Bhushan B. Nanotribology and Nanomechanics: An Introduction
Подождите немного. Документ загружается.

940 Bharat Bhushan
on microwearare shown in Fig. 17.22 [44,47]. The adhesive force of silicon showed
an increase with relative humidity, Fig. 17.22a. This is expected since the surface
of silicon is hydrophilic, as shown in Fig. 17.20a. Greater condensation of water at
the tip–sample interface increases the adhesive force due to the capillary effect. On
the other hand, the adhesive forces of the SAMs showed very weak dependencies
on humidity. This may be because the surfaces of the SAMs are hydrophobic. The
adhesive forces of ODMS/Si and ODDMS/Si showed slight increases from 75 to
90% RH. This increase was absent for PFTS/Si. This may result from the more hy-
drophobic surface properties of PFTS/Si. The Al substrate is partially hydrophobic
and hence does not show much of a dependence on humidity, Fig. 17.22b. The OP
and ODP SAMs deposited on Al substrates showed almost no change in adhesive
force with humidity. The highly hydrophobicnature of these monolayers means that
the contribution of the water menisci to the overall adhesive force is negligible at all
humidities.
The friction force of silicon showed an increase with relative humidity up to
about 75% RH and then a slight decrease beyond this point, see Fig. 17.22a. The
initial increase could result from the increase in adhesive force. The decrease in
friction force at higher humidities could be attributed to the lubricant effects of the
water layer. This effect is more pronounced in the coefficient of friction. Since the
adhesive forceincreased and the coefficient of friction decreased in this range, those
effects cancelled each other out and the resulting friction force showed only slight
changes. On the other hand, the friction forces and coefficients of friction of the
SAMs showed very small changes with relative humidity; similar behavior to that
observed for adhesive force. This suggests that the adsorbed water layer on the sur-
face maintained a similar thickness throughout the relative humidity range tested.
The differences among the SAM types were small within measurement errors, but
a closer look at the coefficient of friction for ODMS/Si showed a slight increase
from 75 to 90% RH compared to PFTS/Si, possibly due to the same reasons as for
the increase in adhesive force. The inherent hydrophobicityof the SAMs means that
they do not show much relative humidity dependence.
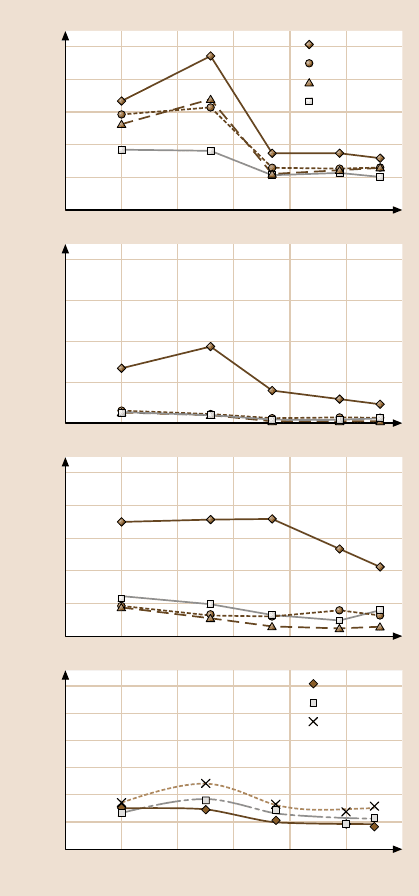
Figure 17.23 shows the effect of temperature on the adhesive force, the friction
force at a normal load of 5 nN and the coefficient of friction [44, 47]. The adhe-
sive force for silicon increased with the temperature from room temperature (RT)
to 55
◦
C, and then decreased from 55 to 75
◦
C before eventually leveling off from
75 to 110
◦
C, see Fig. 17.23a. The adhesive forces of the SAMs showed a similar
tendency except that the initial increase was not pronounced. The initial increase
in adhesive force for silicon at low temperatures is not understood. The decrease
observed for silicon could be attributed to the desorption of water molecules on
the surface. After the water layer has been almost completely depleted, the adhe-
sive force may stay constant. The SAMs with hydrocarbon backbone chains (OP,
ODP, ODMS and ODDMS) showed similar behavior to the Al substrate but the ini-
tial increase in the adhesive force with temperature was much smaller. The SAMs
