Bhushan B. Nanotribology and Nanomechanics: An Introduction
Подождите немного. Документ загружается.

960 Bharat Bhushan and Huiwen Liu
film thickness. In general, the stability and durability of surface films decrease in
the following order: chemically reacted films, chemisorbed films, and physisorbed
films. A good boundary lubricant should have a high degree of interaction between
its molecules and the sliding surface. As a general rule, liquids are good lubricants
when they are polar and, thus, able to grip solid surfaces (or be adsorbed). Polar lu-
bricants contain reactive functional groups with low ionization potential, or groups
having high polarizability [1–3]. Boundary lubrication properties of lubricants are
also dependent upon the molecular conformation and lubricant spreading [4–7].
Self-assembled monolayers (SAMs), Langmuir–Blodgett (LB) films, and per-
fluoropolyether (PFPE) films can be used as boundary lubricants [2,3,8–10]. PFPE
films are commonly used for lubrication of magnetic rigid disks and metal evapo-
rated magnetic tapes to reduce friction and wear of a head–medium interface [10].
PFPEs are well suited for this application because of the following properties: low
surface tension and low contact angle, which allow easy spreading on surfaces and
provide a hydrophobic property; chemical and thermal stability, which minimizes
degradation under use; low vapor pressure, which provides low out-gassing; high
adhesionto substrate via organofunctionalbonds; and good lubricity, which reduces
the interfacial friction and wear [10–12]. While the structure of the lubricants em-
ployed at the head–medium interface has not changed substantially over the past
decade, the thickness of the PFPE film used to lubricate the disk has steadily de-
creased from multilayer thicknesses to the sub-monolayerthickness regime [11,13].
Molecularly thick PFPE films are also being considered for lubrication purposes
of the evolving microelectromechanical systems (MEMS) industry [14]. It is well
knownthat the properties of molecularlythick liquidfilms confined to solid surfaces
can be dramatically differentfrom those ofthe correspondingbulkliquid. In orderto
efficiently develop lubrication systems that meet the requirements of the advanced
rigid disk drive and MEMS industries, the impact of thinning the PFPE lubricants
on the resulting nanotribology should be fully understood [15, 16]. It is also im-
portant to understand lubricant–substrate interfacial interactions and the influence
of the operating environment on the nanotribological performance of molecularly
thick PFPEs.
An overviewof nanotribologicalproperties of SAMs and LB films can be found
in many references, such as [17]. In this chapter, we focus on PFPEs. We first in-
troduce details of the commonly used PFPE lubricants; then present a summary
of nanodeformation, molecular conformation, and lubricant spreading studies; fol-
lowed by an overview of nanotribological properties of polar and nonpolar PFPEs
studied by atomic force microscopy (AFM) and some concluding remarks.
18.2 Lubricants Details
Properties of two commonly used PFPE lubricants (Z-15 and Z-DOL) are reviewed
here. Their molecular structuresare shownschematicallyin Fig. 18.1. Z-15 has non-
polar
−
CF
3
end groups, whereas Z-DOL is a polar lubricant with hydroxyl (
−
OH)

18 Nanoscale Boundary Lubrication Studies 961
Z-15
Z-DOL
H
O
F
C
Fig. 18.1. Schematics of the
molecular structures of Z-15
and Z-DOL. In this figure
the m/n value, shown in
Table 18.1, equals 2/3
Table 18.1. Typical properties of Z-15 and Z-DOL (data from Montefluous S.P.A., Milan,
Italy)
Z-15 Z-DOL (2000)
Formula CF
3
−
O
−
(CF
2
−
CF
2
−
O)
m
−
HO
−
CH
2
−
CF
2
−
O
−
(CF
2
−
O)
n
−
CF
3
∗
(CF
2
−
CF
2
−
O)
m
−
(CF
2
−
O)
n
−
CF
2
−
CH
2
−
OH
∗
Molecular weight (Daltons) 9100 2000
Density (ASTM D891) 20
◦
C
(g/cm
3
)1.84 1.81
Kinematic viscosity
(ASTM D445) (cSt)
20
◦
C 148 85
38
◦
C9034
99
◦
C25−
Viscosity index (ASTM D2270) 320 −
Surface tension (ASTM D1331)
(dyn/cm) 20
◦
C24 24
Vapor pressure (torr)
20
◦
C1.6×10
−6
2×10
−5
100
◦
C1.7×10
−5
6×10
−4
Pour point (ASTM D972)
◦
C −80 –
Evaporation weight loss
(ASTM D972)
149
◦
C, 22 h (%) 0.7–
Oxidative stability (
◦
C) − 320
Specific heat (cal/g
◦
C)
38
◦
C0.21 –
∗
m/n ∼2/3
962 Bharat Bhushan and Huiwen Liu
end groups. Their typical properties are summarized in Table 18.1; it shows that Z-
15 andZ-DOL havealmost thesame densityand surface tension.But Z-15has larger
molecular weight and higher viscosity. Both of them have low surface tension, low
vapor pressure, low evaporation weight loss, and good oxidative stability [10,12].
