Bhattacharjee J.K., Bhattacharyya S. Non-Linear Dynamics Near and Far from Equilibrium
Подождите немного. Документ загружается.

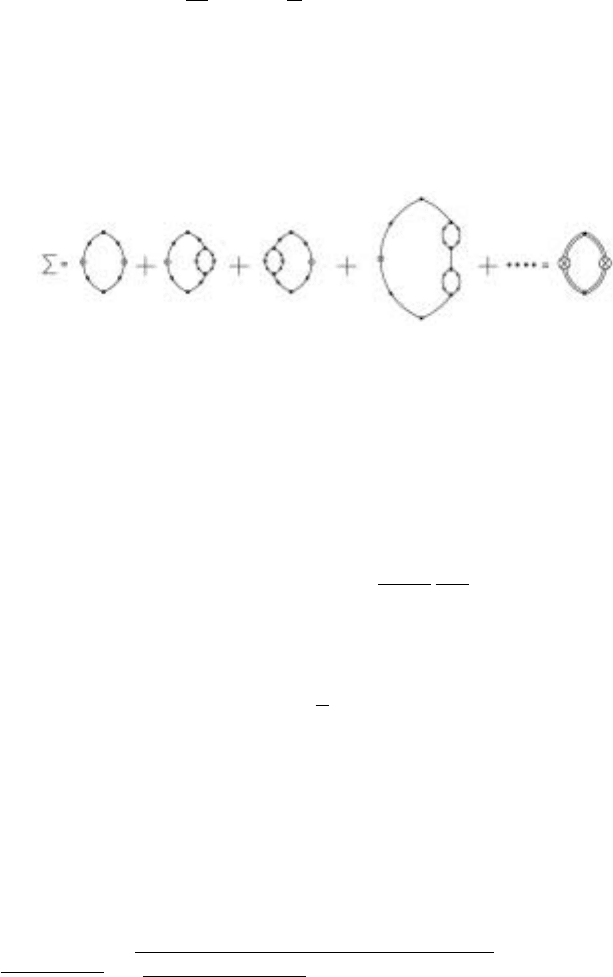
4.3 Spherical Limit 101
of Fig. 4.10c to is O(
1
M
2
M)=O(
1
M
).IfwetakeM →∞limit, this contribution
can be dropped.
Thus, we notice a great simplification in the limit M →∞which is dubbed
the spherical limit. The contribution to (k, ω) is given by graph(a) in Fig. 4.10
and all other graphs where the lines of graph(a) are dressed by a graph of the form
shown in graph(a), e.g. the contribution from graph(b). Thus, in the spherical limit,
the graphical expansion of is as given in Fig. 4.11.
Figure 4.11. Summing the self energy in the spherical limit
which is simply the self consistent mode coupling form of Eq.(4.2.19). If one
is interested in the scaling limit, where the contribution coming from the linear
term in the equation of motion can be dropped,
G
−1
(k, ω) =−iω +(k, ω) (4.3.5)
corresponding to Fig. 4.11
(k, ω) = g
2
(k
2
+m
2
)
ω
1
+ω
2
=ω
p
1
+p
2
=
k
d
D
p
1
(2π)
D
dω
1
2π
(p
2
1
−p
2
2
)
2
× C(p
1
,ω
1
)C(p
2
,ω
2
) (4.3.6)
and from the FDT
C(k,ω) =
1
ω
Im G(k, ω) (4.3.7)
In the spherical limit and in the scaling regime, Eqns.(4.3.5)-(4.3.7) constitute an
exact solution to the problem of critical dynamics in the Heisenberg model.
We end this section by a discussion of whether the spherical limit and self
consistent mode coupling are identical. The answer is no. Self consistent mode
coupling can be carried beyond the single loop approximation shown in Fig. 3.9
and indeed it is often necessary to do that in the field of dynamic critical phenomena
where the experiments are precise and better accuracy than that providedby Fig. 3.9
is necessary. The spherical limit on the other hand is exact. There are no O(g
4
)
in this limit. The self consistent mode coupling approximation
of Fig. 3.9 gives an
exact answer
to a generalized problem in the M →∞limit.
