Bhushan B. Nanotribology and Nanomechanics: An Introduction
Подождите немного. Документ загружается.

920 Bharat Bhushan
Xiao et al. [71] and Lio et al. [72] also studied the effect of the length of the alkyl
chain on the frictional properties of n-alkanethiolate films on gold and n-alkylsilane
films on mica. Friction was foundto be particularlyhigh for short chainsof less than
eight carbon atoms. Thiols and silanes exhibit similar friction force for the same n
when n> 11; for n < 11, silanes exhibit higher friction, about three times larger than
that for thiols for n = 6. The increase in friction was attributed to the large number
of dissipative modes in the less ordered chains that occur when going from a thiol
to a silane anchor or when decreasing n. Longer chains (n > 11), stabilized by van
der Waals attractions, form more compact and rigid layers and are better lubricants.
Schonherr and Vancso [73] also correlated the magnitude of the friction with the
order among the alkane chains. The disorder in short-chain hydrocarbon disulfide
SAMs was found to result in a significant increase in the magnitude of the friction.
Tsukruk and Bliznyuk [74] studied the adhesion and friction between a Si sam-
ple and a Si
3
N
4
tip, in which both surfaces were modified by
−
CH
3
-,
−
NH
2
-and
−
SO
3
H-terminated silane-based SAMs. Various polymer molecules were used for
the backbone. They reported a very broad maximum adhesive force at pH 4–8, with
minimumadhesion at pH> 9 and pH< 3, for all ofthe studiedmating surfaces. This
observation can be understood by considering a balance of electrostatic and van der
Waals interactions between composite surfaces with multiple isoelectric points. The
friction coefficients of NH
2
/NH
2
-andSO
3
H/SO
3
H-mating SAMs are very high in
aqueous solution. Capping NH
2
-modified surfaces (3-aminopropyltriethoxysilane)
with rigid and soft polymer layers resulted in a significant reduction in adhesion,
to a level lower than that of the untreated surface [75]. Fujihira et al. [76] studied
the influence of surface terminal groups of SAMs and functional tips on adhesive
force. It was found that the adhesive force measured in air increases in the order
CH
3
/CH
3
,CH
3
/COOH, COOH/COOH.
Bhushan and Liu [39], Liu et al. [40], and Liu and Bhushan [41, 42] studied
adhesion, friction and wear properties of alkylthiol and biphenylthiol SAMs. They
explained the friction mechanisms using a molecular spring model in which local
stiffness and intermolecularforces governfriction properties.They studiedthe influ-
ence of relative humidity, temperature and velocity on adhesion and friction. They
also investigated the wear mechanisms of SAMs using a continuous microscratch
AFM technique.
Fluorinated carbon (fluorocarbon) molecules are known to have low surface en-
ergy and are commonly used for lubrication [2,9,10]. Bhushan et al. [43,46], Kasai
et al. [44] and Lee et al. [45] studied the friction and wear properties of methyl- and
perfluoro-terminated alkylsilanes on silicon. Bhushan et al. [5] and Kasai et al. [44]
reported that perfluoroalkylsilaneSAMs exhibit lower surface energies, higher con-
tact angles, lower adhesive forces, and lower wear than those of alkylsilanes. Kasai
et al. [44] also reported the influence of relative humidity, temperature and veloc-
ity on adhesion and friction. Tambe and Bhushan [47] studied the nanotribological
properties of methyl-terminated alkylphosphonate on aluminium, which is of in-
dustrial interest. They found that these SAMs perform as well on aluminium as on
17 Self-Assembled Monolayers (SAMs) for Controlling Adhesion 921
silicon. Tao and Bhushan [48] studied degradation mechanisms of SAMs. They re-
ported that oxygen from the air causes thermal oxidation of SAMs.
