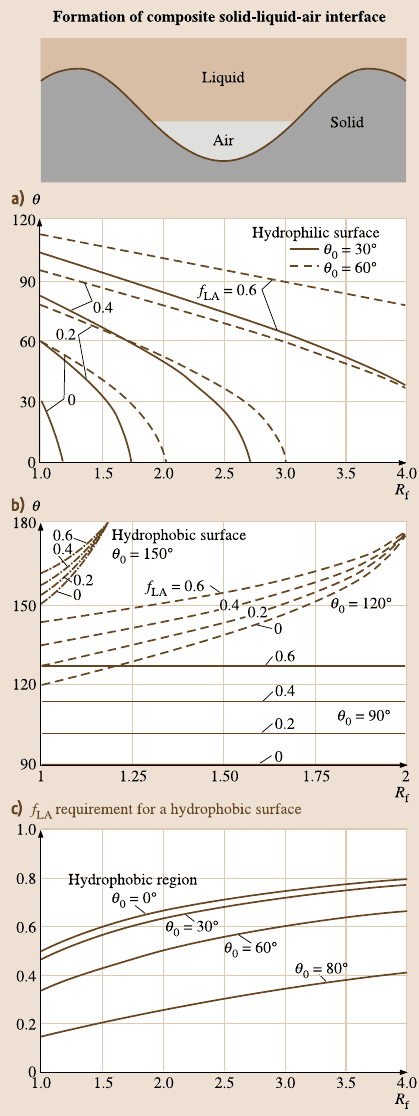
1004 B. Bhushan et al.
Fig. 19.3. (a) Formation of
a composite solid-liquid-air
interface for rough surface,
(b) contact angle for rough
surface (θ) as a function of
the roughness factor (R
f
)
for various f
LA
values on
the hydrophilic surface and
the hydrophobic surface,
and (c) f
LA
requirement for
a hydrophilic surface to be
hydrophobic as a function of
the roughness factor (R
f
)and
θ
0
[67]

19 Lotus Effect: Roughness-Induced Superhydrophobic Surfaces 1005
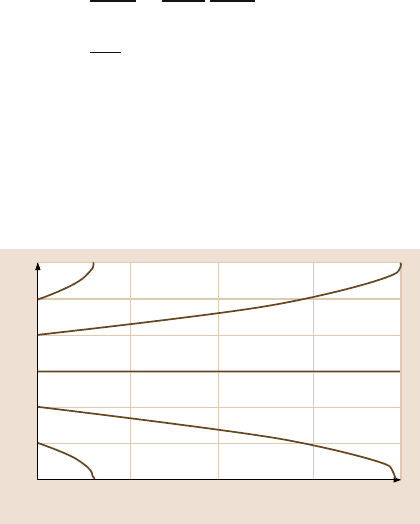
The dependence of the contact angle on the roughness factor for hydrophilic and
hydrophobic surfaces is presented in Fig. 19.3b.
According to (19.12), even for a hydrophilic surface, the contact angle increases
with an increase of f
LA
. At a high value of f
LA
, surface can become hydrophobic;
however, the value required may be unachievable or formation of air pockets may
become unstable. Using the Cassie–Baxter equation, the valueof f
LA
at which a hy-
drophilic surface could turn into a hydrophobicone, is given by [67]
f
LA
≥
R
f
cosθ
0
R
f
cosθ
0
+ 1
for θ
0
< 90
◦
. (19.13)
Figure19.3c shows thevalueof f
LA
requirementas a functionof R
f
for foursurfaces
with different contact angles, θ
o
. Hydrophobic surfaces can be achieved abovea cer-
tain f
LA
values as predicted by (19.13). The upper part of each contact angle line
is hydrophobicregion. For the hydrophobicsurface, contact angle increases with an
increase in f
LA
both for smooth and rough surfaces.
Shuttleworth and Bailey [124] studied spreading of a liquid over a rough solid
surface and found that the contact angle at the absolute minimum of surface en-
ergy corresponds to the values, given by (19.6) (for the homogeneous interface)
or (19.12) (for composite interface). According to their analysis, spreading of a liq-
uid continues, until simultaneously the (19.5) (the Young equation) is satisfied lo-
cally at the triple line and the minimal surface condition is satisfied over the entire
liquid-air interface. The minimal surface condition states, that the sum of inversed
principal radii of curvature, R
1
and R
2
(mean curvature), is constant at any point,
and thus governs the shape of the liquid-air interface.
1
R
1
+
1
R
2
= const . (19.14)
The same condition is also the consequence of the Laplace equation, which relates
pressure change through an interface with its mean curvature.