References
1. R. Kraichnan, J. Math. Phys. 2 124 (1962)
102 4 Mode Coupling Theories
2. C. Y. Mou and P. Weichman, Phys. Rev. Lett. 70 1101 (1993)
3. J. P. Doherty, M. A. Moore J. M. Kim and A. J. Bray, Phys. Rev. Lett. 72 2041 (1994)
4. L. Sasvari, F. Schwabl and P. Szepfalusy, Physica A81 108 (1975)
5. R
´
esibois and C. Piette, Phys. Rev. Lett. 24 514 (1970)
6. K. Kawasaki, Ann. Phys(N.Y.) 61 1 (1970)
7. R. A. Ferrell, Phys. Rev. Lett. 24 1169 (1970)

5
Critical Dynamics in Fluids
5.1 Introduction
We have already encountered the phenomenon of critical slowing down in our
discussion of ferromagnetic transition in chapters 2 and 3. In the study of static
response in second order phase transitions, there is a very strong universality which
tells us that it is only the dimensionality of the order parameter which determines
universality classes. Consequently, the best known critical phenomenon which
is the critical point of a liquid-vapour system (well studied both theoretically and
experimentallyfor about one hundred and twenty five years) has the same behaviour
as the Ising model which was introduced to study the magnetic transition. The order
parameter (the quantity whose expectation value is zero above the transition and
non-zero below) of the liquid vapour system is the difference between the densities
in the liquid and vapour state. This field is a scalar - capable of only being only
positive or negative. The Ising model consists of spins which can point up or down
and thus can be positive or negative and hence in the continuum limit is represented
by a scalar order parameter field. The static critical behaviour of the two systems
are consequently identical. For the dynamic responses, this broad universality does
not hold. The liquid-vapour system has nothing to do with the Ising model.
We have already seen evidence of this in our discussion of the ferromagnetic
transition. The Ginzburg-Landau model for a n-component order parameter is ad-
equate for discussing the static responses near the paramagnet- ferromagnet tran-
sition but is totally irrelevant for studying the dynamic responses. The dynamic
response is dictated by reversible terms in the equation of motion for the order
parameter. The reversible terms are so called because they keep the equilibrium
distribution unchanged in time and are simply the physical terms which determine
the dynamics. In our ferromagnet, this simply meant recognizing that the magne-
tization which is the order parameter responds to the torque (magnetization comes
from the ‘angular momentum’) and writing down equations of motion based on
that ensures a correct handling of the dynamics. This is true for all systems showing

104 5 Critical Dynamics in Fluids
dynamic critical behaviour. In this chapter, we discuss the most well-known system
exhibiting critical behaviour from this point of view and then discuss another well
known system - the superfluid transition - of liquid
4
He which gave rise to the
concept of dynamic scaling.
To start our discussion we focus on a system which is in the same universality
class as the liquid-vapour system - this is the symmetric binary mixture. This is a
50 −50 mixture of atoms A and atoms B, which is completely miscible above a
critical temperature T
c
. Below T
c
, there is phase separation. If C
A
is the concentra-
tion of the A atoms and C
B
the concentration of B atoms, The order parameter is
δC =C
A
−C
B
.ForT>T
c
, δC=0, where the angular brackets denote statisti-
cal averaging. When phase separation occurs for T<T
c
, δC is clearly non-zero.
Thus δCis clearly the order parameter φ of the transition. This clear cut definition
of the order parameter makes us choose this as a better example - for the liquid
vapour system the analogue of δC =C
A
−C
B
is δρ =ρ
L
−ρ
g
, ρ
L
and ρ
g
being
the densities in the liquid and gaseous phase. However, experiments show that the
coexistence curve is not symmetric and the order parameter is really a mixture of
density and energy fluctuations. In fact the relevant combination of density and
energy is very much like the entropy.