Generally, a single-crystalSi(100)wafer with a nativeoxidelayer was usedas a sub-
strate for deposition of molecularly thick lubricant films for nanotribological char-
acterization.Z-15 and Z-DOL filmscan be deposited directlyonto the Si(100) wafer
by the dip-coating technique. The clean silicon wafer is vertically submerged into
a dilute solution of lubricant in hydrocarbonsolvent (HT-70) for a certain time. The
silicon wafers are pulled up vertically from the solution with a motorized stage at
a constant speed for deposition of the desired thicknesses of Z-15 and Z-DOL lu-
bricants. The lubricant film thickness obtained in dip coating is a function of the
concentration and pull-up speed, among other factors. The Z-DOL film is bonded
to the silicon substrate by heating the as-deposited Z-DOL samples in an oven at
150
◦
C for about 30minutes.The native oxide layer of Si(100) wafer reacts with
the
−
OH groups of the lubricants during thermal treatment [18–21]. Subsequently,
fluorocarbon solvent (FC-72) washing of the thermally treated specimen removes
looselyabsorbed species, leavingthe chemically bondedphase on the substrate. The
chemical bonding between Z-DOL molecules and silicon substrate is illustrated in
Fig. 18.2. The bonded and washed Z-DOL film is referred to as Z-DOL(BW) in this
chapter. The as-deposited Z-15 and Z-DOL films are mobile-phase lubricants (i.e.,
liquid-like lubricants), whereas the Z-DOL(BW) films are fully bonded soft solid
phase (i.e., solid-like) lubricants. This will be further discussed in the next section.
Z-DOL molecules
Silica
Silicon
wafer
Si
O
CH
2
CF
2
O
Si
O
CH
2
CF
2
O
Si
O
CH
2
CF
2
O
Si
O
CH
2
O
Si
O
CH
2
CF
2
O
Si
O
CH
2
CF
2
O
Si
O
CH
2
CF
2
O
Si
O
CH
2
O
O
Fig. 18.2. Schematic of
Z-DOL molecules that
are chemically bonded on
Si(100) substrate surface
(which has native oxide) after
thermal treatment at 150
◦
C
for 30min
18 Nanoscale Boundary Lubrication Studies 963
18.3 Nanodeformation, Molecular Conformation,
and Lubricant Spreading
Nanodeformation behavior of Z-DOL lubricants was studied using an AFM by
Blackman et al. [22,23]. Before bringing a tungsten tip into contact with a molecu-
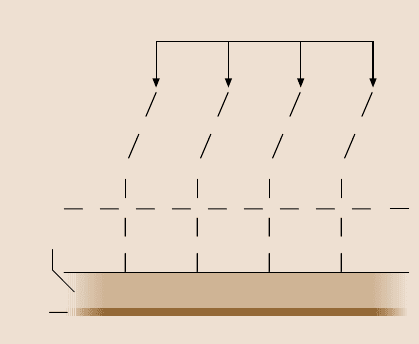
lar overlayer,it was brought into contact with a bare clean-silicon surface, Fig. 18.3.
As the sample approaches the tip, the force initially is zero, but at point A the force
suddenly becomes attractive (top curve), which increases until point B, where the
sample and tip come into intimate contact and the force becomes repulsive. As the
sample is retracted, a pull-off force of 5×10
−8
N (point D) is required to overcome
adhesion between the tungsten tip and the silicon surface. When an AFM tip is
brought into contact with an unbonded Z-DOL film, a sudden jump into adhesive
contact is also observed. A much larger pull-off force is required to overcome the
adhesion. The adhesion is initiated by the formation of a lubricant meniscus sur-
rounding the tip. This suggests that the unbonded Z-DOL lubricant shows liquid-
like behavior. However, when the tip was broughtinto contact with a lubricant film,
which was firmly bonded to the surface, the liquid-like behavior disappears. The
initial attractive force (point A) is no longer sudden, as with the liquid film, but,
rather, gradually increases as the tip penetrates the film.
