ASM Metals HandBook Vol. 17 - Nondestructive Evaluation and Quality Control
Подождите немного. Документ загружается.

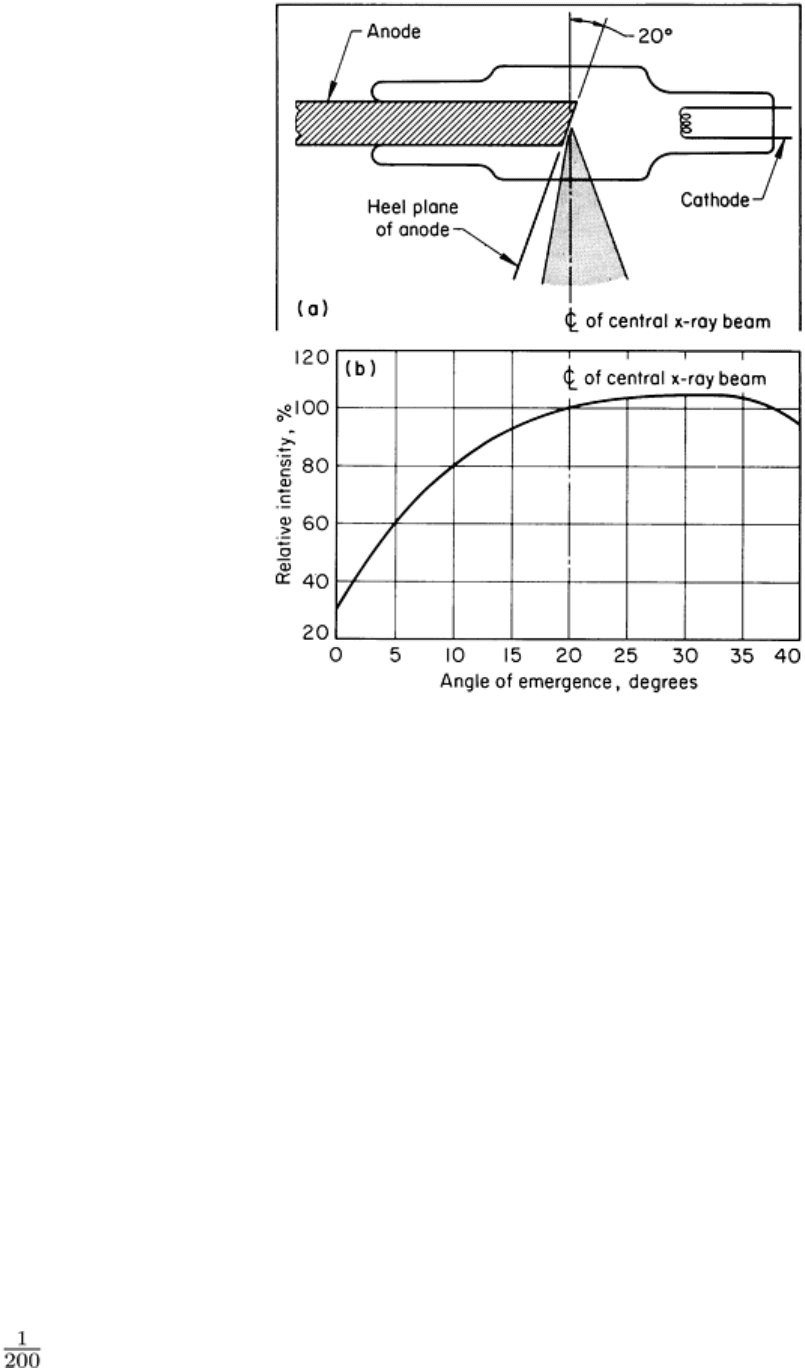
Fig. 15 Heel effect in a typical conventional x-
ray tube having an anode face inclined 70° to the tube axis (20°
anode heel angle). (a) Diagram showing relation of useful beam (crosshatched region) to tube components. (b)
Graph of beam intensity (expressed as a percent
age of the intensity of the central beam) as a function of angle
of emergence relative to the anode heel plane
Radiographs of large-area testpieces that are made at relatively short source-to-detector distances will exhibit less
photographic density (film) or less brightness (real-time) in the region where the incident radiation is less intense because
of the heel effect. This can lead to errors in interpretation unless the heel effect is recognized. The consequences of heel
effect can be minimized by using a source-to-detector distance that ensures that the desired area of coverage is entirely
within the useful beam emanating from the x-ray tube or by making multiple exposures with a reduced area of coverage
for each exposure.