To discuss the dynamic response in the binary mixture, we imagine the system
to be in equilibrium and then we imagine applying an external force. The force
is
F per unit mass on every atom A and −
F on every atom B. The system now
settles in to a steady state where there will be a finite current corresponding to the
concentration difference ψ. The A-current and B-current are equal and opposite
and the current
j
ψ
corresponding to the concentration difference ψ is
j
ψ
=
j
A
−
j
B
=2
j
A
(5.1.1)
For small values of the force
F , the current is proportional to the force and we can
write
j
ψ
=λ
F (5.1.2)
the constant of proportionality defining the transport coefficient for the concen-
tration difference. The same current can be generated if instead of an external
force, we have a concentration gradient in the system. In this case, the current is
proportional to the gradient of chemical potential and we have
j
ψ
=−λ
∇µ =−=
λ
χ
ψ
∇ψ (5.1.3)
where
χ
ψ
=
∂µ
∂ψ
P,T
(5.1.4)

5.1 Introduction 105
is the isothermal susceptibility of the system. The equation of motion for ψ can be
found from the conservation law
∂ψ
∂t
=−
∇.
j
ψ
=
λ
χ
ψ
∇
2
ψ
= D
ψ
∇
2
ψ (5.1.5)
where D
ψ
is the familiar concentration diffusion coefficient. In terms of the trans-
port coefficient λ,wefind
D
ψ
=
λ
χ
ψ
. (5.1.6)
The relaxation rate for the Fourier component ψ(k) is clearly D
ψ
k
2
.
If instead of a concentration current, we talk about a heat current, then the trans-
port coefficient is the thermal conductivity λ
T
, the corresponding susceptibility is
the constant pressure specific heat and for the thermal diffusion coefficient is
D
T
=
λ
T
C
P
. (5.1.7)
For the momentum current, the transport coefficient is the shear viscosity η and the
corresponding susceptibility is simply the density of the system. The momentum
diffusion coefficient is the kinematic viscosity ν and we have
ν =
η
ρ
. (5.1.8)
We now need to discuss what happens to the transport coefficient near the critical
point. Close to the critical point, regions where fluctuations are correlated become
very large and the correlation length ξ which characterizes the system becomes
infinite at T =T
c
. Close to T =T
c
, ξ ∼t
−ν
where
t =
T −T
c
T
c
.
Over a region of size ξ in a D dimensional space, the applied force
f is given by
f =ξ
D
ψ
F (5.1.9)
where
F is the applied force per unit mass. In the steady state, where there is a
uniform velocity v, there is an equal and opposite resistive Stokes’ law force which
is given by
f
r
∝vηξ
D−2
(5.1.10)
106 5 Critical Dynamics in Fluids
The current
j is given by
j =ψ v=
ψ
η
ξ
2−D
f
r
=
ψ
η
ξ
2−D
f
=
ξ
2
ψ
2
η
F (5.1.11)
leading to the transport coefficient
λ =
ξ
2
η
ψ
2
. (5.1.12)
The expectation value ψ
2
is proportional to the susceptibility per unit volume
and thus
ψ
2
∝
χ
ψ
ξ
D
∝ξ
2−η
0
−D
. (5.1.13)
From static critical phenomenon
χ
ψ
∝ξ
2−η
0
,
where η
0
is the anomalous dimension index, the subscript put to differentiate it
from the shear viscosity η. The transport coefficient is given by
λ =
ξ
2
η
ψ
2
∝
ξ
4−D−η
0
η
(5.1.14)
Assuming that the shear viscosity has no critical variation (or a very weak one
even if it has one, since it is not the response function directly associated with the
order parameter), we see that the transport coefficient diverges for D<4 −η
0
4(η
0
O(10
−2
)).
The above discussion shows two things
• i) the transport of order parameter is strongly affected by critical fluctuations
and the transport coefficient diverges at the critical point.
• ii) The velocity fluctuations are involved in the process since the current is
provided by the velocity. The velocity fluctuations are not critical but couple
to the order parameter. Without detailed calculations, it is not possible to say
how the transport of the momentum fluctuations is affected, and what would
be if any, the critical behaviour of the shear viscosity.
5.2 Equations for Transport Coefficients 107
5.2 Equations for Transport Coefficients
We thus see that to proceed further and get a quantitative estimate of the different
transport coefficients, the coefficients associated with concentration flow, the heat
flow, the momentum flow, and also the density fluctuations determining the sound
propagation - equations of motion need to be established. For the concentration
difference ψ for the binary liquid, the equation of motion in Eq.(5.1.5) needs
to be augmented by including the coupling to the velocity field. This is a very
straightforward modification of the time derivative in Eq.(5.1.5). Instead of the
partial time derivative at a given point in space, we need the total time derivative
which includes the change brought about by the transport by the velocity field.