To date, the nanotribological properties of alkanethiol, biphenylthiol, alkylsi-
lane and perfluoroalkylsilane SAMs have been widely studied. In this chapter, we
review, in some detail, the nanotribological properties of various SAMs that have
alkyl and biphenyl spacer chains with different surface terminal groups (
−
CH
3
,
−
CF
3
) and head groups (
−
S
−
H,
−
Si
−
O
−
,
−
OH, and P
−
O
−
), which were inves-
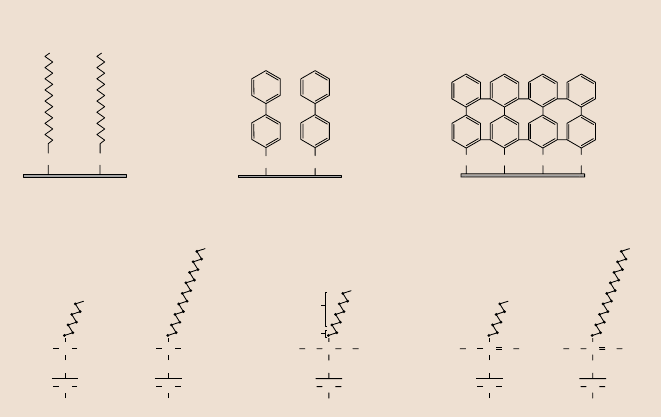
tigated by AFM at various operating conditions, Fig. 17.8a,b [5,39–42,44,47,48].
Hexadecanethiol (HDT), 1,1
-biphenyl-4-thiol(BPT) andcrosslinked BPT (BPTC)
were deposited on Au(111) substrates by immersing the substrate in a solution
containing a precursor (ligand) that reacts with the substrate surface. Crosslinked
BPTC wasproduced by irradiatingBPT monolayerswith low-energyelectrons.Per-
fluorodecyltricholorosilane (PFTS), CF
3
−
(CF
2
)
7
−
(CH
2
)
2
−
SiCl
3
, n-octyldimethyl
(dimethylamino) silane (ODMS), CH
3
−
(CH
2
)
n
−
Si(CH
3
)
2
−
N(CH
3
)
2
(n = 7), and
n-octadecylmethyl(dimethylamino)silane (n = 17) (ODDMS) were deposited on Si
by exposing thesubstrate to the vaporof the reactive chemicalprecursor. Octylphos-
phonate (OP)
CH
3
−(CH
2
)
n
−
O
|
P
||
O
−OH(n = 7)
a)
b)
Hexadecane thiol
(HDT)
1,1'–biphenyl–4–thiol
(BPT)
Cross-linked 1,1'–biphenyl–4–thiol
(BPTC)
CH
3
Si
O
SiCH
3
(CH
2
)
17
CH
3
Si
O
Si
(CF
2
)
7
CF
3
OO
(CH
2
)
2
Al
O
P
(CH
2
)
7
OO
CH
3
Al
O
P
(CH
2
)
17
OO
CH
3
CH
3
Si
O
SiCH
3
CH
3
(CH
2
)
7
n-Dimethyl(dimethylamino)silane Perfluorodecyltrichlorosilane n-Phosphonate
Octodecyl (ODDMS) Deca (PFTS) Octadecyl (ODP)Octa (ODMS) Octa (OP)
CH
3
S
Alkyl
–(CH
2
)
n
–
Au(111)
CH
3
S
Biphenyl
–(C
6
H
6
)
2
–
Au(111)
S
S
Au(111)
SSSS
Fig. 17.8. Schematics of the structures of (a) hexadecane and biphenyl thiol SAMs on
Au(111) substrates, and (b) perfluoroalkylsilane and alkylsilane SAMs on Si with native ox-
ide substrates, as well as alkylphosphonate SAMs on Al with native oxide
922 Bharat Bhushan
and octadecylphosponate(n = 17)(ODP) were depositedon Al by liquiddeposition.
Thermally evaporated Au(111)films on Si(111) substrate were selected because the
epitaxial film is smooth and defect-free, which is desirable for SAM applications.
Bulk Si(100) and Al with natural oxide layers were selected because they are used
in the construction of MEMS/NEMS.