with fluorocarbon backbonechains showed almost no temperature dependence. The
adhesive force shows some temperature dependence for the SAMs with hydrocar-
17 Self-Assembled Monolayers (SAMs) for Controlling Adhesion 941
Adhesive force (nN)
50.0
40.0
30.0
20.0
10.0
Temperature (°C)
0
120
Friction force (nN)
Coefficient of friction
Adhesive force (nN)
a)
00.0
0.00
0.00
0
8.00
6.00
4.00
2.00
0.10
0.08
0.06
0.04
0.02
120
100
80
60
40
20
b)
20 40 60 80 100
Normal load = 5 nN
Al substrate
Si
ODDMS/Si
ODMS/Si
PFTS/Si
ODP
OP
Al
Fig. 17.23. Effect of temper-
ature on the adhesive force,
the friction force and the
coefficient of friction for
various SAMs on (a)Si
substrates [44], and (b)Al
substrates
bon backbone chains. This increase in adhesive force is believed to be caused by
the melting of the SAM film. The typical melting point for a linear carbon chain
molecule such as CH
3
(CH
2
)
14
CH
2
OH is 50
◦
C [92]. As the temperature increases,
the SAM film softens, thereby increasing the real area of contact and consequently
the adhesive force. Once the temperature is higher than the melting point, the lu-
942 Bharat Bhushan
brication regime is changed from boundary lubrication in the solid SAM to liquid
lubrication in the melted SAM [41].
The friction force of silicon increased with temperature and then steadily de-
creased. The friction force is highly affected by changes in adhesion. The decrease
in friction could therefore result from the depletion of the water layer. The coeffi-
cient of friction for silicon remained constant and then decreased, starting at about
80
◦
C. For the SAMs, the coefficient of friction exhibited a monotonic decrease
with temperature. The decrease in the friction and the coefficient of friction for
the SAMs possibly results from the decrease in stiffness. As mentioned before, the
spring model suggests lower friction for more compliant SAMs [41]. The different
types of SAM types behaved reasonably similarly. PFTS kept its stiffness more than
ODMS and ODDMS when the temperature was increased [93], but this was not
a particularly pronounced effect in the results.
Figure 17.23b shows the effect of temperature on the adhesive force for SAMs
on Al. The adhesive force increased for Al substrate up to 50
◦
C and then decreased
to a stable value for higher temperatures. This initial increase in the adhesive force
with temperature for Al is not understood. The inherently high hydrophobicity of
the SAMs over the corresponding substrates meant that they did not show much of
a relative humidity or temperature dependence.
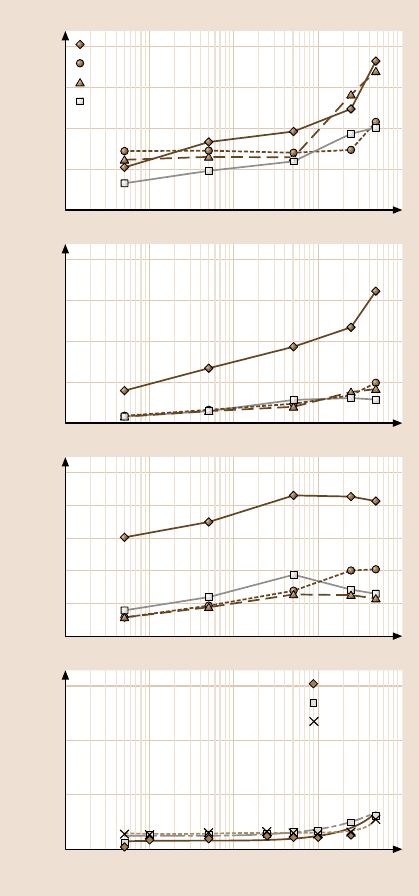
Figure 17.24 shows the effect of sliding velocity on the adhesive force, the fric-
tion force and the coefficient of friction [44,47]. The adhesive force for silicon re-
mained relatively constant at low sliding velocities, and then increased rapidly: see
Fig. 17.24a. A similar trend was observed for the SAMs. The increase in adhesive
force for silicon is believed to be due to a tribochemical reaction at the interface
between the tip and sample [23] and the increased contact area due to mechanical
plowing. For the SAMs, the increased adhesive force at high velocities may result
from viscous drag of SAM molecules [94]. SAMs can be detached from the surface
and attached to an AFM tip. In addition, the increasedcontact area may be causedby
greater penetration of the AFM tip into the SAM. The rate of increase was larger for
ODMS than for PFTS, presumably because of the higher stiffness and more dense
structure of PFTS.