Accordingto Blackmanet al. [22,23],if the substrateand tip were infinitely hard
with no compliance and/or deformation in the tip and sample supports, the line for
B to C would be vertical with an infinite slope. The tangent to the force–distance
curve at a given point is referred to as the stiffness at that point and was determined
D
0
Wire deflection (nm)
Tip-sample separation distance (nm)
2
0
2
0
2
010203040
0
Force (nN)
100
Clean Si(100)
Unbonded perfluoropolyether
Bonded perfluoropolyether
A
C
B
A
A
0
100
0
100
Fig. 18.3. Deflection (normal
load) as a function of tip–
sample separation-distance
curves comparing the behav-
ior of clean Si(100) surface
to a surface lubricated with
free and unbonded PFPE lu-
bricant, and a surface where
the PFPE lubricant film was
thermally bonded to the sur-
face [22]
964 Bharat Bhushan and Huiwen Liu
by fitting a least-squares line through the nearby data points. For silicon, the de-
formation is reversible (elastic), since the retracting (outgoing) portion of the curve
(C to D) follows the extending (ingoing) portion (B to C). For bonded lubricant
film, at the point where the slope of the force changes gradually from attractive to
repulsive, the stiffness changes gradually, indicating compression of the molecular
film. As the load is increased, the slope of the repulsive force eventually approaches
that of the bare surface. The bonded film was found to respond elastically up to the
highestloads of 5 µN that couldbe applied.Thus, bonded lubricantbehavesas a soft
polymer solid.
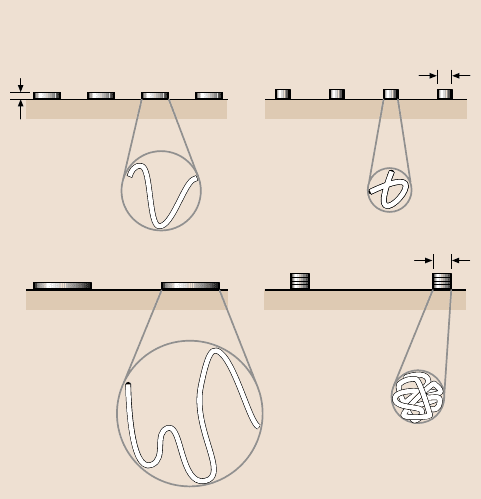
Figure 18.4 illustrates two extremes for the conformation on a surface of a lin-
ear liquid polymer without any reactive end groups and at submonolayer cover-
ages [4,6]. At one extreme, the molecules lie flat on the surface, reaching no more
than their chain diameter δ above the surface. This would be the case if a strong
attractive interaction exists between the molecules and the solid. On the other ex-
treme, when a weak attraction exists between polymer segments and the solid,
the molecules adopt a conformation close to that of the molecules in the bulk,
with the microscopic thickness equal to about the radius of gyration R
g
. Mate and
Novotny [6] used AFM to study conformation of 0.5–1.3 nm-thick Z-15 molecules
δ
Bulk conformation
Low molecular weight
Side view
High molecular weight
Flat conformation
~2R
g
~2R
g
side view
Side view
Expanded
top view
Expanded
top view
Fig. 18.4. Schematic representation of two extreme liquid conformations at the surface of the
solid for low and high molecular weights at low surface coverage. δ is the cross-sectional
diameter of the liquid chain, and R
g
is the radius of gyration of the molecules in the bulk [6]
18 Nanoscale Boundary Lubrication Studies 965
on clean Si(100) surfaces. They found that the thickness measured by AFM is
thicker than that measured by ellipsometry, with the offset ranging from 3–5nm.