17.4.1 Measurement Techniques
Static Contact Angle Measurement Using DI Water
The static contact angle, a measure of how water repellent a material is, was meas-
ured using a Rame–Hart model 100 contact angle goniometer (Mountain Lakes,
NJ, USA) [77,78]. Ten microliter droplets of DI water were typcially used for the
contact angle measurements. At least two measurements of the contact angle were
taken. The contact angles were reproducible within ±2
◦
.
AFM Adhesion and Friction Measurements
Adhesion and friction tests were conducted using a commercial AFM system
(Dimension 3000, Nanoscope IIIa controller, DI, Santa Barbara, USA). Square-
pyramidal Si
3
N
4
tips with a 30–50nm tip radius were used on a gold back-coated
triangularSi
3
N
4
cantileverwith a typicalspring constant of 0.58 N/m. The adhesion
can be calculated using either force calibration plots or from the negative intercepts
on friction force versus normal load plots. Both methods generally yield similar re-
sults. The force calibration plot technique was used in this study. The coefficient of
friction was obtained from the slope of a plot of the friction force versus the normal
load. Normal loads typically ranged from 5 to 100nN. Friction force measurements
were performed at a scan rate of 1 Hz along the fast scan axis and over a scan size of
2 µm. The fast scan axis was perpendicular to the longitudinal direction of the can-
tilever. The friction force was calibrated by the method described in Bhushan [4].
Effects of Relative Humidity, Temperature and Sliding Velocity
The influence of the relative humidity on the adhesive force, the friction force and
the wear was studied in an environmentally controlled chamber. Relative humidity
was controlled by introducing a mixture of dry and moist air into the chamber. The
temperature was maintained at 22±1
◦
C. The sample was kept in the environmental
chamber at the desired humidity for at least 2 h prior to the tests so that the system
could reach equilibrium.
In order to study the effect of temperature on adhesion and friction force, the
samples were placed on a thermal stage during the measurements. A glass plate
was placed under the thermal stage to preventthe heat from being transported away.
The temperature range studied was from 20 to 110
◦
C. The relative humidity was
maintained at 50±5% during the measurements.
The effect of sliding velocity on friction force was monitored in ambient con-
ditions using a high-velocity piezo stage designed to achieve high relative sliding
17 Self-Assembled Monolayers (SAMs) for Controlling Adhesion 923
velocities on a commercial AFM set-up [79]. The traveling distance of the sample
(the scan size)was set at 25µm, while the scan frequencywas varied between0.1Hz
(5µm/s) and 100Hz (5000µm/s).
AFM Wear Measurements
Wear tests were conducted using a diamond tip with a nominal radius of 50nm
and a cantilever with a nominal stiffness of 10N/m. Wear tests were performed on
a scan area of 1 µm×1 µm at the desired normal load and at a scan rate of 1 Hz.
After each wear test, an area of 3 µm×3µm was imaged and the average wear depth
was calculated.
Degradation and Environmental Studies
The lubricant degradation experiments were carried out in a high-vacuum tribotest
apparatus [82, 83]. The system was equipped with a mass spectrometer so that
gaseous emissions from the interface could be monitored in situ during the slid-
ing in high vacuum. The normal loads and friction forces at the contacting interface
were measured using resistive-type strain-gauge transducers. Sliding tests were con-
ducted by rubbing the sample against a flat sample of Si(100) at a vacuum pressure
of 2×10
−7
Torr at a sliding speed of 0.3m/s. The environmental effects were inves-
tigated in high vacuum (2×10
−7
Torr), argon, dry air (less than 2% RH), ambient
air (30% RH) and in high humidity air (70% RH).
17.4.2 Hexadecane Thiol and Biphenyl Thiol SAMs on Au(111)
Hexadecane thiol on Au(111) was selected as it is a widely studied film. Biphenyl
thiol was selected to study the effect of rigidity on nanotribological performance.
The biphenyl thiol film was crosslinked to further increase its stiffness.
Surface Roughness, Adhesion and Friction
Surfaceheight and friction force images of SAMs were recorded simultaneously on
an area of 1 µm×1 µm by an AFM, and adhesive forces were measured using an
AFM in force calibration mode [39].