The coefficient of friction increased with sliding velocity and then reached
a plateau for Si, ODMS/Si and ODDMS/Si. As the sliding speed is increased, ex-
tra work is needed for SAM reorientation, which may lead to increased friction.
For PFTS, the coefficient of friction decreased at large sliding velocities, result-
ing in a peak. This peak structure may result from the viscoelastic properties of
SAMs [95].
Figure 17.24b shows the effect of sliding velocity on the friction force. Friction
force was found to remain constant over a range of sliding velocities for Al sub-
strate as well as the OP and ODP SAMs deposited on Al substrates. The increase in
friction force at high velocities (> 1mm/s) is the result of asperity impacts and cor-
respondingly high frictional energy dissipation at the sliding interface for Al [96].
For the OP and ODP SAMs, the increase in friction force is believed to result from
the SAM molecules reorienting under the tip load and during the tip motion. The
17 Self-Assembled Monolayers (SAMs) for Controlling Adhesion 943
Adhesive force (nN)
80.0
60.0
40.0
20.0
Sliding velocity (μm/s)
10
0
Friction force (nN)
Coefficient of friction
Friction force (nN)
a)
00.0
0.00
0.00
0
8.00
6.00
4.00
2.00
0.10
0.08
0.06
0.04
0.02
15.0
10.0
5.0
b)
10
1
10
2
10
3
10
4
Normal load = 5 nN
Al substrate
Si
ODDMS/Si
ODMS/Si
PFTS/Si
ODP
OP
Al
Fig. 17.24. Effect of sliding
velocity on the adhesive
force, the friction force and
the coefficient of friction for
various SAMs on (a)Si
substrates [44], and (b)Al
substrates (22
◦
C, 50% RH,
70 nN normal load)
SAM reorientation can act as an additional hindrance to tip motion when the AFM
tip reverses during scanning, resulting in higher friction. The molecules can get en-
tangled and/or get detached from the substrate and attach to the AFM tip. Tambe
and Bhushan [94] extended the molecular spring model presented by Bhushan and
944 Bharat Bhushan
Liu [39] to explain this velocity-dependent increase in friction force for compliant
SAM molecules.
Overall, the SAMs deposited on the Al substrate showed much lower friction
at all sliding velocities than those deposited on the Si substrates. As discussed, the
primary reason for this is believed to be the ease with which the molecules on Al
substrate can rotate dueto the absence of either the
−
CH
3
groupsor anycrosslinking
at the head groups. The higher stiffness of the fluorocarbon backbone chains than
the hydrocarbonbackbone chains [44] is also believed to result in the higher friction
for PFTS than ODMS and ODDMS.
