ASM Metals HandBook Vol. 17 - Nondestructive Evaluation and Quality Control
Подождите немного. Документ загружается.

are washed off, and the film is dried. Detailed information on the processing of radiographic film is available in the
Appendix to this article.
Although x-ray film is sensitive to light, the characteristics of its emulsion are different from those of emulsions used in
photography. Industrial x-ray film is of two general types: that used for direct exposure (called direct-exposure, no-screen,
or nonscreen film) and that used with fluorescent screens (often called screen-type film).
Most industrial x-ray film is of the direct-exposure type and is available in various combinations of film speed, gradient,
and graininess. The choice of film for a given application depends on the type of radiography to be performed. Film types
and selection are discussed in the section "Film Radiography" in this article.
Screen-type films, which are marketed primarily for medical radiography, are only occasionally used for industrial
applications--for example, when a low-power x-ray machine is used and exposure time is excessive with direct-exposure
x-ray film. Screen-type films are more sensitive to light than to x-radiation and are particularly sensitive to the
wavelengths emitted by the fluorescent screen with which they are used. Although blue-sensitive emulsion types are the
ones most often used for industrial radiography, other emulsions sensitive to ultraviolet and to green screen light are
available.
Radiographic Paper. Ordinary photographic paper can be used to record x-ray images, although its characteristics are
not always satisfactory. Photographic paper has a low speed, and the resulting image is low in contrast. However,
photographic paper used with fluorescent screens can be effective for some applications.
Specially designed radiographic paper (also known as paper-base film or opaque-base film) is essentially opaque and has
only a single emulsion. The emulsion is made of gelatin and silver salts, as in x-ray film, but it also contains developer
chemicals. After exposure, the developing action is triggered by an alkaline solution called an activator. Instead of being
fixed after development of the radiographic paper, chemicals remaining in the emulsion are made chemically inactive by
stabilization processing, which is performed very rapidly in a small automatic processor. The images are similar to those
on film radiographs and will not deteriorate for 2 to 3 months. If it is desired to store the radiographs for a longer period,
they can be fixed in ordinary x-ray fixer, washed, and dried.
Although paper radiographs can be exposed directly to x-radiation, it is preferred to make the exposure with fluorescent
screens that produce ultraviolet light. Fluorescent-screen exposures are preferred because exposure time is short and
radiographic contrast is high. With direct exposure, the radiographic contrast is low and the corresponding exposure time
is long.
The image is viewed in reflected light like a photograph; however, the greatest amount of detail can be seen when the
image is viewed in specular light from a mirrorlike reflector (like a spotlight) directed at about 30° to the surface and to
one side of the radiograph. The density (blackening) of paper radiographs, which is known as reflection density, is quite
different from the transmission density of film, but the differences do not cause problems in interpretation.
Radiographic paper can exhibit adequate sensitivity, which in many respects matches or exceeds that of fast direct-
exposure x-ray films. Radiographic paper does not match the sensitivities of slow x-ray films, but because of their speed,
convenience, and low cost, radiographic papers are being used both for the radiography of materials that do not require
critical examination and for in-process control.
A new (3M) dry silver film has recently been introduced that is developed with heat in about 12 s. It also is exposed with
a fluorescent screen and achieves resolutions up to 7 line pairs per millimeter (or a resolution of 0.0055 in.). Viewing is
by reflected or transmitted light.
Xeroradiography (dry radiography) is a form of imaging that uses electrostatic principles for the formation of a
radiographic image. In film radiography, a latent image is formed in the emulsion of a film; in xeroradiography, the latent
image is formed on a plate coated with selenium. Before use, the plate is given an even charge of static electricity over the
entire surface. As soon as the plate is charged, it becomes sensitive to light as well as to x-radiation and must be protected
from light by a rigid holder similar to a film cassette. In practice, the holder is used for radiography as though it contained
film. X-radiation will differentially discharge the plate according to the amount of radiation received by different areas.
This forms an electrostatic latent image of the testpiece on the plate.
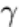
The exposed plate is developed by subjecting the plate, in the absence of light, to a cloud of very fine plastic powder, each
particle in the cloud being charged opposite to the electrostatic charges remaining on the plate. The charged plastic
powder is attracted to the residual charges on the plate. The visible radiographic image at this state is not permanent,
because the powder is simply held in place by electrostatic charges. However, the image can be made permanent by
placing a piece of specially treated paper over the plate and transferring the powder to the paper, which is then heated to
fix the powder in place.
Selenium-coated plates can be easily damaged by fingerprints, dirt, and abrasion. For this reason, automated equipment is
used for charging and for development and image transfer to paper.