The heel effect also influences image sharpness when there is some geometric enlargement. The projected size of the
focal spot is reduced near the "heel," which reduces the geometric unsharpness of the radiographic image nearest the heel.
Conversely, the radiographic image is less sharp on the cathode side of the radiation beam.
Stem Radiation. In an x-ray tube, electrons that produce the useful x-rays are known as primary electrons. The kinetic
energy of the primary electrons is converted partly to radiation and partly to heat when these electrons collide with the
target. However, some of the primary electrons collide with other components inside the tube envelope, giving rise to
slower-moving electrons known as secondary electrons or stray electrons. Some of these stray electrons bounce around
inside the tube and eventually strike the anode. This produces x-rays, called stem radiation, that are of relatively long
wavelength because of the low kinetic energy of the stray electrons that produced them. Stem radiation, which is about
as intense as the useful beam, is produced all over the anode surface, not only at the focal spot. Because stem
radiation is projected from a comparatively large area instead of emanating only from the focal spot, it invariably causes
image unsharpness.

Some x-ray tubes are designed to eliminate this phenomenon by the use of hollow-end anodes called hooded anodes (Fig.
16). Hooded anodes reduce stem radiation both by shielding the target from some of the stray electrons and by absorbing
much of the radiation produced by stray electrons that enter through the open end of the anode cavity.
Fig. 16 Schematic of an x-ray tube having a hooded anode to minimize stem radiation
Tube Rating. X-ray tubes produce a great amount of heat. At 100-kV tube voltage, only about 1% of the electrical
energy is converted to x-rays; the remaining 99% is converted to heat. Heat removal constitutes the most serious
limitation on x-ray tube design. The size of the focal spot and the design of the anode are the main factors that determine
the rating of a particular x-ray tube. A tube rating is a limiting or maximum allowable combination of tube voltage
(kilovoltage) and tube current (milliamperage).
Most industrial tubes are rated for continuous service at the maximum tube current that will give reasonable target life at
maximum tube voltage. Therefore, if a tube has a continuous rating of 10 mA at 150-kV constant potential, the rating is
1500 W. Any product of kilovoltage and milliamperage equal to 1500 will not exceed the heat limit on the anode. The
tube should be able to operate continuously at 20 mA and 75 kV, 30 mA and 50 kV, and so on.
However, another limitation on operating characteristics is that, to sustain the same tube current, the filament must be
heated to a higher temperature at low tube voltages than at higher tube voltages. Therefore, the tube current at low voltage
must be limited to achieve satisfactory service life. Figure 17 shows a tube-rating chart for continuous operation in which
the total length of time for each exposure is not a factor in determining operating conditions. On the left side of the chart,
at low tube voltages, operating conditions are determined by filament life; on the right side of the chart, at high tube
voltages, anode heating (focal-spot loading) limits operating conditions. Any combination of tube voltage and tube
current below the curve can be used without impairing the service life of either anode or filament. In practice, unless there
are special considerations, values approaching the continuous-duty rating are ordinarily used to minimize exposure time.