AFM Wear Measurements
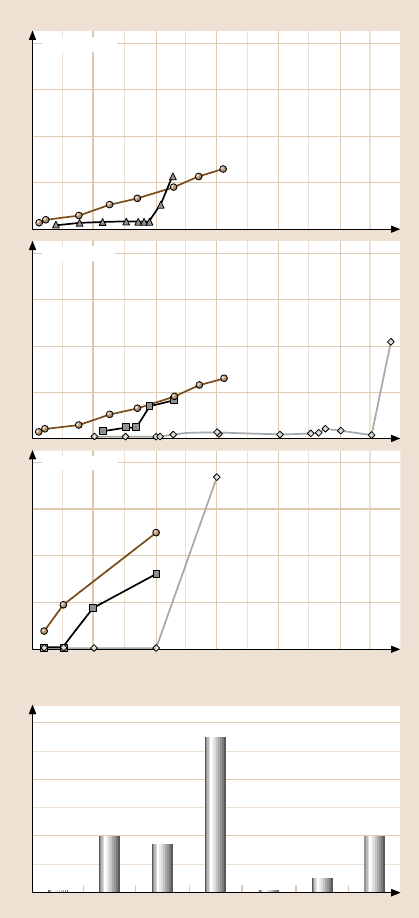
Figure17.25ashowsthe relationshipsbetweensurfaceheight andnormal load found
for various SAMs during wear tests [44, 47]. As shown in the figure, the SAMs
exhibit a critical normal load beyond which the surface height decreases drasti-
cally. Figure 17.25a also shows the wear behavior of the Al and Si substrates. Un-
like the SAMs, the substrates show a monotonic decrease in surface height with
increasing normal load, with wear initiating from the very beginning, even for
low normal loads. Si (Young’s modulus of elasticity, E = 130GPa [97], hardness,
H = 11GPa [28]) is relatively hard in comparisonto Al (E = 77 GPa, H = 0.41GPa)
and hence the decrease in surface height for Al is much larger than that for Si for
similar normal loads.
The critical loads corresponding to the sudden failure of the SAM are shown in
Fig. 17.25b. Amongst all the SAMs, ODDMS shows the best performance in the
wear tests, and this is believed to be due to the chain length effect (it has a longer
chain). OP and ODP show very similar wear behavior to ODMS and ODDMS.
ODP exhibits a higher critical load than OP because of its longer chain length. The
mechanism of failurefor compliantSAMs duringwear tests was presented earlier,in
Fig. 17.18. It is believed that the SAMs usually fail due to shearing of the molecule
at the head group; that is, the molecules are sheared off the substrate. Table 17.14
gives the bond strengths for various intermolecular bonds. The weakest bonds are at
the interface, and hence failure is expected to occur at the interface first.
To study the effect of relative humidity on wear, wear tests were performed at
various humidities. The bottom of Fig. 17.22a shows the critical normal load as
a function of relative humidity. The critical normal load shows a weak dependency
on the relative humidity for ODMS/Si and PFTS/Si, and was larger for ODMS/Si
than for PFTS/Si throughout the range of humidities used. For ODDMS/Si, the
critical normal load showed an increase with relative humidity. This suggests that
water molecules can penetrate into ODDMS, which then might work as lubri-
cant [41,100]. This effect was absent for PFTS/Si and ODMS/Si.