The image produced by xeroradiography can be either positive or negative, depending on the color of the plastic powder
(toner) and the color of the paper upon which the image is deposited. If a negative xeroradiographic image is desired,
dark-colored paper with light-colored toner can be used; a positive image would require light-colored paper and dark-
colored toner.
Because of an electrostatic edge effect, xeroradiographic images exhibit excellent detail; even small indications in the
image are sharply outlined. Optimum results from xeroradiography are more likely when the user develops special
techniques rather than employing the radiographic techniques normally used with film.
Lead Screens
It is the combination of filtration and intensification that makes lead screens the most widely used in industrial
radiography. Lead absorbs radiation to a greater extent than most other materials, with the amount of absorption
depending largely on the penetrating quality of the radiation (photon energy or wavelength). High-energy (short-
wavelength) radiation passes through lead much more readily than low-energy radiation; in other words, low-energy
radiation is more readily absorbed by a lead screen than high-energy radiation. Because scattered radiation from a
testpiece is always of a lower energy than the incident beam passing through a testpiece, a lead screen will absorb a
relatively high percentage of unwanted scattered radiation, but will absorb a somewhat lower percentage of the image-
forming radiation. This effect is known as filtration, and lead screens are sometimes referred to as lead-filter screens.
Filtration of Secondary Radiation. As discussed in the earlier section "Principles of Shadow Formation" in this
article, secondary radiation can arise by internal scatter from within the testpiece and by back scatter from objects behind
the film, detector, or screen. Because of the need to filter out both internal scatter and back scatter, two screens are
normally used in film radiography. The screen that faces the top of the film is referred to as the front screen, and the
screen behind the film toward the table or floor is referred to as the back screen. Both screens absorb scattered radiation.
In practice, the front screen is sometimes the thinner of the two because the image-forming radiation must always pass
through this screen. The usual thickness of the front lead screen is 0.13 or 0.25 mm (0.005 to 0.010 in.), but may be
greater or less depending on the type of material being inspected and the photon energy of the incident beam. The main
function of the beam screen is to absorb unwanted back scatter; back screens can be of any thickness that performs this
function adequately, although usually they are of the same thickness as the front screen.
In the radiography of thin-gage or low-density materials, in which low photon energies are used, care must be exercised to
ensure that the front screen does not excessively filter the image-forming radiation. Excessive filtration affects subject
contrast and tends to reduce radiographic sensitivity. In such cases, lead screens less than 0.13 mm (0.005 in.) thick
should be used; lead screens 0.05 or 0.025 mm (0.002 or 0.001 in.) thick or less are available. For thicker testpieces or
denser material, where higher photon energies are used, the front screen can be thicker than 0.13 mm (0.005 in.), ranging
as high as 1 mm (0.040 in.) when betatrons or high-MeV linear accelerators are used. The back screen can be 0.5 to 10
mm (0.020 to 0.400 in.) thick in these cases.
Intensification. When lead is excited by x-ray or -ray radiation, it produces electrons. The number of electrons
emitted is directly proportional to the photon energy of the radiation that passes through the testpiece and reaches the
screens.
In film radiography, the emitted electrons expose additional silver halide crystals in the film emulsion. After
development, film densities are greater than they would have been without the intensifying action of emitted electrons.
Intensification not only increases overall photographic density, thus requiring shorter exposure times for producing a
given density, but also enhances radiographic contrast, thus improving the ability to resolve small flaws.

In real-time radiography with a fluorescent-screen system, a similar effect occurs. The phosphor material used in
fluorescent screens is generally more sensitive to electrons than to primary x-rays. With high-energy x-rays, the electrons
generated from a suitable lead screen (or other heavy metal, such as tantalum or tungsten) can be used to enhance the
imaging process. These screens are often useful in real-time radiography with MeV radiation.
Because lead screens have properties of both filtration and intensification, there is a combination of thickness of the
subject material and photon energy of the incident radiation at which intensification just balances filtration and there is no
net advantage. With steel testpieces in film radiography, this null point is generally considered to occur at a combination
of 6 mm ( in.) testpiece thickness and 140-keV x-rays when a 0.13 mm (0.005 in.) thick front screen and a 0.25 mm
(0.010 in.) thick back screen are used. At lower tube voltages or with thinner testpieces, the filtration effect is dominant,
resulting in longer exposure times. At high voltages or with thicker testpieces, the intensification factor becomes
dominant, and exposure times can be reduced to as much as one-third of the time for direct exposure (no screens), at a
tube voltage of 200 to 300 kV. With cobalt-60 radiation and steel testpieces, the exposure time using lead screens is about
one-third that for direct exposure.