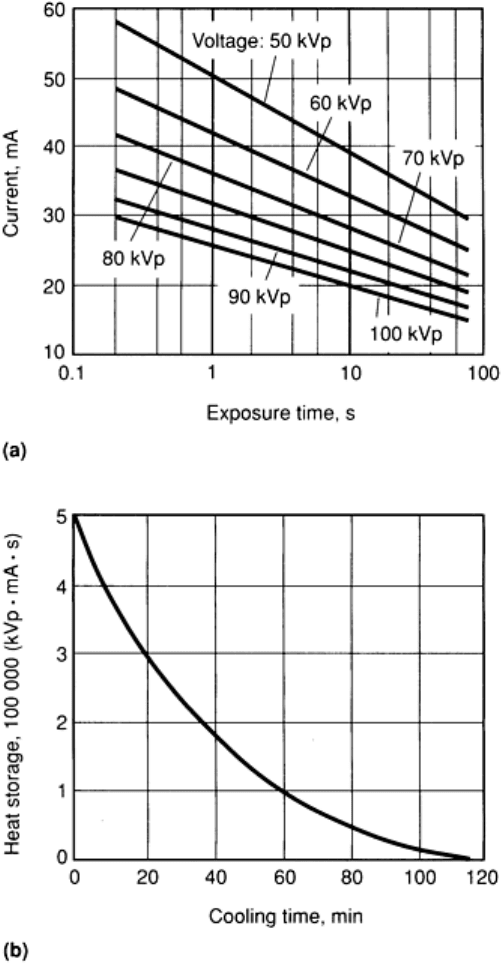
Fig. 17 Tube-rating chart for a typical beryllium-window tube
with a 0.3 mm (0.012 in.) focal spot. Tube rating
is for continuous operation with a full-wave-rectified single-
phase power supply. Below 22.5 kVp, tube current
is limited by desired filament life; above 34.5 kVp, tube current is limited by focal-spot loa
ding. Filament
voltage is 1.7 to 2.6 V, and filament current is 3.2 to 4.0 A.
Some tube manufacturers use an alternative form of rating (intermittent rating), in which the time involved in making a
particular film (or paper) exposure enters into the rating. As illustrated in Fig. 18(a), for each combination of tube current
and tube voltage there is a maximum exposure time that must not be exceeded. Longer exposure times can cause the
target to melt. Normally, there is a minimum waiting time between exposures for tubes rated for intermittent operation.
For the tube rating shown in Fig. 18(a), the waiting time is specified as 5 s when maximum exposure times are used.
Some manufacturers have developed rating systems in which the minimum waiting time can be calculated.
Fig. 18 Tube-rating chart (a) and cooling chart (b) for a typical industrial x-ray tube having a 1
.5 mm (0.06
in.) focal spot. Tube rating is for intermittent operation with a full-wave-rectified single-
phase power supply; a
minimum 5-s waiting time between exposures is specified. Cooling chart is for an oil-
filled housing without an
air circulator. When housing is fitted with an optional air circulator (fan), cooling times are reduced to one-
half
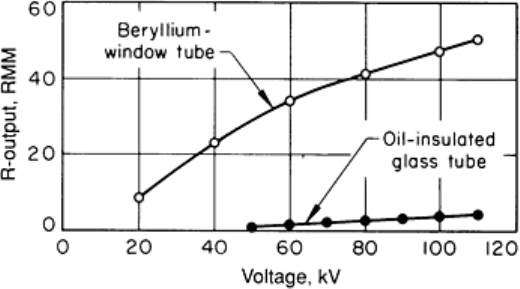
the values determined from the chart.
Tubes that are rated for intermittent operation can accumulate only a certain amount of heat, then they must be allowed to
cool before additional exposures are made. Figure 18(b) is the cooling chart used in conjunction with the tube-rating chart
in Fig. 18(a). According to the cooling chart, the tube can store 500,000 units of heat (one heat unit equals 1 mA · 1 kVp ·
1 s). Therefore, if a series of exposures is to be made at 70 kVp, 30 mA, and 5 s, each exposure will expend energy equal
to 10,500 heat units. The number of successive film exposures that can be made, with a 5-s wait between exposures, is 47
(500,000 heat units divided by 10,500 heat units per exposure). After the 47th exposure, the tube must be allowed to cool
according to the cooling curve in Fig. 18(b). The tube can be allowed to cool for any length of time, and then another
exposure can be made as long as the additional exposure does not increase the stored heat above 500,000 heat units. After
the tube has reached its capacity for heat storage, waiting times between successive exposures must be in accordance with
the cooling curve. In this case, as shown in Fig. 18(b), 2 h would be required (or 1 h with an optional air circulator) for the
tube to cool completely.