Detailed analysis is presented later in this chapter, but the measured rough-
nesses, thickness, tilt angles and spacer chain lengths of Si(111), Au(111) and var-
ious SAMs are listed in Table 17.10 [39]. The roughness of BPT is very close to
that of Au(111), but the roughness of BPTC is lower than that of Au(111) and BPT;
this is caused by electron irradiation. Table 17.10 indicates that the roughness of
HDT is much higher than the substrate roughness of Au(111). This is caused by
local aggregation of organic compounds on the substrates during SAM deposition.
Table 17.5 also indicates that the thicknesses of biphenyl thiol SAMs are generally
smaller than those of the alkylthiol, because of the shorter spacer chain in biphenyl
thiol.
924 Bharat Bhushan
Table 17.10. R
a
roughnesses, thicknesses, tilt angles and spacer chain lengths of SAMs
Samples R
a
roughness
a
Thickness
b
Tilt angle
b
Spacer length
c
(nm) (nm) (degrees) (nm)
Si(111) 0.07
Au(111) 0.37
HDT 0.92 1.89 30 1.91
BPT 0.36 1.25 15 0.89
BPTC 0.14 1.14 25 0.89
a
Measured by an AFM with a 1 µm×1 µm scan size, using a Si
3
N
4
tip under 3.3 nN normal
load
b
The thickness and tilt angles of BPT and BPTC are reported by Geyer et al. [67]. The thick-
ness and tilt angles of HDT are reported by Ulman [25]
c
The spacer chain lengths of alkylthiols were calculated by the method reported by Miura
et al. [80]. The spacer chain lengths of biphenyl thiols were calculated from the data reported
by Ratajczak-Sitarz et al. [81]
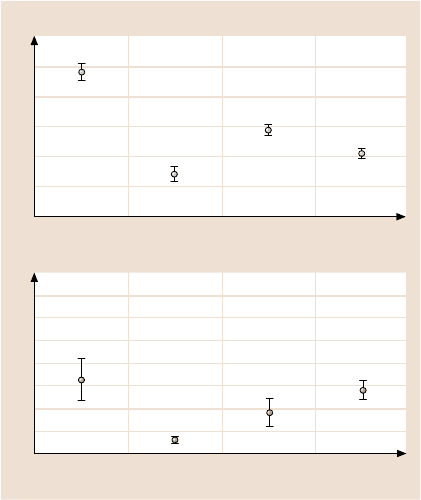
Average values and standard deviations for the adhesive force and coefficient of
friction are presented in Fig. 17.9 [39]. Based on the data, the adhesive forces and
friction coefficients of SAMs are less than those of their corresponding substrates.
Among the various films, HDT exhibits the lowest values. The adhesive force F
a
is ranked as follows: F
a–Au
> F
a–BPT
> F
a–BPTC
> F
a–HDT
. The rankings for the
friction coefficients μ are: μ
Au
>μ
BPTC
>μ
BPT
>μ
HDT
. Note that many SAMs have
similar rankings for both adhesive force and coefficient of friction. This suggests
that alkylthiol and biphenyl SAMs would both make effective molecular lubricants
for micro/nanodevices.
Liquid capillary condensation is one source of adhesion and friction in micro/
nanoscale contact. For a sphere in contact with a flat surface, the attractive Laplace
force caused by a water capillary is
F
L
= 2πRγ
la
(cosθ
1
+ cosθ
2
) , (17.1)
where R is the radius of the sphere, γ
la
is the surface tension of the liquid against
air, and θ
1
and θ
2
are the contact angles between the liquid and flat and spherical
surfaces, respectively [9,10]. In an AFM adhesive study, the tip–flat sample contact
is just like a sphere in contact with a flat surface, and the liquid is water. Since
a single tip is used in the adhesionmeasurements,cosθ
2
can be treated as a constant.
Therefore,
F
L
= 2πRγ
la
(1+ cosθ
1
)−2πRγ
la
(1−cosθ
2
)
= 2πRγ
la
(1+ cosθ
1
)−C , (17.2)
where C is a constant.