With light metals such as aluminum, the null point occurs at a greater thickness than for steel. With metals of greater
atomic number than steel, it occurs in thinner sections. For both lighter and heavier metals, the null point will occur at a
radiation energy different from 140-keV x-rays. Although electrons are produced throughout the volume of lead on both
screens when excited by x-rays or -rays, electrons produced by radiation having photon energies below 1 MeV are
largely low-energy electrons. Such electrons are readily absorbed by the volume of lead in the screen. Only those
electrons produced at the surface adjacent to the film escape to intensify the latent image on the film. Therefore, the closer
the film emulsion is to the surface of the lead, the more effectively the electrons interact with the emulsion. This is why
lead screens should always be in intimate contact with the film.
Low-energy electrons have little penetrating capability. They will affect the emulsion closest to the screen, but will not
penetrate the film base to affect the emulsion on the other side. Although electrons have little penetrating capability, they
will penetrate interleaving paper, so this should always be removed from the film to avoid a paper-pattern image on the
radiograph. Similarly, dust, dirt, lint, and other foreign material between the film and screen must be avoided to prevent
extraneous images (artifacts) on the radiograph.
Precautions. Lead sheet of usual screen thicknesses is easily bent. For this reason, lead screens are often backed by
cardboard or other material to facilitate handling. Even so, the care and handling of lead screens is important. Deep
scratches or dents must be avoided because they can appear as artifacts on the radiographic image. Wrinkles or folds in
the screens can also be detected on the radiograph. Chemical spills, dust, and dirt must be carefully removed from the
screen before use. Oxidation of the screen, which occurs with age and appears as a gray coating, does not seem to affect
the utility of the screen. Some screens are coated with a special material to prevent oxidation. Although coated screens
exhibit a reduced capability for intensification, they are easier to keep clean and free of tiny scratches. The use of spray
lacquers or acrylics to protect the surface of the screens should be avoided; such coatings often have a highly detrimental
effect on the radiographic image.
The composition of the lead used for screens is important. Pure lead is soft and may rub off on the film to produce lead
smudge on the radiograph. Lead screens made from 94Pb-6Sb alloy are most commonly used because they are harder and
more resistant to scratching. However, care must be taken that there is no segregation of antimony (which appears as
shiny or different-colored streaks on the screen), because antimony segregation produces low-density streaks on the
radiograph.
Screens must be flat and free of roll marks or chatter. Variations in screen thickness result in areas of poor contact with
the film, which can produce fuzzy areas in the radiograph.
Prolonged contact with lead screens can produce an effect called lead-screen fog. Consequently, films should never be left
in contact with lead screens longer than is reasonably necessary. This is particularly important under conditions of high
temperature and humidity, or within 24 h after cleaning screens with very fine steel wool or other abrasive.
As a general rule, lead screens should be used whenever they can improve the resolution of detail, even though the
exposure time may be longer. A single back screen can be used for intensification purposes, but in the absence of a front
screen, forward scatter may be present in the radiograph. Sometimes, the single back screen technique is useful in the
radiography of very thin materials at low photon energies. If scatter is a problem, other means of control (such as a copper
filter located at the tube) are quite effective. Lead screens should always be used in radiography using high photon

energies (above 300-keV x-rays or with most -ray sources) to avoid extraneous paper patterns or other effects due to
irradiation of the film holder.
Certain types of film packaging incorporate thin lead foil (0.025 or 0.050 mm, or 0.001 or 0.002 in. in thickness) on both
sides of the film, with the film-foil composite contained in a lighttight envelope. These prepackaged composites are
convenient because the envelopes containing screens and film can be used as a film holder or cassette. In addition,
loading in the darkroom is eliminated, and there is less likelihood of foreign particles being present to create artifacts on
the radiograph.
Lead Oxide Screens
A variation of lead screens is lead oxide screens, which are made by evenly coating a paper base with lead oxide. The
result is an extremely flexible screen, equivalent to about 0.013 mm (0.0005 in.) of lead foil, that is lightweight and free
of antimony segregation. Lead oxide screens are available only in lighttight envelopes containing screens on both sides of
the film.
The principle of lead oxide screens is essentially the same as for lead screens. The main differences are a lesser degree of
filtration than lead screens and a greater intensification below 140 keV as well as slightly lesser intensification at 300 keV
than with 0.13 mm (0.005 in.) thick lead screens. Although lead oxide screens are particularly advantageous for the
radiography of light alloys or thin material, they can also be used in the radiography of heavier material.
Screens of Metals Other Than Lead
Foils of many of the heavier metals can be as effective as lead for radiographic screens, but usually are not as practical,
either because the foils are not as flexible or because of cost.
Gold screens perform as well or better than lead screens, but gold costs considerably more and tends to work harden
when bent, which eventually cracks the screen.