They found that the offset was the same for very thin submonolayer coverages. If
the coverage is submonolayer and inadequate to make a liquid film, the relevant
thickness is then the height (h
e
) of the molecules extended above the solid sur-
face. The offset should be equal to 2h
e
, assuming that the molecules extend the
same height above both the tip and silicon surfaces. They therefore concluded that
the molecules do not extend more than 1.5–2.5nm above a solid or liquid surface,
much smaller than the radius of gyration of the lubricants, which ranges between
3.2and7.3nm, and to the approximate cross-sectional diameter of 0.6–0.7nmfor
the linear polymer chain. Consequently, the height that the molecules extend above
the surface is considerably less than the diameter of gyration of the molecules and
only a few molecular diameters in height, implying that the physisorbed molecules
on a solid surface have an extended, flat conformation. They also determined the
disjoining pressure of these liquid films from AFM measurements of the distance
needed to break the liquid meniscus that forms between the solid surface and the
AFM tip. (Also see [7].) For a monolayer thickness of about 0.7nm, the disjoining
pressure is about 5 MPa, indicating strong attractive interaction between the liquid
molecules and the solid surface. The disjoining pressure decreases with increasing
film thickness in a manner consistent with a strong attractive van der Waals interac-
tion between the liquid molecules and the solid surface.
Rheological characterization shows that the flow activation energy of PFPE lu-
bricants is weakly dependent on chain length and is strongly dependenton the func-
tional end groups [25]. PFPE lubricant films that contain polar end groups have
lowermobility than thosewith nonpolarend groupsof similar chainlength [26]. The
mobility of PFPE also depends on the surface chemical properties of the substrate.
The spreading of Z-DOL on amorphous carbon surface has been studied as a func-
tion of hydrogen or nitrogen content in the carbon film, using scanning microel-
lipsometry [24]. The diffusion coefficient data presented in Fig. 18.5 is thickness-
dependent. It shows that the surface mobility of Z-DOL increased as the hydrogen
content increased, but decreased as nitrogen content increased. The enhancement
of Z-DOL surface mobility by hydrogenation may be understood from the fact that
the interactions between Z-DOL molecules and the carbon surface can be signif-
icantly weakened, due to a reduction of the number of high-energy binding sites
on the carbon surface. The stronger interactions between the Z-DOL molecules and
carbon surface, as the nitrogen content in the carbon coating increases, leads to the
lowering of Z-DOL surface mobility.
Molecularly thick films may be sheared at very high shear rates, on the order of
10
8
–10
9
s
−1
during sliding, such as during magnetic disk drive operation. During
such shear, lubricant stability is critical to the protection of the interface. For proper
lubricant selection, viscosity at high shear rates and associated shear thinning need
to be understood. Viscosity measurements of eight different types of PFPE films
show that all eight lubricants display Newtonian behavior and their viscosity re-
mains constant at shear rates up to 10
7
s
−1
[27,28].
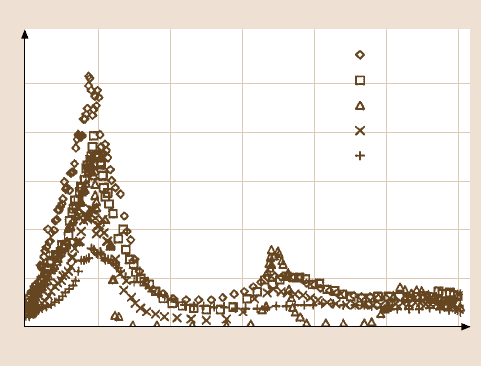
966 Bharat Bhushan and Huiwen Liu
D(h) (10
–12
m
2
/s)
Film thickness (nm)
a-C:H (50%)
a-C:H (10%)
a-C
a-C:N (10%)
a-C:N (50%)
12
10
8
6
4
2
0
6810
12
42
0
Fig. 18.5. Diffusion coefficient D(h) as a function of lubricant film thickness for Z-DOL on
different carbon films [24]
18.4 Boundary Lubrication Studies
With the development of AFM techniques, studies have been carried out to investi-
gate the nanotribological performance of PFPEs. Mate [29,30], O’Shea et al. [31,
32], Bhushan et al. [15, 33], Koinkar and Bhushan [20, 34], Bhushan and Sun-
dararajan [35], Bhushan and Dandavate [36], and Liu and Bhushan [21] used an
AFM to provide insight into how PFPE lubricants function at the molecular level.
Mate [29,30] conducted friction experiments on bonded and unbonded Z-DOL and
found that the coefficient of friction of the unbonded Z-DOL is about two times
largerthan thebonded Z-DOL (also see [31,32]). Koinkar and Bhushan [20,34] and
Liu and Bhushan [21] studiedthe friction and wear performanceof a Si(100) sample
lubricated with Z-15, Z-DOL, and Z-DOL(BW) lubricants. They found that using
Z-DOL(BW) could significantly improve the adhesion, friction, and wear perfor-
mance of Si(100). Theyalso discussed the lubrication mechanisms on the molecular
level. Bhushan and Sundararajan[35] and Bhushan and Dandavate[36] studied the
effect of tip radius and relative humidity on the adhesion and friction properties of
Si(100) coated with Z-DOL(BW).