17 Self-Assembled Monolayers (SAMs) for Controlling Adhesion 945
Decrease in surface height (nm)
20
15
10
5
0
a)
Normal load (μN)
0
60
20
15
10
5
0
10 20 30 40 50
20
15
10
5
0
60
40
20
0
Si PFTS ODMS ODDMS Al OP ODP
SiPFTS
Si
ODMS
ODDMS
Al
OP
ODP
b)
Si substrate
Si substrate
Al substrate
Normal load (μN)
Fig. 17.25. (a) Decrease in
surface height as a function
of normal load after one scan
cycle for various SAMs on
Si and Al substrates, and
(b) comparison of critical
loads for failure during wear
tests for various SAMs

946 Bharat Bhushan
Table 17.14. Typical bond strengths
a
in SAMs
Hexadecanethiol Biphenylthiol Perfluoroalkylsilane Alkylsilane Akylphosphonate
(HDT) (BPT) (PFTS) (ODMS or ODDMS) (OP and ODP)
Bond (kJ/mol) (kJ/mol) Bond (kJ/mol)
Interfacial bonds
S
−
Au 184
b
184
b
Si
−
O 242
c
242
c
242
c
S
−
C 286
a
800
d
800
d
800
d
C
6
H
5
−
S 362
a
Si
−
C 414
a
414
a
Al
−
O 511
a
P
−
C 513
a
P
−
O 599
a
Bonds in backbone
C
−
CC
−
C
CH
2
−
CH
2
326
e
CH
2
−
CH
2
326
e
326
e
CH
3
−
CH
2
≈ 305
a
CF
2
−
CF
2
≈ 326
f
C
6
H
5
strong CF
2
−
CH
2
≈ 326
f
CF
3
−
CF
2
≈ 326
f
CH
3
−
CH
2
≈ 305
a
a
Lide [92]
b
Chemical adsorption bond from Lio et al. [72]
c
Chemical adsorption bond from Hoshino [98]
d
In diatomic molecules
e
Cottrell [99]
f
Because of the C
−
C bond it is expected to be close to that of CH
2
−
CH
2
17 Self-Assembled Monolayers (SAMs) for Controlling Adhesion 947
17.4.4 Degradation and Environmental Studies
Degradation Studies
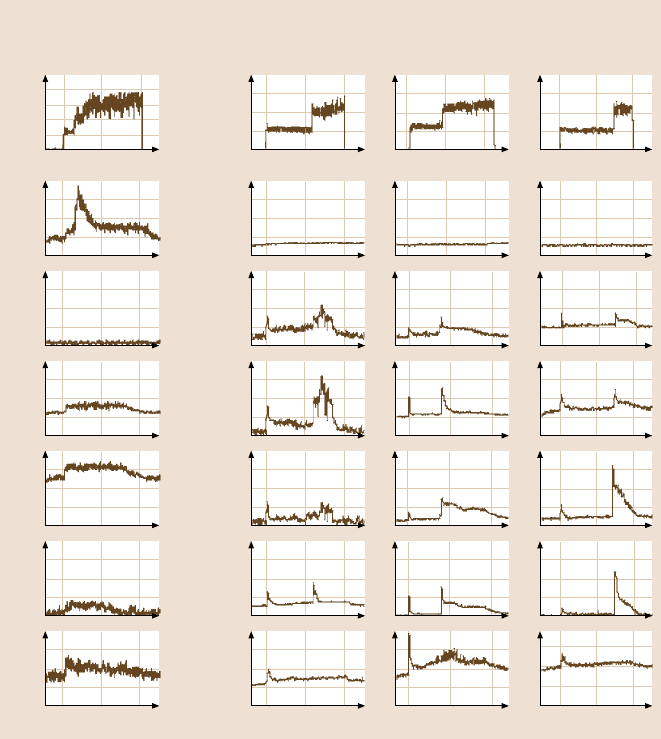
The coefficientof friction and the gaseousproducts detectedfor HDT/Au are shown
in Fig. 17.26a [48]. A normal pressure of 50kPa was applied to the HDT films.
The coefficient of friction increased after a sliding distance of about 10m. During
sliding, (CH
2
)
15
S, C
2
H
3
,CH
3
,CH
2
and H
2
were detected by a mass spectrometer.