Tantalum screens exhibit slightly lower filtration but higher intensification than lead. However, tantalum foil is stiff
and springy, which does not allow it to be shaped to fit around a testpiece. Tantalum foil can be used in solid cassettes,
can be polished, and is quite resistant to minor abrasion, but is expensive to use.
Depleted-uranium screens exhibit greater filtration than lead screens. However, uranium is brittle, is somewhat
difficult to obtain in thin sheets, and is also expensive to use.
Copper screens have been used, especially with cobalt-60 radiography. Copper has a lower degree of filtration than
lead and a lower intensification factor, but copper provides greater radiographic sensitivity.
Composite screens of lead, copper, and aluminum (and sometimes other metal foils) to control the radiation reaching
the film have occasionally been used with supervoltage x-ray machines for the inspection of thick steel testpieces.
Fluorescent Intensifying Screens
The efficiency of film and paper radiography can be improved by fluorescent intensifying screens, which emit radiation in
the ultraviolet, blue, or green portion of the electromagnetic spectrum. These screens are called fluorescent intensifying
screens because they fluoresce, or produce light, when excited by x-rays or -rays. Certain compounds, such as calcium
tungstate or barium lead sulfate, often containing trace elements of some other chemical or phosphor, have the property of
emitting light immediately upon excitation by short-wavelength radiation. Crystals of these chemicals are finely
powdered, mixed with a binder, and coated on some mildly flexible support such as cardboard or plastic to make a
fluorescent screen. A thin, tough, transparent overcoat is applied to the sensitized surface of the screen to prevent damage
to the crystals during use.
Screen Speeds. Fluorescent intensifying screens, widely used in medical radiography, are available in a variety of
speeds. Perhaps the most common of these are blue-emitting screens, which can be characterized in speed as very slow,
slow, medium, medium high, high, and super. In industrial radiography, the screen most often used (with the appropriate
blue-sensitive screen-type film) is the medium-speed fluorescent screen.
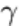
By the addition of proprietary rare-earth phosphors to the fluorescent crystals, green-emitting screens are produced that,
when used with the appropriate green-sensitive film, have relatively fast screen speeds characterized as high speed,
superspeed, and beyond superspeed.
With the addition of barium lead sulfate and a proprietary trace element, the crystals can be made to fluoresce in the
ultraviolet range. Used with the appropriate ultraviolet-sensitive film or radiographic paper, these screens have speeds that
are equivalent to the slow and medium-high-speed blue-emitting screens.
For the greatest radiographic effect, fluorescent screens should be used with a film that is sensitive to the particular
wavelengths of light emitted by the screen. In general, though not always true, the slower the screen-film combination,
the better the radiographic definition. However, even slow-speed combinations can reduce exposure times by 20 to 98%
compared to the fastest direct-exposure film, depending on photon energy, type of screen, type of film, and testpiece
material.
Disadvantages. The main reason for using fluorescent intensifying screens in industrial radiography is to reduce
exposure times. However, in comparison with lead screens, fluorescent screens have the following disadvantages. First,
the screen light reaches the film as cones of light from each fluorescing crystal. Because this tends to blur the image,
radiographic definition using lead screens is superior. As in real-time radiography, inherent unsharpness in a fluorescent
screen often overshadows any effect of geometric unsharpness.
Second, the purely statistical variations in the number of x-ray quanta (the total amount of x-radiation) absorbed from one
small area to another on the screen result in uneven brightness, which in turn is recorded on the film as screen mottle (also
known as quantum mottle). Screen mottle is particularly apparent at photon energies in the range 150 to 300 keV. There is
essentially no screen mottle associated with lead screens.
Finally, lead screens filter out scattered radiation in addition to intensifying the radiation beam, but fluorescent screens
have little or no filtering capability and intensify scattered radiation in the same proportion as transmitted radiation.
However, they can be placed between lead screens, using the lead to protect against secondary radiation and back scatter.
Precautions in Use. The combination of a screen and the film that is sensitive to the wavelength of the screen light
results in minimum exposures. However, fluorescent screens can be used with the direct-exposure films ordinarily used in
industrial radiography. With direct-exposure film, the intensification factor may range from 2 to 25 times, but screen
mottle and noticeable light spreading will be less because of the lower speed of the screen-film combination.
Fluorescent intensifying screens are often permanently mounted inside rigid film holders (cassettes). Cassettes of this type
are designed to provide even pressure between screen and film to maintain intimate contact during exposure. Lack of
contact results in unsharpness in the image and should be carefully avoided. Fluorescent screens may not be permanently
mounted and can be used in regular film holders. Under these conditions, however, maintenance of good contact between
fluorescent screen and film is more difficult.