This leads to the equation of motion
∂ψ
∂t
+(v.
∇)ψ =D∇
2
ψ =
λ
χ
∇
2
ψ (5.2.1)
We now need to write down the equation of motion for the velocity field. This is
the Navier-Stokes equation. For the time being we will ignore the sound wave and
assume incompressible flow i.e.
∇.v =0 (5.2.2)
The velocity fluctuations are small and all flows are in the very low Reynold’s
number regime. So the usual non-linearity (v.
∇)v [and also
∇P , P being the
pressure, which for an incompressible flow is proportional to (v.
∇)v] is negligible
and for the i
th
component of the velocity vector
∂v
i
∂t
=T
ij,j
(5.2.3)
where T
ij
is the stress tensor. The usual contribution to the stress tensor is
η
∂v
i
∂x
j
+
∂v
j
∂x
i
.
What we need to know is how does the concentration fluctuation affect the stress
tensor, because it is through the coupling to the order parameter field that we expect
behaviour to arise. To this end, we need to know the equilibrium free energy. This
is the Ginzburg-Landau free energy augmented by a quadratic term in velocity to
account for the probabilistic distribution of the velocity fluctuations. We will drop
the quadratic term in the Ginzburg-Landau free energy since it is not particularly
relevant directly to the dynamics. It’s effect on the statics will be taken care of by
assuming that the mass term in the free energy has the correct critical behaviour.
The free energy F is
F =
d
D
x
κ
2
ψ
2
+
1
2
(∇ψ)
2
+
ρ
2
v
2
(5.2.4)
108 5 Critical Dynamics in Fluids
As stated above the term ψ
4
is missing in F, but its effect on the statics is included
by stipulating that the mass term κ ∝t
ν
where ν is the correct critical exponent
(ν 0.62 for n =1, i.e. scalar order parameter) and t is the reduced temperature
t =
T −T
c
T
c
,
T
c
being the critical point temperature. To find the stress tensor, we imagine the
fluid away from the critical point and apply an external potential V(x), to which the
system responds by setting up a spatially dependent ψ(x), such that the contribution
to the energy is ψ(x)V x. The free energy F in the presence of V(x) is
F(ψ,
∇ψ, V (x)) =F(ψ,
∇ψ, V =0) +ψV (5.2.5)
The force
K is obtained from the gradient of F and
K
i
=−
∂F
∂x
i
=−
∂F
∂ψ
∂ψ
∂x
i
−
j
∂F
∂
∂ψ
∂x
j
∂
∂x
i
∂ψ
∂x
j
−ψ
∂V
∂x
i
(5.2.6)
In the presence of V(x), the system will have to adjust itself to minimize
F(ψ,
∇ψ, V (x))d
D
x
and that leads to the canonical Euler-Lagrange equation
∂F
∂ψ
=
i
∂
∂x
i
∂F
∂(
∂ψ
∂x
i
)
(5.2.7)
Using this condition in Eq.(5.2.6)
K
i
=−
j
∂
∂x
j
∂F
∂(
∂ψ
∂x
j
)
∂ψ
∂x
i
−
j
∂F
∂(
∂ψ
∂x
j
)
∂
∂x
j
∂ψ
∂x
i
−ψ
∂V
∂x
i
=−
j
∂
∂x
j
∂F
∂(
∂ψ
∂x
j
)
∂ψ
∂x
i
−ψ
∂V
∂x
i
=−
j
∂T
ij
∂x
j
−ψ
∂V
∂x
i
(5.2.8)
where
T
ij
=
∂F
∂(
∂ψ
∂x
j
)
∂ψ
∂x
i
(5.2.9)
For a more general way of finding the non-linear contribution to the velocity equa-
tion due to concentration fluctuations we need to ensure that the free energy F is

5.2 Equations for Transport Coefficients 109