The partial pressure of the HS fragments is of interest since it corresponds to the
interface bonds, and so it is reported here. The increase in (CH
2
)
15
S was much
HDT/Au
0.8
0.6
0.4
0.2
0
Coefficient of friction
200
150
100
50
0
(CH
2
)
15
S
4
3
2
1
0
HS
4
3
2
1
0
C
2
H
3
4
3
2
1
0
CH
3
4
3
2
1
0
CH
2
8
6
4
2
0
H
2
50 1000
Sliding distance (m)
0.8
0.6
0.4
0.2
0
Coefficient of friction
20
15
10
5
0
SiO
8
6
4
2
0
C
2
H
5
12
9
6
3
0
C
2
H
3
24
18
12
6
0
CH
3
8
6
4
2
0
CH
2
24
16
12
6
0
H
2
50 1000
Sliding distance (m)
0.8
0.6
0.4
0.2
0
Coefficient of friction
20
15
10
5
0
SiO
4
3
2
1
0
CF
3
4
3
2
1
0
HCF
2
4
3
2
1
0
CF
2
8
6
4
2
0
CH
2
20
15
10
5
0
H
2
50 1000
Sliding distance (m)
0.8
0.6
0.4
0.2
0
Coefficient of friction
20
15
10
5
0
SiO
8
6
4
2
0
C
2
H
5
12
9
6
3
0
C
2
H
3
24
18
12
6
0
CH
3
8
6
4
2
0
CH
2
24
16
8
4
0
H
2
50 1000
Sliding distance (m)
Partial pressure (
× 10
–10
Torr) Partial pressure (× 10
–10
Torr)
PFTS/Si ODMS/Si ODDMS/Si
Vacuum pressure = 2 × 10
–7
Torr
Normal pressure = 50 kPa
Vacuum pressure = 2 × 10
–7
Torr
Normal pressure = 50 kPa
Partial pressure (× 10
–10
Torr) Partial pressure (× 10
–10
Torr)
a) b)
Fig. 17.26. Coefficients of friction and mass spectral data on (a) HDT/Au (1.9nm),
(b) PFTS/Si(1.8 nm), ODMS/Si(≈1.9 nm)and ODDMS/Si(≈2.1 nm) in high vacuum [48]
948 Bharat Bhushan
more than that of other species, due to the breaking of the S
−
Au bond. The partial
pressures of C
2
H
3
,CH
3
,CH
2
,andH
2
were also foundto increase duringthe sliding.
There was no noticeable change in the partial pressure of HS.
The HDT film was deposited on an Au(111) layer. The bond strength of
S
−
Au is 184kJ/mol (Table 17.14), which is lower than those of the C
−
C bonds
(425kJ/mol), C
−
H bonds (422 kJ/mol), and C
−
S bonds (286kJ/mol) in the alkyl
chains. Since the S
−
Au bond is the weakest bond in the alkanethiolchain, the whole
chain should be sheared away from the substrate. Because the upper atomic mass
unit (amu) limit of the mass spectrometer used is 250, we monitored (CH
2
)
15
S
(amu = 242), which is the chain with CH
3
sheared away. The generation rate of
(CH
2
)
15
S is much larger than that of other species. This suggests that the mechan-
ical shear of the whole alkanethiol chain be the dominant factor causing the failure
oftheHDTfilm.ThecleavageoftheS
−
Au bondshas been reported in theliterature.
Basedon the bondstrengths,as well asthe abovestudies, mechanicalshearing ofthe
C
−
C bonds and C
−
H bonds probably does not happen during sliding. The reaction
induced by low-energy electrons, generated by triboelectrical emission during the
sliding, could be responsible for the degradation of the alkanethiol chain. Thermal
desorption of HDT from Au is another possibility for the degradation mechanism
of HDT.
The coefficientof frictionand gaseous productsgeneratedfor PFTS/Si, ODMS/
Si and ODDMS/SI are shown in Fig. 17.26b [48]. The coefficients of friction for
PFTS/Si, ODMS/Si, and ODDMS/Si increase sharply after a certain sliding dis-
tance, which indicates the degradation of the film. At the same time, gaseous prod-
ucts of CF
3
,HCF
2
,CF
2
,CH
2
and H
2
were detected for PFTS/Si, and C
2
H
5
,C
2
H
3
,
CH
3
,CH
2
and H
2
were detected for ODMS/Si and ODDMS/Si.
PFTS/Si showed lower friction than ODMS/Si in the tests. ODDMS/Si showed
lower friction than both PFTS/Si and ODMS/Si. This is because of the chain length
effect; as mentioned earlier, it has been reported that for SAMs the coefficient of
friction decreases with the carbon backbone chain length (n) when the carbon atoms
are less than 12. For chains with more than 12 carbons, increasing the number of
carbon atoms will not influence the coefficient of friction to any noticeable extent.