The fluorescent crystals on the surface of the screens can be easily damaged by handling, and the screen cannot be
repaired. Because fluorescent screens are expensive, great care must be taken to avoid scratches, abrasion, or chemical
spills, which can damage the screen and affect the radiographic image. It is even more important to avoid fingerprints,
stains, grease, dirt, and dust on the surface of the screens. Because these all absorb screen light, their image will be
transmitted to the radiograph. If the screen becomes dirty it may be cleaned, but the manufacturer's cleaning
recommendations should be followed to avoid damage to the protective overcoat.
Because of the high radiation intensity used in industrial radiography, great care must be exercised not to expose any
portion of a fluorescent screen to the full intensity of the primary beam. When exposed to high-intensity radiation, a
screen may continue to fluoresce after being removed from the beam; this semipermanent effect, known as screen lag,
will produce false images on subsequent radiographs.
Fluorescent intensifying screens are most often used for the radiography of thick testpieces when the x-ray machine has
limited penetrating capability. With medium-speed, blue-sensitive fluorescent intensifying screens (with appropriate
screen-type film), it is possible to radiograph 75 mm (3 in.) of steel with 250-keV x-rays in a reasonable exposure time.
Fluorescent screens are rarely used with -rays or with photon energies exceeding 1 MeV because of screen mottle and
low intensification factors. In all cases where fluorescent screens are used, reciprocity law failure is likely to occur;
reciprocity law failure (discussed in the section "X-Ray Tubes" in this article) is not a problem in direct or lead-screen
exposures.
Fluorometallic Screens
Fluorometallic screens consist basically of lead screens placed on either side of a fluorescent-screen/film combination.
The theory behind combining lead and fluorescent screens is that the lead preferentially absorbs scattered radiation
(which fluorescent screens will not do) and that the fluorescent screen next to the film intensifies the radiation beam to
shorten the exposure.
Commercial screens incorporating lead and fluorescent materials laminated together require significantly shorter exposure
times than lead screens, and the image quality exceeds that of fluorescent screens. When direct-exposure film is used with
fluorometallic screens, the exposure time can be reduced to as little as one-seventh of that for lead screens with almost the
same radiographic sensitivity. When screen-type film is used, exposure time can be reduced to as little as one-ninth of that
for other methods. The speed factor varies with type of film, type of fluorometallic screen, and photon energy of
radiation. With high-voltage x-rays, and with cobalt-60 -rays, the speed factor is about two to seven.
Radiographic Inspection
Revised by the ASM Committee on Radiographic Inspection
*
Real-Time Radiography
Various types of image conversion techniques allow the viewing of radiographic images while the testpiece is being
irradiated and moved with respect to the radiation source and the radiation detector. These radioscopic techniques can be
classified as real-time radiography (also known as real-time radioscopy) and near real-time radiography (or near real-time
radioscopy). The distinction between these two classifications is that the formation of near real-time images occurs after a
time delay, and this requires limitation of the test object motion. An example of near real-time radiographic imaging
involves the use of discrete detectors (primarily linear arrays) that scan the area being irradiated. The outputs are then
processed digitally to form images in near real time. This technique is often referred to as digital radiography.
Background. The predecessor of the modern methods of real-time radiography is fluoroscopy. This technique, which is
now largely obsolete, involves the projection of radiographic images on a fluorescent screen (Fig. 27). The screen consists
of fluorescent crystals, which emit light in proportion to the intensity of the impinging radiation. The radiographic image
can then be viewed, with appropriate measures taken to protect the viewer from radiation.
Fig. 27 Diagram of the components and principles of operation of a fluoroscope
The main problem with fluoroscopes is the low level of light output from the fluorescent screen. This requires the
suppression of background light and about 30 min for the viewer's eyes to become acclimated. Moreover, radiation safety
dictates viewing through leaded glass or indirectly by mirrors. Because of these limitations, other methods have been
developed to improve safety and to amplify the images from fluorescent screens.
One of the early systems (1950s) involved the development of image-intensifier tubes. Image-intensifier tubes (Fig. 28)
are glass-enclosed vacuum devices that convert a low-intensity x-ray image or a low-brightness fluorescent-screen image
into a high-brightness visible-light image. Image intensification is achieved by a combination of electronic amplification
and image minification. The image brightness at the output window of an image-intensifier tube is about 0.3 × 10
3
cd/m
2
(10
-1
lambert) as compared to about 0.3 cd/m
2
(10
-4
lambert) for conventional fluoroscopic screen.
These early image intensifiers were originally developed for
medical purposes and were limited to applications with low-
energy radiation (because of low detection efficiencies at
high energies). Consequently, industrial radiography with
these devices was restricted to aluminum, plastics, or thin
sections of steel.