In this section, we review, in some detail, the adhesion, friction, and wear prop-
erties of Z-15 and Z-DOL at various operating conditions (rest time, velocity, rela-
tive humidity, temperature, and tip radius). The experiments were carried out using
a commercial AFM system with pyramidal Si
3
N
4
and diamond tips. An environ-
mentally controlled chamber and a thermal stage were used to perform relative hu-
midity and temperature effect studies.
18 Nanoscale Boundary Lubrication Studies 967
18.4.1 Friction and Adhesion
To investigate the friction properties of Z-15 and Z-DOL(BW) films on Si(100),
the curves of friction force versus normal load were measured by making friction
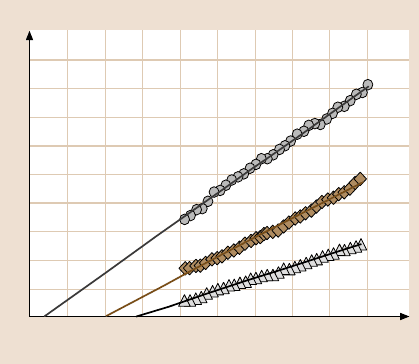
measurementsat increasing normalloads [21].The representativeresults of Si(100),
Z-15, and Z-DOL(BW) are shown in Fig. 18.6. An approximately linear response
of all three samples is observed in the load range of 5–130nN. The friction force of
solid-like Z-DOL(BW) is consistently smaller than that for Si(100), but the friction
force of liquid-like Z-15 lubricant is higher than that of Si(100). Sundararajan and
Bhushan [37] have studied the static friction force of silicon micromotors lubricated
with Z-DOL by AFM. They also found that liquid-like lubricants of Z-DOL signif-
icantly increase the static friction force, whereas solid-like Z-DOL(BW) coatings
can dramatically reduce the static friction force. This is in good agreement with
the results of Liu and Bhushan [21]. In Fig. 18.6, the nonzero value of the friction
force signal at zero external load is due to the adhesive forces. It is well known that
the following relationship exists between the friction force F and external normal
load W [2,3]
F = μ(W + W
a
) , (18.1)
where μ is the coefficient of friction and W
a
is the adhesive force. Based on this
equation and the data in Fig. 18.6, we can calculate the μ and W
a
values. The coeffi-
cients of friction of Si(100),Z-15, and Z-DOL are 0.07, 0.09, and 0.04, respectively.
Based on (18.1), the adhesive force values are obtained from the horizontal inter-
cepts of the curves of friction force versus normal load at a zero value of friction
force. Adhesive force values of Si(100), Z-15, and Z-DOL are 52 nN, 91nN, and
34nN, respectively.
The adhesive forces of these samples were also measured using a force calibra-
tion plot (FCP) technique. In this technique, the tip is brought into contact with the
Friction force (nN)
25
20
15
10
5
0
2μm /s, 22°C, RH 45– 55%
Z-15, μ = 0.09
Si (100), μ = 0.07
Z-DOL(BW), μ = 0.04
Normal load (nN)
–100 100 150500–50
Fig. 18.6. Curves of friction
force versus normal load
for Si(100), 2.8-nm-thick
Z-15 film, and 2.3-nm-thick
Z-DOL(BW) film at 2 µm/s,
and in ambient air sliding
against a Si
3
N
4
tip. Based on
these curves, the coefficient
of friction μ and adhesion
force of W
a
can be calcu-
lated [21]

968 Bharat Bhushan and Huiwen Liu
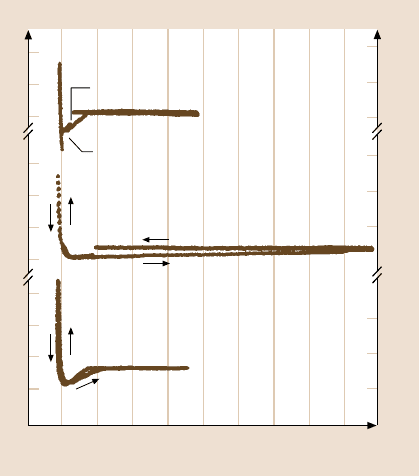
sample and the maximum force needed to pull the tip and sample apart is measured
as the adhesive force. Figure 18.7 shows the typical FCP curves of Si(100), Z-15,
and Z-DOL(BW) [21]. As the tip approaches the sample within a few nanome-
ters (point A), an attractive force exists between the tip and the sample surfaces.