Stability of the composite interface is an important issue. Even though it may be
geometrically possible for the system to become composite, it may be energetically
profitable for the liquid to penetrate into valleys between asperities and to form the
homogeneous interface. Marmur [86] formulated geometrical conditions for a sur-
face, under which the energy of the system has a local minimum and the compos-
ite interface may exist. Patankar [109] pointed out that, whether the homogeneous
or composite interface exists, depends on the system’s history, i.e., on whether the
dropletwas formed at the surface or deposited.However, the above-mentionedanal-
yses do not provide with an answer, which of the two possible configurations, ho-
mogeneous or composite, will actually form.
Limitations of the Wenzel and Cassie Equations
The Cassie equation (19.8) is based on the assumption that the heterogeneous sur-
face is composed of well-separated distinct patches of different material, so that
the free surface energy can be averaged. It has been argued also that when the size
1006 B. Bhushan et al.
of the chemical heterogeneities is very small (of atomic or molecular dimensions),
the quantity that should be averaged is not the energy, but the dipole moment of
a macromolecule [62], and (19.8) should be replaced by
(1+ cosθ)
2
= f
1
(1+ cosθ
1
)
2
+ f
2
(1+ cosθ
2
)
2
. (19.15)
Experimental studies of polymers with different functional groups showed a good
agreement with (19.15) [130].
Later investigations put the Wenzel and Cassie equations into a thermodynamic
framework, however, they showed also that there is no one single value of the con-
tact angle for a rough or heterogeneous surface [66,83,86]. The contact angle can
be in a range of values between the receding contact angle, θ
rec
, and the advancing
contact angle, θ
adv
. The system tends to achieve the receding contact angle when
liquid is removed (for example, at the rear end of a moving droplet), whereas the
advancing contact angle is achieved when the liquid is added (for example, at the
front end of a moving droplet). When the liquid is neither added nor removed, the
system tends to have static or “most stable” contact angle, which is given approx-
imately by (19.5)–(19.10). The difference between θ
adv
and θ
rec
is known as the
“contact angle hysteresis” and it reflects a fundamental asymmetry of wetting and
dewetting and the irreversibility of the wetting/dewetting cycle. Although for sur-
faces with the roughnesscarefully controlled on the molecular scale it is possible to
achieve contact angle hysteresis as low as < 1
◦
[54], hysteresiscannot be eliminated
completely, since even the atomically smooth surfaces have a certain roughness and
heterogeneity. The contact angle hysteresis is a measure of energy dissipation dur-
ing the flow of a droplet along a solid surface. A water-repellent surface should have
a low contact angle hysteresis to allow water to flow easily along the surface.
It is emphasized that the contact angle provided by (19.5)–(19.10) is a macro-
scale parameter, so it is called sometimes “the apparent contact angle.” The actual
angle, under which the liquid-air interface comes in contact with the solid surface
at the micro- and nanoscale can be different. There are several reasons for that.
First, water molecules tend to form a thin layer upon the surfaces of many materi-
als. This is because of a long-distance van der Waals adhesion force that creates the
so-called disjoining pressure [42]. This pressure is dependent upon the liquid layer
thickness and may lead to formation of stable thin films. In this case, the shape of
the droplet near the triple line (line of contact of the solid, liquid and air, shown
later in Fig. 19.6) transforms gradually from the spherical surface into a precursor
layer, and thus the nanoscale contact angle is much smaller than the apparent con-
tact angle. In addition, adsorbed water monolayers and multilayers are common for
many materials.Second, evencarefully preparedatomically smooth surfaces exhibit
certain roughness and chemical heterogeneity. Water tends to cover at first the hy-
drophilic spots with high surface energy and low contact angle [35]. The tilt angle
due to the roughness can also contribute into the apparent contact angle. Third, the
very concept of the static contact angle is not well defined. For practical purposes,
the contact angle, which is formed after a droplet is gently placed upon a surface
and stops propagating, is considered the static contact angle. However, depositing
19 Lotus Effect: Roughness-Induced Superhydrophobic Surfaces 1007
the droplet involves adding liquid while leaving it may involve evaporation, so it is
difficult to avoid dynamic effects. Fourth, for small droplets and curved triple lines,
the effect of the contact line tension may be significant. Molecules at the surface
of a liquid or solid phase have higher energy because they are bonded to fewer
molecules, than those in the bulk. This leads to the surface tension and surface en-
ergy. In a similar manner, molecules at the edge have fewer bonds than those at the
surface, which leads to the line tension and the curvature dependence of the surface
energy. This effect becomes important when the radius of curvature is comparable
with the so-calledTolman’s length, normallyof the molecular size [3]. However, the
triple line at the nanoscale can be curved so that the line tension effects become im-
portant [111]. The contact angle with accountfor the contact line effect for a droplet
with radius R is given by cos θ = cosθ
0
+ 2τ/(Rγ
LA
), where τ is the contact line
tension and θ
0
is the value given by the Young equation [28]. Thus while the contact
angle is a convenient macroscale parameter, wetting is governed by interactions at
the micro- and nanoscale, which determine the contact angle hysteresis and other
wetting properties (Table 19.1).