By the mid-1970s, other technological developments led to
further improvements in real-time radiography. These
advances included high-energy x-ray sensitivity for image
intensifiers, improved screen materials, digital video
processing for image enhancement, and high-definition
imaging with microfocus x-ray generators.
Modern Image Intensifiers. Although the early image
intensifiers were suitable for medical applications and the
inspection of light materials and thin sections of steel, the
image quality was not sufficient for general use in
radiography. Therefore, image intensifiers had to be
redesigned for industrial material testing (Ref 3). The
modern image intensifier is a very practical imaging device
for radiographic inspection with radiation energies up to 10
MeV. With the image intensifier, a 2% difference in
absorption can be routinely achieved in production inspection applications. The typical dynamic range of an image
intensifier before image processing is about 2000:1.
A schematic of a modern x-ray image intensifier is shown in Fig. 29, along with a graph indicating the level of signal
strength as the radiation passes through a sample and impinges on the entrance screen of the image-intensifier tube. These
screens are usually made of a scintillating material, such as cesium iodide (CsI), and fixed to a photocathode. The energy
of the incident radiation quanta generates electrons that produce light in the CsI entrance screen (approximately 150
photons per absorbed gamma quantum). To avoid degradation of the image quality by lateral dispersion of the light in the
conversion screen, the CsI scintillating material, with a cubic crystalline structure, is grown under controlled conditions,
resulting in small, needle-shaped elements. This structure causes the scintillating screen to act as a fiber-optical faceplate.
Light generated in one crystal needle does not spread laterally, but is confined to the needle in a direction parallel to the
incident radiation. Therefore, the thickness of the conversion screen does not cause appreciable deterioration in the spatial
resolution of the system.
Fig. 28 Schematic of an image-intensifier tube
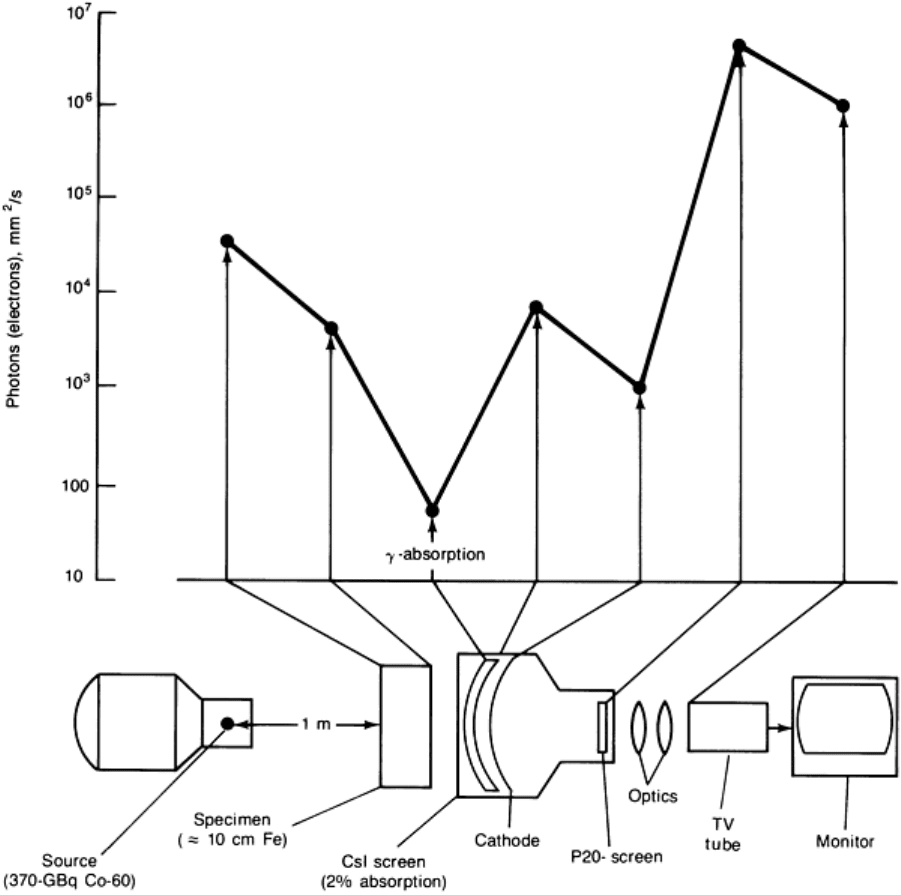
Fig. 29 Schematic of a typical radioscopic system using an x-ray image intensifier
The light from the scintillating screen then impinges on a photocathode in contact with the entrance screen. The
photocathode emits photoelectrons. The electron image produced at the cathode is reduced by a factor of ten and is
intensified by means of an accelerating voltage. The final phosphor screen presents a relatively bright image
(approximately 5 million photons per second per square millimeter), caused by the impinging electrons. The image then
passes through an optical system, which directs the image to a television camera tube, such as a vidicon or plumbicon
tube. Vidicons have a dynamic range of 70:1; plumbicons, 200:1.