The tip is pulled toward the sample, and contact occurs at point B on the graph. The
adsorption of water molecules and/or presence of liquid lubricant molecules on the
sample surface can also accelerate this so-called snap-in, due to the formation of
meniscus of the water and/or liquid lubricant around the tip. From this point on,
the tip is in contact with surface, and as the piezo extends further, the cantilever is
further deflected. This is represented by the slope portion of the curve. As the piezo
retracts, at point C the tip goes beyond the zero deflection (flat) line, because of the
attractive forces, into the adhesive force regime. At point D, the tip snaps free of the
80
40
–40
–80
80
40
–40
–80
80
40
–40
–80
0 400
500
300
200
100
0 400
500
300
200
100
0 400
500
300
200
100
Z-position (nm)
Cantilever deflection (nN)
Z-DOL(BW)
Z-15
Si(100)
Trace
Retrace
C
A
D
B
Fig. 18.7. Typical force cali-
bration plots of Si(100),
2.8-nm-thick Z-15 film, and
2.3-nm-thick Z-DOL(BW)
film in ambient air. The adhe-
sive forces can be calculated
from the horizontal distance
between points C and D, and
the cantilever spring constant
of 0.58 N/m [21]
18 Nanoscale Boundary Lubrication Studies 969
adhesive forces and is again in free air. The adhesive force (pull-off force) is deter-
mined by multiplying the cantilever spring constant (0.58N/m) by the horizontal
distance between points C and D, which correspondsto the maximum cantilever de-
flection toward the samples before the tip is disengaged. Incidentally, the horizontal
shift between the loading and unloading curves results from the hysteresis of the
PZT tube.
The adhesive forces of Si(100), Z-15, and Z-DOL(BW) measured by FCP and
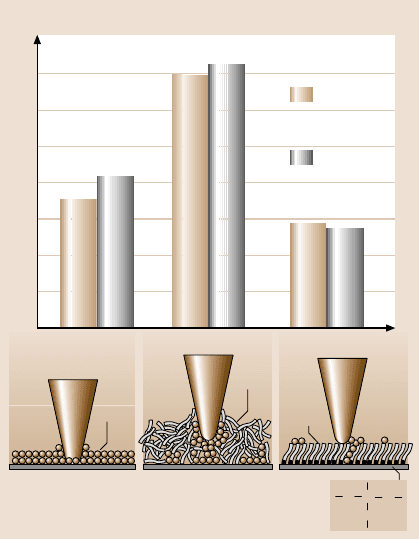
plots of friction force versus normal load are summarized in Fig. 18.8 [21]. The re-
sults measured by these two methods are in good agreement. Figure 18.8 shows that
the presence of mobile Z-15 lubricant film increases the adhesive force compared to
that of Si(100). In contrast, the presence of solid-phase Z-DOL(BW) film reduces
the adhesive force compared to that of Si(100). This result is in good agreement
with the results of Blackman et al. [22] and Bhushan and Ruan [38]. Sources of ad-
hesive forces between the tip and the sample surfaces are van der Waals attraction
and long-rangemeniscusforces [2,3,16].The relativemagnitudesof the forces from
the two sourcesare dependenton variousfactors, includingthe distance between the
tip and the sample surface, their surface roughness, their hydrophobicity, and rela-
tive humidity [39]. For most surfaces with some roughness, meniscus contribution
dominates at moderate to high humidities.
100
75
50
25
0
Z-DOL(BW)Z-15Si(100)
22 °C,
RH 45 – 55%
Adhesive force (nN)
Force
calibration
plot
Friction
force plot
Z-DOL
O
Si
O
O
Z-15
H
2
O
Fig. 18.8. Summary of the
adhesive forces of Si(100),
2.8-nm-thick Z-15 film, and
2.3-nm-thick Z-DOL(BW)
film measured by force cali-
bration plots and friction
force versus normal load in
ambient air. The schematic
(bottom)showstheeffect of
meniscus formed between
the AFM tip and the sample
surface on the adhesive and
friction forces [21]