unaffected by the non-linear terms. With
˙
ψ given in Eq.(5.2.1) we have (keeping
only the non-linear terms in the equation of motion)
0 =
∂
∂t
Fd
D
x =
ρv
i
˙v
i
+κ
2
ψ
˙
ψ +
∂ψ
∂x
j
∂
˙
ψ
∂x
j
d
D
x
=
ρv
i
˙v
i
−κ
2
ψv
i
∂ψ
∂x
i
−
∂ψ
∂x
j
∂
∂x
j
v
i
∂ψ
∂x
i
d
D
x
=
ρv
i
˙v
i
−κ
2
ψv
i
∂ψ
∂x
i
+v
i
∂ψ
∂x
i
∂
2
ψ
∂x
i
∂x
j
d
D
x (5.2.10)
keeping in mind that v is compressible thus gives the equation of motion (so far as
the non-linear terms go)
ρ ˙v
i
=P
il
−
∂ψ
∂x
l
∂
2
ψ
∂x
j
∂x
j
+κ
2
ψ
∂ψ
∂x
l
(5.2.11)
The missing linear term in the above equation comes from the shear contribution
to the stress tensor and
˙v
i
=ν∇
2
v
i
+P
il
κ
2
ψ
∂ψ
∂x
l
−
∂ψ
∂x
l
∇
2
ψ
(5.2.12)
The deterministic equations shown in Eqns.(5.2.11) and (5.2.12) cannot maintain
the equilibrium distribution because the linear terms are dissipative. These are
hydrodynamic terms and are associated with the long wavelength behaviour. The
short wavelength fluctuations give rise to noise terms in the equations of motion
for ψ and v. These noise terms with appropriate correlations to ensure fluctua-
tion dissipation theorem maintain the equilibrium. The final equations of motion,
consequently, are
˙
ψ =
λ
χ
∇
2
ψ −(v.
∇)ψ +N
ψ
(5.2.13)
˙v
i
= ν∇
2
v
i
+P
il
κ
2
ψ
∂ψ
∂x
l
−
∂ψ
∂x
l
∇
2
ψ
+ N
v
i
(5.2.14)
The correlations are
N
ψ
(x,t)N
ψ
(
x
,t
)=−2λ∇
2
δ(x −
x
)δ(t−t
)
N
v
i
(x,t)N
v
j
(
x
,t
)=−2ν∇
2
δ(x −
x
)δ(t−t
)P
ij
(5.2.15)
where P
ij
is the projection operator
P
ij
=δ
ij
−
∂
2
∂x
i
∂x
j
which is necessary to maintain the transverse nature of the velocity field.
110 5 Critical Dynamics in Fluids
In momentum space, the equations of motion are
˙
ψ(
k) =−
λ
χ
k
2
ψ(
k) −i
p
v
j
(
k −p) p
j
ψ(p) + N
ψ
(
k)
=−λ(k
2
+κ
2
)k
2
ψ(
k) −i
p
k
j
v
j
(
k −p) ψ( p) + N
ψ
(
k) (5.2.16)
˙v
j
(
k) =−νk
2
v
j
(
k) +iκ
2
p
p
j
ψ(
k −p) ψ( p)
+
i
2
P
jl
(k)
p
p
l
[(
k −p)
2
−p
2
]ψ(
k −p) ψ( p) + N
v
j
(
k) (5.2.17)
In the above, we have used
χ
−1
=k
2
+κ
2
.
If the non-linear terms are absent, then the Green’s functions and correlation func-
tions can be written down as
G
ψ
(k,κ,ω) =−iω +λ(k
2
+κ
2
)k
2
C
(0)
ψ
(k,κ,ω) =
2λk
2
ω
2
+λ
2
k
4
(k
2
+κ
2
)
2
[G
(0)−1
v
]
ij
= (−iω +ηk
2
)P
ij
(k)
and [C
(0)
v
]
ij
=
2ηk
2
ω
2
+η
2
k
4
P
ij
(k) (5.2.18)
where the momentum space projection operator is
P
ij
(k) =δ
ij
−
k
i
k
j
k
2
The dynamic critical exponents for the order parameter and velocity fields are
different at this zeroth level. At the critical point, κ =0, the relaxation rate for
the ψ field is λk
4
, giving z
ψ
=4. For the velocity field relaxation rate is ηk
2
,
giving z
v
=2. The non-linear terms are likely to affect λ and η, and we use the
self-consistent technique developed in Chapter 4 to find the answer in the next
section.
5.3 One-Loop Perturbation Theory
In this section, we will investigate the single loop perturbation theory contribution
to the transport coefficients λ and η. To do so, we first look at the lowest order