Range of Applicability of the Wenzel and Cassie Equations
Gao and McCarthy [53] showed experimentally that the contact angle of a droplet
is defined by the triple line and does not depend upon the roughness under the bulk
of the droplet. A similar result for chemically heterogeneous surfaces was obtained
by Extrand [47]. Gao and McCarthy [53] concluded that the Wenzel and Cassie–
Baxter equations “should be used with the knowledge of their fault.” The question
remained, however, under what circumstances the Wenzel and Cassie–Baxter equa-
tions can be safely used and under what circumstances do they become irrelevant.
For a liquid front propagating along a rough two-dimensional profile
(Fig. 19.4ab), the derivative of the free surface energy (per liquid front length), W,
by the profile length, t, yields the surface tension force σ = dW/ dt = γ
SL
−γ
SA
.The
quantity of practical interest is the component of the tension force that corresponds
Table 19.1. Wetting of a superhydrophobic surface as a multiscale process [102,106]
Scale level Characteristic
length
Parameters Phenomena Interface
Macroscale Droplet
radius (mm)
Contact angle,
droplet radius
Contact angle
hysteresis
2D
Microscale Roughness
detail (µm)
Shape of the
droplet, position
of the liquid-air
interface (h)
Kinetic effects 3D solid
surface,
2D liquid
surface
Nanoscale Molecular
heterogeneity
(nm)
Molecular descrip-
tion
Thermodynamic
and dynamic
effects
3D
1008 B. Bhushan et al.
to the advancing of the liquid front in the horizontal direction for dx. This com-
ponent is given by dW/dx = (dW/dt)(dt/ dx) = (γ
SL
−γ
SA
)dt/dx. It is noted that
the derivative R
f
= dt/ dx is equal to Wenzel’s roughness factor in the case when
the roughness factor is constant throughout the surface. Therefore, the Young equa-
tion, which relates the contact angle with solid, liquid, and air interface tensions,
γ
LA
cosθ = γ
SA
−γ
SL
, is modified as [96]
γ
LA
cosθ = R
f
(
γ
SA
−γ
SL
)
. (19.16)
The empirical Wenzel equation (19.6) is a consequence of (19.16) combined with
the Young equation.
Nosonovsky [96] showed that for a more complicated case of a non-uniform
roughness, given by the profile z(x), the local value of r(x) = dt/ dx
= (1+ (dz/dx)
2
)
1/2
matters. In the cases that were studied experimentally by Gao
and McCarthy [53] and Extrand [47], the roughness was present (r > 1) under the
bulk of the droplet, but there was no roughness (r = 0) at the triple line, and the
contact angle was given by (19.6) (Fig. 19.4c). In the general case of a 3D rough
surface z(x, y), the roughness factor can be defined as a function of the coordinates
r(x, y) = (1+ (dz/ dx)
2
+ (dz/ dy)
2
)
1/2
.
Whereas (19.6) is valid for uniformly rough surfaces, that is, surfaces with
r = const, for non-uniformly rough surfaces the generalized Wenzel equation is for-
mulated to determine the local contact angle (a function of x and y) with a rough
surfaces at the triple line [96]
cosθ = r(x,y)cosθ
0
. (19.17)
The difference between the Wenzel equation (19.6) and the Nosonovsky equa-
tion (19.17) in that the later is valid for a non-uniformroughness with the roughness
factor as the function of the coordinates. Equation (19.17) is consistent with the ex-
perimental results of the scholars, who showed that roughness beneath the droplet
does not affect the contact angle, since it predicts that only roughness at the triple
line matters. It is consistent also with the results of the researchers who confirmed
the Wenzel equation (for the case of the uniform roughness) and of those who re-
ported that only the triple line matters (for non-uniformroughness) (Table 19.2).