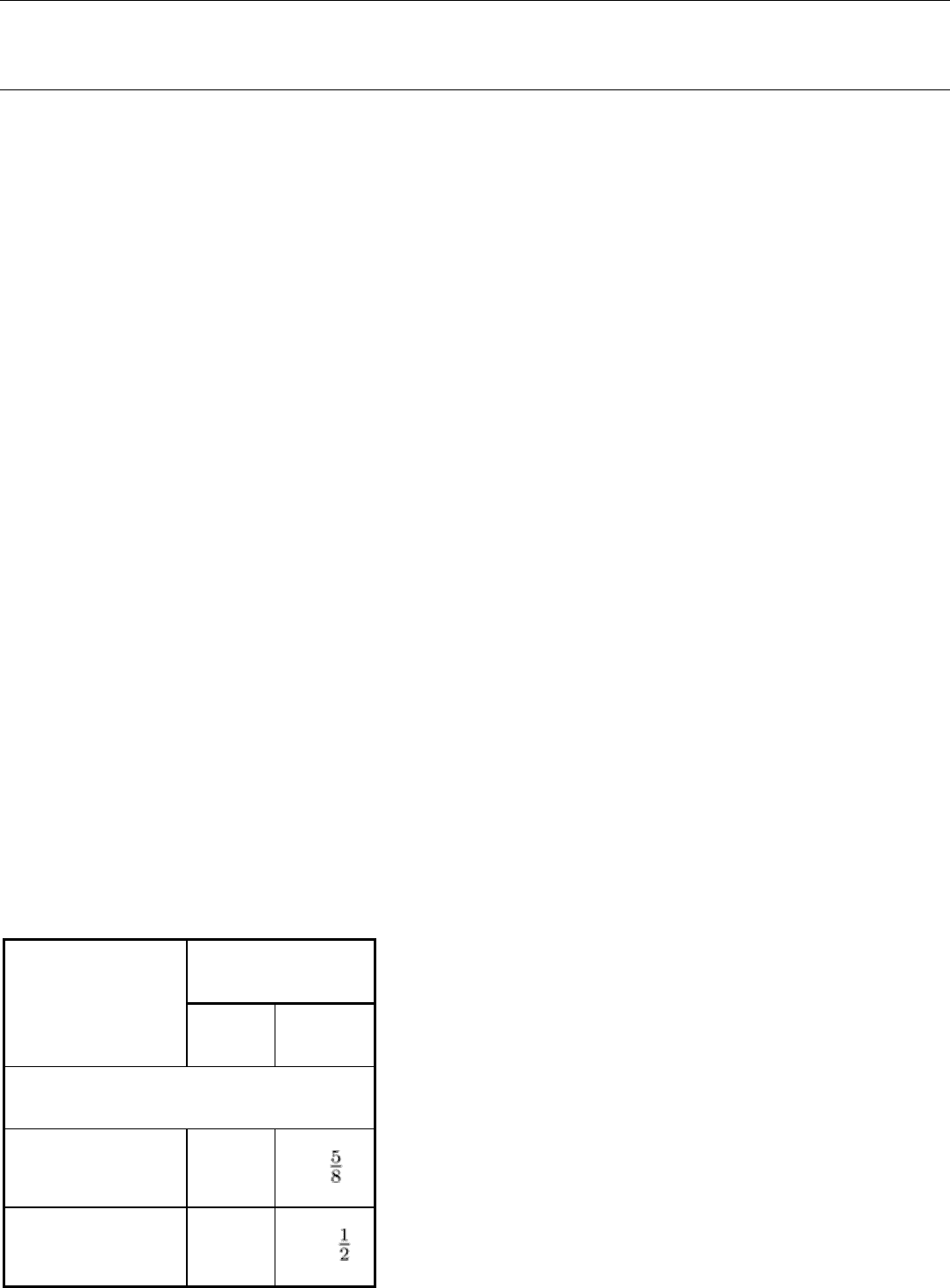
Inherent Filtration. In the radiography (film or real-time) of thin or lightweight materials, which requires low-energy
radiation, filtration by the glass walls of the x-ray tube becomes a problem. Ninety-five percent of a 30-kV x-ray beam is
absorbed by the glass walls of an ordinary x-ray tube. Consequently, in a tube used to radiograph thin or lightweight
materials, a beryllium window is fused into the glass wall in the path of the x-ray beam. Beryllium is one of the lightest of
metals and is more transparent to x-rays than any other metal. The beryllium-window tube has a minimum of inherent
filtration and allows most of the very low energy x-rays to escape from the tube, as shown by the comparison with an
ordinary oil-insulated glass tube in Fig. 19. The results are quite noticeable with both film and real-time radiography,
particularly in contrast improvement. This effect is sometimes noticeable even beyond 150 kV. Very costly 250- or 300-
kV tubes with beryllium windows are sometimes used when thin, light materials as well as thick, dense materials must be
inspected with the same apparatus. In beryllium-window tubes designed for very low voltage work, the window can be as
thin as 0.25 mm (0.010 in.). Consequently, to avoid rupture due to external pressure on the evacuated tube, thin beryllium
windows are also of small diameter; this restricts the size of the x-ray beam and the corresponding field diameter.