Based on the following Young–Dupre equation, the work of adhesion W
a
(the
work required to pull apart a unit area of the solid–liquid interface) can be written
17 Self-Assembled Monolayers (SAMs) for Controlling Adhesion 925
Adhesive force (nN)
60
40
20
0
Au
HDT BPT BPTC
Coefficient of friction
0.08
0.06
0.04
0.02
0
Materials
Au
HDT BPT BPTC
Fig. 17.9. Adhesive forces
and coefficients of friction for
Au(111) and various SAMs
as [84]
W
a
= γ
la
(1+ cosθ
1
) . (17.3)
This indicates that W
a
is determined by the SAM contact angle; in other words it is
influenced by the surface chemistry properties (polarization and hydrophobicity)of
the SAM. By substituting (17.3) into (17.2), F
L
can be expressed as
F
L
= 2πRW
a
−C (17.4)
When the influence of other factors, such as van der Waals force, on the adhesive
force is very small, then the adhesive force F
a
≈ F
L
. Thus the adhesive force F
a
should be proportional to the work of adhesion W
a
.
The contact angle is a measure of the wettability of a solid by a liquid, and it
determines the W
a
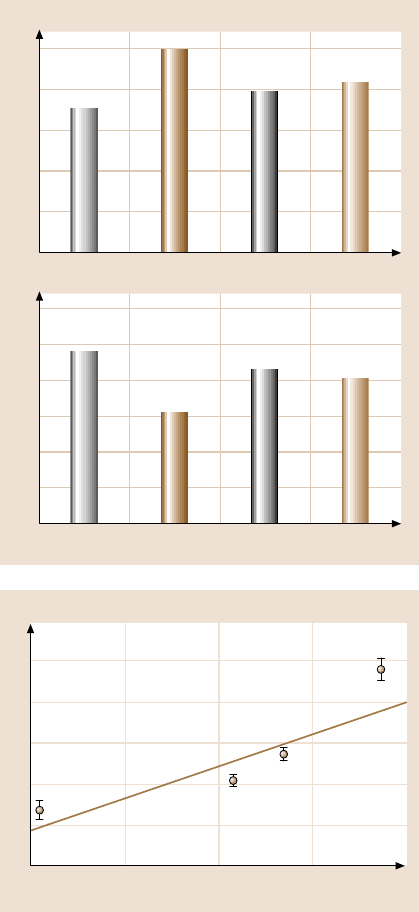
value [77,78]. The contact angles for distilled water on Au(111)
and SAMs have been measured, and are summarized in Fig. 17.10a [39]. For water,
γ
la
= 72.6mJ/m
2
at 22
◦
C.Therefore, usingthisvalue and(17.3),it is possibleto ob-
tain W
a
data, and these are presented in Fig. 17.10b. The W
a
values can be ranked in
the following order: W
a–Au
(97.1) > W
a–BPT
(86.8) > W
a–BPTC
(82.1) > W
a–HDT
(61.4).
Except for W
a–Au
, this order mimics the order of adhesion force in Fig. 17.9. The re-
lationship between F
a
and W
a
is summarized in Fig. 17.11 [39]. It indicates that the
adhesive force F
a
(nN) increases with the work of adhesion W
a
(mJ/m
2
) as follows:
F
a
= 0.57W
a
−22 . (17.5)
926 Bharat Bhushan
100
80
60
40
20
0
120
100
80
60
40
20
0
BPTCBPTHDTAu
Materials
a)
Contact angle (deg)
b)
W
a
(mJ/m
2
)
Fig. 17.10. (a) The static
advancing contact angle, and
(b) the work of adhesion for
Au(111) and various SAMs.