The Cassie equation for the composite surface can be generalized in a similar
manner introducing the spatial dependence of the local densities, f
1
and f
2
of the
solid-liquid interface with the contact angle, as a function of x and y,givenby
cosθ
composite
= f
1
(x, y)cosθ
1
+ f
2
(x, y)cosθ
2
, (19.18)
where f
1
+ f
2
= 1,θ
1
and θ
2
are contact angles of the two components [96].
The important question remains, what should be the typical size of rough-
ness/heterogeneity details in order for the generalized Wenzel and Cassie equa-
tions (19.17)–(19.18) to be valid? Some scholars have suggested that roughness/
heterogeneity details should be comparable with the thickness of the liquid-air
interface and thus “the roughness would have to be of molecular dimensions to
19 Lotus Effect: Roughness-Induced Superhydrophobic Surfaces 1009
Table 19.2. Summary of experimental results for uniform and non-uniform rough and chem-
ically heterogeneous surfaces. For non-uniform surfaces, the results shown for droplets larger
than the islands of non-uniformity. Detailed quantitative values of the contact angle in various
sets of experiments can be found in the referred sources [96]
Experiment Roughness/
hydropobicity
at the triple
line and at
the rest of
the surface
Roughness
at the bulk
(under the
droplet)
Experimental
contact angle
(compared
with that at
the rest of
the surface)
Theoretical
contact
angle,
Wenzel/
Cassie
equations
Theoretical
contact
angle,
generalized
Wenzel/
Cassie
(19.17)–(19.18)
Gao and Hydrophobic Hydrophilic Not changed Decreased Not changed
McCarthy Rough Smooth Not changed Decreased Not changed
[53] Smooth Rough Not changed Increased Not changed
Extrand Hydrophilic Hydrophobic Not changed Increased Not changed
[47] Hydrophobic Hydrophilic Not changed Decreased Not changed
Bhushan Rough Rough Increased Increased Increased
et al. [22]
Barbieri Rough Rough Increased Increased Increased
et al. [6]
alter the equilibrium conditions” [7], whereas others have claimed that rough-
ness/heterogeneity details should be small comparing with the linear size of the
droplet [6, 16, 18,19, 66–69, 83]. The interface in our analysis is an idealized 2D
object, which has no thickness. In reality, the triple line zone has two characteristic
dimensions: the thickness (of the order of molecular dimensions) and the length (of
the order of the droplet size).
The apparent contact angle, given by (19.17)–(19.18), may be viewed as the re-
sult of averaging of the local contact angle at the triple line by its length, and thus the
size of the roughness/heterogeneity details should be small comparing to the length
(and not the thickness) of the triple line. A rigorous definition of the generalized
equation requires the consideration of several scale lengths. The length dx needed
for averaging of the energy gives the length over which the averaging is performed
to obtain r(x, y). This length should be larger than roughness details. However, it is
still smaller than the droplet size and the length scale at which the apparent contact
angle is observed (at which local variations of the contact angle level out). Since
of these lengths (the roughness size, dx, the droplet size) the first and the last are
of practical importance, we conclude that the roughness details should be smaller
than the droplet size. When the liquid-air interface is studied at the length scale
of roughness/heterogeneity details, the local contact angle, θ
0
, is given by (19.6)–
(19.10). The liquid-air interface at that scale has perturbations,caused by the rough-
ness/heterogeneity, and the scale of the perturbations is the same as the scale of
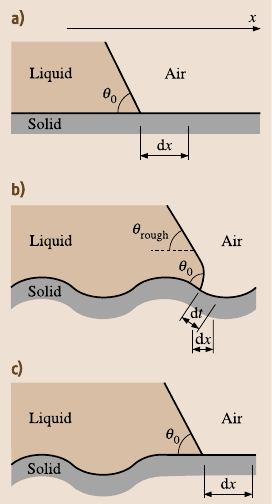
1010 B. Bhushan et al.
Fig. 19.4. Liquid front in contact with a (a) smooth
solid surface (b) rough solid surface, propagation
for a distance dt along the curved surface corre-
sponds to the distance dx along the horizontal sur-
face. (c) Surface roughness under the bulk of the
droplet does not affect the contact angle
the roughness/heterogeneitydetails. However, when the same interface is studied at
a larger scale, the effect of the perturbation vanishes, and apparent contact angle is
given by (19.17)–(19.18)(Fig. 19.4c). This apparent contact angle is defined at the
scale length, for which the small perturbationsof the liquid-air interface vanish, and
the interface can be treated as a smooth surface. The valuesof r(x, y), f
1
(x, y), f
2
(x, y)
in (19.17)–(19.18)are average values bythe area (x, y) with size larger than a typical
roughness/heterogeneity detail size. Therefore, the generalized Wenzel and Cassie
equations can be used at the scale, at which the effect of the interface perturbations
vanish,or,in otherwords,when thesizeof thesolid surfaceroughness/heterogeneity
details is small comparing with the size of the liquid-air interface, which is of the
same order as the size of the droplet.