Fig. 19 Comparison of the R-output of a beryllium-window tube with that of an ordinary oil-insulated glass x-
ray tube
In general, the higher the tube voltage, the larger the tube must be, and consequently the thicker the glass walls must be to
support the internal elements and to withstand external atmospheric pressure. Furthermore, as the tube voltage increases,
there is more likely to be high-voltage arcing from the tube to various parts of the housing. High-voltage tubes are
generally surrounded by oil, which not only insulates the tube from the housing but also is often the primary means of
extracting heat from the anode. In some tube assemblies, the oil surrounding the tube may be 50 mm (2 in.) thick or more.
Both the oil surrounding the tube and the window in the housing that allows the useful beam of radiation to escape act as
filters, particularly affecting the long-wavelength portion of the emitted radiation. Above about 200 kV, inherent filtration
by components of the tube assembly is less important, primarily because inherent filtration by the testpiece would not
allow the long-wavelength portion of the spectrum to reach the film anyway.
Radiographic Inspection
Revised by the ASM Committee on Radiographic Inspection
*
High-Energy X-Ray Sources
Above about 400 kV, the conventional design of an x-ray tube and its high-voltage iron-core transformer becomes
cumber-some and large. Although x-ray machines with iron-core transformers have been built for 600 kV (maximum),
there are no commercial versions operating above 500 kV. For higher-energy x-rays, other designs are used. Some of the
machine designs for the production of high-energy x-rays include:
• Linear accelerators
• Betatrons
• Van de Graaff generators
• X-ray tubes with a resonant transformer
Linear accelerators, which produce high-velocity electrons by means of radio-frequency energy coupled to a waveguide,
have extended industrial radiography to about 25-MeV photon energy. Betatrons, which accelerate electrons by magnetic
induction, are used to produce 20- to 30-MeV x-rays. Portable linear accelerators and betatrons are also used in field
inspection. The electron energy of portable units is of the order of 1.5 MeV for portable linear accelerators and 2 to 6
MeV for portable betatrons. Van de Graaff generators and x-ray tubes with resonant transformers are less widely used in
industrial radiography. The Van de Graaff generator is an electrostatic device that operates from 500 kV to about 6 MV.
X-ray tubes with resonant transformers were developed in the 1940s, and some units are still in operation. The output of
these units is limited to about 4000 keV (4 MeV) in maximum photon energy that can be produced. Above these energies,
the efficiency of resonant transformers is somewhat impaired. The radiation spectrum is broad, containing a large amount
of radiation produced at much lower energies than the maximum. In addition, the accelerating potential fluctuates, which
makes focusing of the electron beam difficult and creates a focal spot that is much larger than desired for optimum
radiographic definition.
In terms of penetrating capability, expressed as the range of steel thickness that can be satisfactorily inspected, Table 2
compares high-energy sources with conventional x-ray tubes. The maximum values in Table 2 represent the thicknesses
of steel that can be routinely inspected using exposures of several minutes' duration and with medium-speed film. Thicker
sections can be inspected at each value of potential by using faster films and long exposure times, but for routine work the
use of higher-energy x-rays is more practical. Sections thinner than the minimum thicknesses given in Table 2 can be
penetrated, but radiographic contrast is not optimum.