Contrast Sensitivity. Figure 29 shows the importance of detection efficiency with regard to the contrast sensitivity of
the radioscopic system. The example is for a 370 GBq (10 Ci) cobalt-60 source, but a similar analysis can be performed
for an x-ray source. A cobalt-60 source with an activity of 3700 GBq (100 Ci) generates 60,000 photons per second per
square millimeter at 1 m with energies of 1.1 and 1.3 MeV. After passing through a 100 mm (4 in.) thick steel specimen,
the radiation is attenuated in intensity by a factor of 15. Therefore, only 4000 photons per second per square millimeter
are available for imaging. The detection efficiency of the CsI entrance screen in a typical modern image-intensifier tube is
approximately 2%. This reduces the photon intensity to 80 per second per square millimeter. With this intensity, the laws
of statistics (see the section "Quantum Noise (Mottle) and the Detective Quantum Efficiency" in the article "Industrial
Computed Tomography" in this Volume) predict a noise-to-signal ratio of approximately 11%, which limits the attainable
contrast resolution.
From Fig. 29, it is apparent that the critical factor for determining the contrast resolution is the relatively low detection
efficiency of the entrance screen. To overcome this limitation, one must either increase the detection efficiency of the
conversion screen or collect the photons over a longer period by summing television frames to improve the photon
statistics in each picture element. The detection efficiency of the entrance screen can be improved by reducing the energy
of the radiation or by making the entrance screen thicker. The minimum energy of the radiation is generally dictated by
the need to have sufficient energy to penetrate the subject and to provide sufficient intensity for imaging at the conversion
screen. Therefore, it is generally impossible to reduce the energy to improve conversion efficiency. Although a thicker
entrance screen would improve detection efficiency, it would reduce the attainable spatial resolution. In general,
improving the detection efficiency and improving the spatial resolution are competitive processes. The most conventional
means of enhancing contrast resolution is through the summation of television frames with a digital image-processing
system.
The spatial resolution of a real-time system is principally defined by the size and thickness of the detectors (or grain
size of fluorescent crystals), the raster scan of the television system, and, for quantum statistical reasons, the intensity of
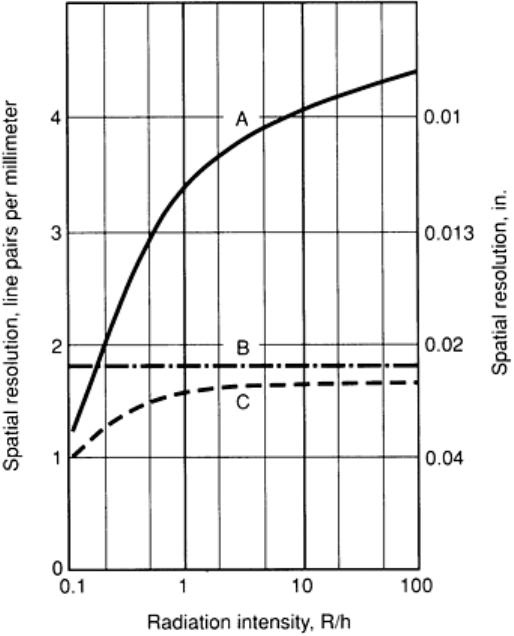
the radiation. The dependencies are plotted in Fig. 30 for a typical image intensifier. Curve A shows a spatial resolution of
about four line pairs per millimeter (or a resolution of 0.01 in.) at 10 R/h without television monitoring. Curve C shows
the typical resolution of an image intensifier with television monitoring.
Fig. 30 Spatial resolution dependence of an image-intensifier syst
em as a function of radiation intensity at the
entrance screen. A, image-
converter resolution; B, 625 TV lines limit; C, combined resolution of image
converter and TV line
Real-Time Radiography With Fluorescent Screens. Because of the development of low-level television camera
tubes and low-noise video circuitry, the dim images on a fluorescent screen can be monitored with video systems. The
contrast sensitivity and the spatial resolution of fluorescent-screen systems are comparable to those of image intensifiers,
but the use of fluorescent screens is limited to lower radiation energies (below about 320 keV without intensifying screens
and about 1 MeV with intensifying screens). Nevertheless, fluorescent screens can provide an unlimited field of view,
while image intensifiers have a field of view limited to about 300 mm (12 in.). Example 1 in this article describes an
application in which a fluorescent screen (with isocon camera) was preferred over an image intensifier. The dynamic
range of systems with fluorescent screens can vary from 20:1 for raw images to 1000:1 with digital processing and a large
number of frames averaged.