PFTS/Si showed greater durability than ODMS/Si. It is harder to rotate a per-
fluorinated carbon backbone (due to the larger size of F versus H) which implies
that this structure is more rigid than a hydrocarbon backbone [91]. Chambers [101]
has reported that the C
−
C bond strength increases when hydrogen is replaced with
fluorine.This suggests that the rigidperfluorinatedcarbon backbone may be respon-
sible for the increased durability. The length of the alkyl chain also influences the
desorption energies of alkanes. Based on studies of the adsorption of alkanes on
Cu(100), Au(111), Pt(110) and others, the physisorption energy increases with the
alkyl chain length [102–104]. Therefore, ODDMS are more durable than ODMS.
During sliding on PFTS films, gaseous products of CF
3
,HCF
2
,CF
2
,CH
2
and
H
2
were detected. From the structure of perfluoroalkylsilane, the only source of H
on the molecular chain which would cause a partial pressure increase of H
2
is the
(CH
2
)
2
, which is located at the bottom of the chain. Since the partial pressure of
17 Self-Assembled Monolayers (SAMs) for Controlling Adhesion 949
H
2
increases immediately after sliding and remains high until the end of sliding, it
is probably generated by low-energy electrons arising from triboelectrical emission.
The partial pressure of CH
2
exhibits a sharp peak at the beginning of sliding and
at the moment when friction changes. Meanwhile, the partial pressures of CH
3
,
HSF
2
and CF
2
increased significantly when the friction increased. For ODMS and
ODDMS, C
2
H
5
,C
2
H
3
,CH
3
,CH
2
and H
2
were detected during sliding. The partial
pressures of the carbon-related products increase considerably when the friction
is increased. SiO, which is associated with interface bonds, shows no noticeable
change during sliding.
Perfluoroalkylsilanes and alkylsilanes are attached to the naturally oxidized sili-
conbySi
−
O bonds. The Si
−
O bond strength varies widely (Table 17.14) depend-
ing on the formation conditions. In the alkylsilane chain, the C
−
Si bond strength
(414kJ/mol) is slightly lower than the C
−
C bond strength. Based on Table 17.14,
the interfacialbonds (Si
−
O) are weakerthan the C
−
C bondsin the backbone.There-
fore, it is believed that film cleavage occurs at the interface. We have previously
reported evidence of the cleavage of interfacial bonds using an AFM. To explain
the hydrogen, C
1
and C
2
hydrocarbon (in the tests for PFTS/Si, ODMS/Si and
ODDMS/Si) or fluorocarbon (in the tests for PFTS/Si) products, Kluth et al. [105]
suggested that the alkylsilane (or perfluoroalkylsilane) chains break and create rad-
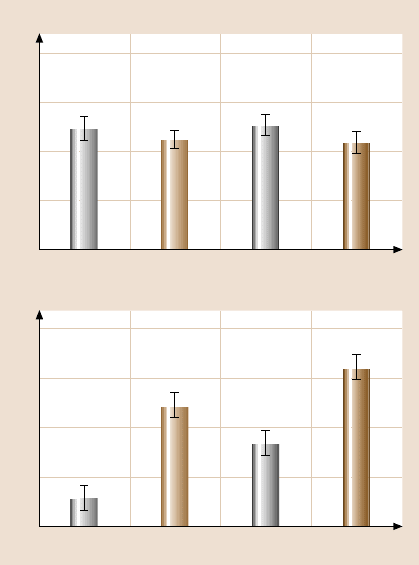
0.4
0.3
0.2
0.1
0
ODDMS/Si
100
75
50
25
0
ODMS/SiPFTS/SiHDT/Au
ODDMS/SiODMS/SiPFTS/SiHDT/Au
Coefficient of friction before failure of the film
Durability in sliding distance (m)
Fig. 17.27. Coefficient of
friction (upper) and dura-
bility (lower) comparisons
for HDT/Au, PFTS/Si,
ODMS/Si and ODDMS/Si
in high vacuum. Error bars
represent ±3σ basedonfive
measurements (normal pres-
sure 50 kPa) (after [48])