Each point in this figure
represents the mean value
of six measurements. The
uncertainty associated with
the average contact angle is
±2
◦
W
a
(mJ/m
2
)
Adhesive force (nN)
60
40
20
0
60
70 80 90 100
Au(111)
HDT
BPT
BPTC
Fig. 17.11. Relationship
between the adhesive force
and the work of adhesion for
different specimens
These experimental results agree well with the modeling prediction presented
earlier in (17.4). It proves that, on the nanoscale, and under ambient conditions, the
adhesiveforces of SAMs are mainly influencedby the water capillary force. Though
neither HDT nor BPT has polar surface groups, the surface terminal group of HDT
has a symmetrical structure, which causes a smaller electrostatic attractive force and
17 Self-Assembled Monolayers (SAMs) for Controlling Adhesion 927
yields a smaller adhesive force than BPT. It is believed that the easy attachment of
Au to the tip is one of the reasons for the unexpectedly large adhesive force, which
means that it does not fit the linear relationship described by (17.5).
Stiffness, Molecular Spring Model and Micropatterned SAMs
The friction mechanisms of SAMs were also examined. Monte Carlo simulation
of the mechanical relaxation of a CH
3
(CH
2
)
15
SH self-assembled monolayer,as per-
formedby Siepmanand McDonald [85],indicatedthat SAMs compressand respond
nearly elastically to microindentation by an AFM tip when the load is below a criti-
cal normal load. Compression can lead to major changes in the mean molecular
tilt (the orientation), but the original structure is recovered as the normal load is
removed.
Stiffness properties were measured by an AFM in force modulation mode [4,
5,41]. They reported that BPT was stiffer than HDT. Since BPT has rigid benzene
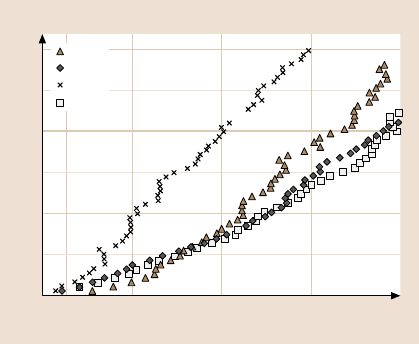
structure,it is more difficultto compressthanHDT. Figure17.12 showsthe variation
in the displacement with normal load during indentation mode. It clearly indicates
that SAMs can be compressed. At a given normal load, SAMs with long carbon
chain structures such as HDT are easy to compress compared to SAMs with rigid
benzene ring structures, such as BPT. Garcia-Parajo et al. [86] have also reported
on the compression and relaxation of octadecyltrichlorosilane (OTS) film obtained
from loading and unloading tests.
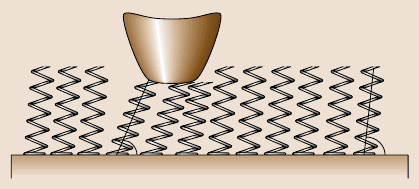
A molecular spring model is presented in Fig. 17.13 to explain the difference
in friction between the SAMs observed in the friction and stiffness measurements
by AFM and the Monte Carlo simulation. It is believed that the self-assembled
molecules on a substrate act just like assembled molecular springs anchored to the
substrate [39]. A Si
3
N
4
tip sliding on the surface of a SAM is like a tip sliding on
the top of molecular springs or a brush. The molecular spring assembly has com-
pliant features and can experience compression and orientation under normal load.
Normal load (nN)
Displacement (nm)
0.0
2.0
30.0
20.0
10.0
0.0
0.5 1.0 1.5
Au
HDT
BPT
BPTC
Fig. 17.12. Normal load
versus displacement curves
for Au(111) and various
SAMs
928 Bharat Bhushan
α
2
α
1
Substrate
Fig. 17.13. Molecular spring model for SAMs. In this figure, α
1
<α
2
, which is caused by re-
orientation under the normal load applied by the AFM tip. The reorientation of the molecular
springs reduces the shearing force at the interface, which in turn reduces the friction force.
The molecular spring constant and the intermolecular forces determine the magnitude of the
coefficient of friction. In this figure, the size of the tip and the molecular springs are not drawn
tothesamescale[39]
The orientation of the molecular springs or brush reduces the shearing force at the
interface, which in turn reduces the friction force. The possibility of orientation is
determined by the spring constant of a single molecule (local stiffness), as well as
the interactions between neighboring molecules, which is reflected in the packing
density or packing energy. It should be noted that the orientation can lead to con-
formational defects along the molecular chains, which lead to energy dissipation. In
the study of BPT by AFM, it was found that the friction force is significantly re-
duced after the first several scans, but the surface height does not show any apparent
change. This suggests that molecular orientation can be facilitated by initial sliding
and is reversible [42].