Television Cameras. The types of low-level television cameras available include image orthicons, image isocons, and
secondary electron-coupled vidicons. Radiographic inspection is also performed with x-ray sensitive television cameras,
which require no extra conversion or optics. X-ray sensitive television cameras are limited in image size and sensitivity
and are primarily used in the inspection of low-density materials and electronic components.
Image orthicons and image isocons are return beam tubes with internal electron multipliers. The orthicon is useful to light
levels of about 1.076 × 10
-3
lm/m
2
(10
-4
ftc), and the isocon is useful to 1.076 × 10
-4
lm/m
2
(10
-5
ftc). The isocon provides
the best noise performance and resolution of all tubes for low-light levels and static scenes, but degrades for moving
scenes and is highly complex. Both orthicons and isocons exhibit little target overloading damage. Both the image
orthicon and isocon camera tubes must be carefully adjusted for optimum performance; the isocon is the most difficult to
adjust and demands a skilled technician. Both are temperature sensitive and should be operated under stabilized
conditions. The dynamic range for the isocon is about 1000:1.
Secondary electron-coupled vidicons employ an internal initial stage of target amplification, using a secondary target.
They are similar in performance to isocons, are less complex, but differ in lag and require special protection circuitry to
prevent target destruction from overloading. Their primary use is in low-light level, low-contrast applications down to
1.076 × 10
-4
lm/m
2
(10
-5
ftc).
Fluorescent screens used in real-time radiography are similar in construction and properties to fluorescent
intensifying screens for film radiography. The commonly used fluorescent screens consist of a plastic substrate coated
with a powdered luminescent material and an epoxy binder. The spatial resolution of the screen depends on the grain size
of the luminescent material and the range and distribution of the conversion electrons and light photons. The x-ray
absorption properties of the screen depend on the atomic weight and density of the luminescent material. The light
conversion efficiency, the color of the emitted light, and the speed of the screen are additional properties that are
dependent on the characteristics of the luminescent material.
A wide variety of fluorescent screens are available. Screens using zinc sulfide as the fluorescent material have a high light
output and a short afterglow, but the contrast and sharpness of this type of screen are poor. Zinc sulfide screens also use
cadmium, which has a higher atomic number and a higher density than zinc. Other phosphor materials used include
calcium tungstate and phosphors with rare-earth materials. Until the 1970s, many applications utilized the popular
calcium tungstate screens. With the availability of rare-earth materials in high states of purity and at reasonable costs,
however, one can design new phosphors with improved x-ray absorptions, greater density, and higher x-ray to light
conversion efficiencies. Screens have been made of such materials as gadolinium oxysulfide doped with terbium,
LaOBr:Tm, BaFCl:Eu, or YTaO
2
:Nb. These screens are discussed more fully in Ref 4. It should be noted that many rare-
earth screens are not as effective as calcium tungstate in absorbing very high energy x-rays.
Because the intensity of the image on a fluorescent screen is rather low, very sensitive TV cameras are required for
remote viewing. Different types of low light level cameras are available that have one- or two-stage light amplifiers or
extremely sensitive camera tubes. When designing a fluoroscopic system, it is important to recognize that high light
amplification from a double-stage amplifier generally yields poor spatial resolution and contributes additional noise to the
image. In many cases, depending on the size of the fluorescent screen, the camera can also be the limiting factor in image
quality. Therefore, both the entrance screen and the camera must be optimized for the particular inspection application.
Digital Radiography. A third method of radiographic imaging involves the formation of an image by scanning a linear
array of discrete detectors along the object being irradiated. This method directly digitizes the radiometric output of the
detectors and generates images in near real time. Direct digitization (as opposed to digitizing the output of a TV camera or
image intensifier) enhances the signal-to-noise ratio and can result in a dynamic range up to 100,000:1. The large
dynamic range of digital radiography allows the inspection of parts having a wide range of thicknesses and densities.
Discrete detector arrangements also allow the reduction of secondary radiation from scattering by using a fan-beam
detector arrangement like that of computed tomography (CT) systems. In fact, industrial CT systems are used to obtain
digital radiographs (see the article "Industrial Computed Tomography" in this Volume).
The detectors used in digital radiography include scintillator photodetectors, phosphor photodetectors, photomultiplier
tubes, and gas ionization detectors. Scintillator and phosphor photodetectors are compact and rugged, and they are used in
flying-spot and fan-beam detector arrangements. Photomultiplier tubes are fragile and bulky, but do provide the capability