Nosonovsky and Bhushan [106] used the surface energy approach to find the do-
main of validity of the Wenzel and Cassie equations (uniformlyrough surfaces) and
generalized it for a more complicated case of non-uniform surfaces. The general-
ized equations explain a wide range of existing experimental data, which could not
be explained by the original Wenzel and Cassie equations.
19.2.3 Contact Angle Hysteresis
The contact angle hysteresis is another important characteristic of a solid-liquid
interface (Fig. 19.5a). The contact angle hysteresis occurs due to surface roughness
and heterogeneity. Low contact angle hysteresis results in a very low water roll-
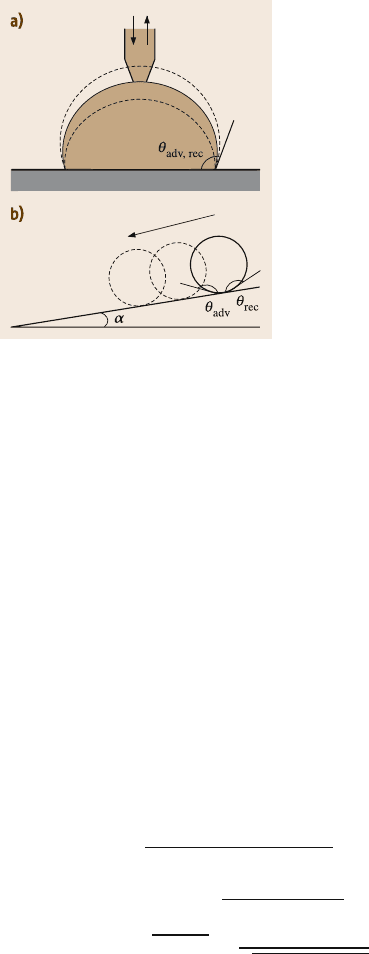
19 Lotus Effect: Roughness-Induced Superhydrophobic Surfaces 1011
Fig. 19.5. (a) Liquid droplet in contact with rough
surface (advancing and receding contact angles are
θ
adv
and θ
rec
, respectively) and (b) tilted surface
profile (the tilt angle is α) with a liquid droplet
off angle, which denotes the angle to which a surface may be tilted for roll-off of
water drops (i.e., very low water contact angle hysteresis) [18, 19, 46,68, 69, 73]
(Fig. 19.5b). Low water roll-off angle is important in liquid flow applications such
as in micro/nanochannels and surfaces with self cleaning ability.
There is no simple expression for the contact angle hysteresis as a function of
roughness; however, certain conclusions about the relation of the contact angle hys-
teresis to roughness can be made. Using (19.12), the difference of cosines of the
advancing and receding angles is related to the difference of those for a nominally
smooth surface, θ
adv0
and θ
rec0
, as [16,100]
cosθ
adv
−cosθ
rec
= R
f
(1− f
LA
)(cosθ
adv0
−cosθ
rec0
)+ H
r
, (19.19)
where H
r
is the effect of surface roughness, which is equal to the total perimeter of
the asperity per unit area. It is observed from (19.12) and (19.19), that increasing
f
LA
→ 1 results in increasing the contact angle (cosθ →−1,θ → π) and decreasing
the contact angle hysteresis (cosθ
adv
−cosθ
rec
→ 0). In the limiting case of very
small solid-liquid fractional contact area under the droplet, when the contact angle
is large (cosθ ≈−1+ (θ−π)
2
/2, sinθ ≈π−θ) and the contact angle hysteresisis small
(θ
adv
≈ θ ≈ θ
rec
), (19.12) and (19.19) are reduced to
θ −π =
2(1− f
LA
)(R
f
cosθ
0
+ 1) (19.20)
θ
adv
−θ
rec
= (1− f
LA
)R
f
cosθ
a0
−cosθ
r0
sinθ
=
1− f
LA
R
f
cosθ
r0
−cosθ
a0
√
2(R
f
cosθ
0
+ 1)
. (19.21)
For the homogeneous interface, f
LA
= 0, whereas for composite interface f
LA
is not a zero number. It is observed from (19.20)–(19.21) that for homogeneous
interface, increasing roughness (high R
f
) leads to increasing the contact angle hys-
teresis (high values of θ
adv
−θ
rec
), while for composite interface, an approach to