Basedonthestiffness measurement results presented in Fig. 17.12 and the view
of molecular structures given in Fig. 17.13, biphenyl is a more rigid structure due
to the contribution of the two rigid benzene rings. Therefore, the spring constant
of BPT is larger than that of HDT. The hydrogen (H
+
) in the biphenyl chain has
an electrostatic attraction to the π electrons in the neighboring benzene ring. Thus
the intermolecular forces between biphenyl chains are stronger than those between
alkyl chains. The larger spring constant of BPT and stronger intermolecular forces
mean that it requiresa larger external force to allow it to orient, thus causing a higher
coefficient of friction. The crosslinking of BPT leads to a larger packing energy for
BPTC. Therefore BPTC orientation requires a larger external force: the coefficient
of BPTC is higher than BPT.
An elegant way to demonstrate the influence of molecular stiffness on friction is
to investigate SAMs with different structures on the same wafer. A micropatterned
SAM was prepared for this purpose. First biphenyldimethylchlorosilane (BDCS)
was deposited on the silicon using a typical self-assembly method [41]. Then the
film was partially crosslinked using a mask technique by low-energy electron irra-
diation. Finally, micropatterned BDCS films that had the different coating regions
on the same wafer were realized. The local stiffness properties of these micropat-
terned samples were investigated by a force modulation AFM technique [87]. The
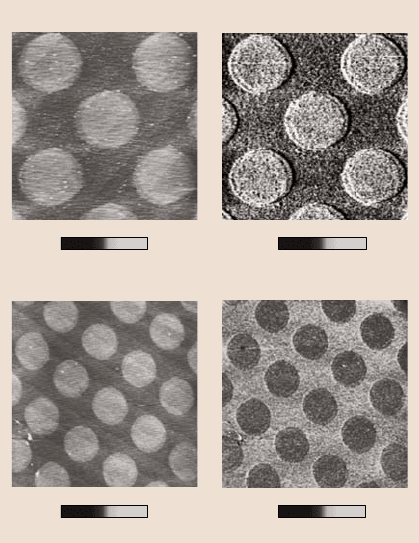
17 Self-Assembled Monolayers (SAMs) for Controlling Adhesion 929
Surface height Stiffness
0
6
0
6μm
a)
Surface height Friction force
b)
0 10 nm
010010μm
stiff soft
0 10 nm
0
4.5 nN
Fig. 17.14. (a)AFM
grayscale surface height
and stiffness images, and
(b) AFM grayscale surface
height and friction force
images of micropatterned
BDCS [41]
variationin the deflection amplitude provides a measure of the relativelocal stiffness
of the surface. Surface height, stiffness and friction images of the micropatterened
biphenyldimethylchlorosilane (BDCS) specimen were obtained and are presented
in Fig. 17.14 [41]. The circular areas correspond to the as-deposited film, and the
remaining area to the crosslinked film. Figure 17.14a indicates that crosslinking
caused by the low-energy electron irradiation leads to a decrease of about 0.5nm
in the surface height of the BDCS film. The corresponding stiffness images indi-
cate that the crosslinked area has a higher stiffness than the as-deposited area. Fig-
ure 17.14b indicates that the as-deposited area has lower friction force. Obviously,
these data for the micropatterned sample prove that the local stiffness of the SAM
influences its friction performance. Higher stiffness leads to larger friction force.
These results correlate well with the suggested molecular spring model.
In summary, it was found that SAMs exhibit compliance and can experience
compression and orientation under normal load. SAM orientation reduces the shear
stress at the interface, so SAMs provide good lubricants. The molecular spring con-
stant (local stiffness) and intermolecular forces can both influence the friction coef-
ficient of a SAM.