Table 2 Penetration ranges of x-ray tubes and high-energy sources
Penetration range in steel
Maximum accelerating
potential
mm
in.
X-ray tubes
150 kV Up to 15
Up to
250 kV Up to 40
Up to 1
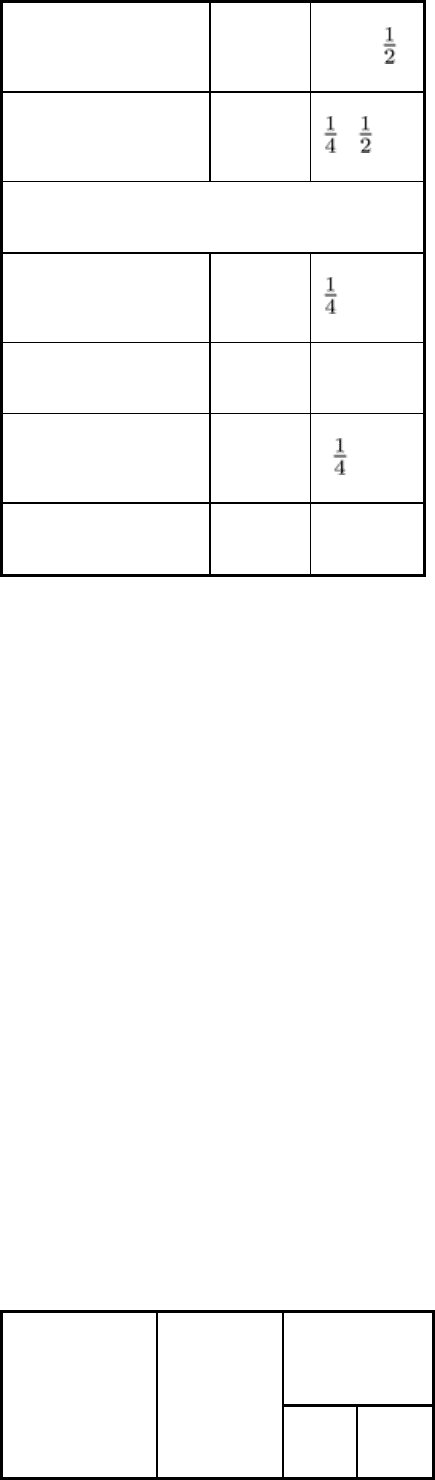
400 kV Up to 65
Up to 2
1000 kV (1 MV) 5-90
-3
High-energy sources
2.0 MeV 5-250
-10
4.5 MeV 25-300
1-12
7.5 MeV 60-460
2 -18
20.0 MeV 75-610 3-24
R-Output of High-Energy Sources. The R-output of a pulsed x-ray generator such as a linear accelerator or betatron
depends on several factors, including pulse length and frequency of pulse repetition, although the output of x-ray
generators of similar type and design varies approximately as V
2.7
. For high-energy sources, electron beam current
generally is not a satisfactory measure of R-output, and comparisons of beam current with the tube current of
conventional x-ray tubes have little meaning. In fact, even the measurement of R-output is complicated and difficult,
especially for energies exceeding 3 MeV. It is often more realistic to estimate the output of high-energy sources from film
exposure data.
In terms of effective output, linear accelerators have the highest beam strength; machines with estimated outputs as high
as 25,000 RMM have been built. Betatrons, although capable of producing higher-energy radiation than linear
accelerators, are limited in beam strength to about 500 RMM. By contrast, 200 RMM is about the maximum output of
conventional rectified, Villard-circuit or constant-potential equipment, of resonant-transformer-based machines, or of
machines powered by an electrostatic Van de Graaff generator. Portable betatrons and linear accelerators have an R-
output in the range of 1 to 3 RMM.
With most high-energy sources (in which the x-ray beam is emitted from the back side of the target in approximately the
same direction of travel as the impinging stream of electrons), the combined effects of self-absorption and electron
scattering within the target itself produce a variation in intensity with angle relative to the axis of the central beam that
parallels the heel effect in conventional x-ray tubes. The magnitude of the effect varies with electron energy and is
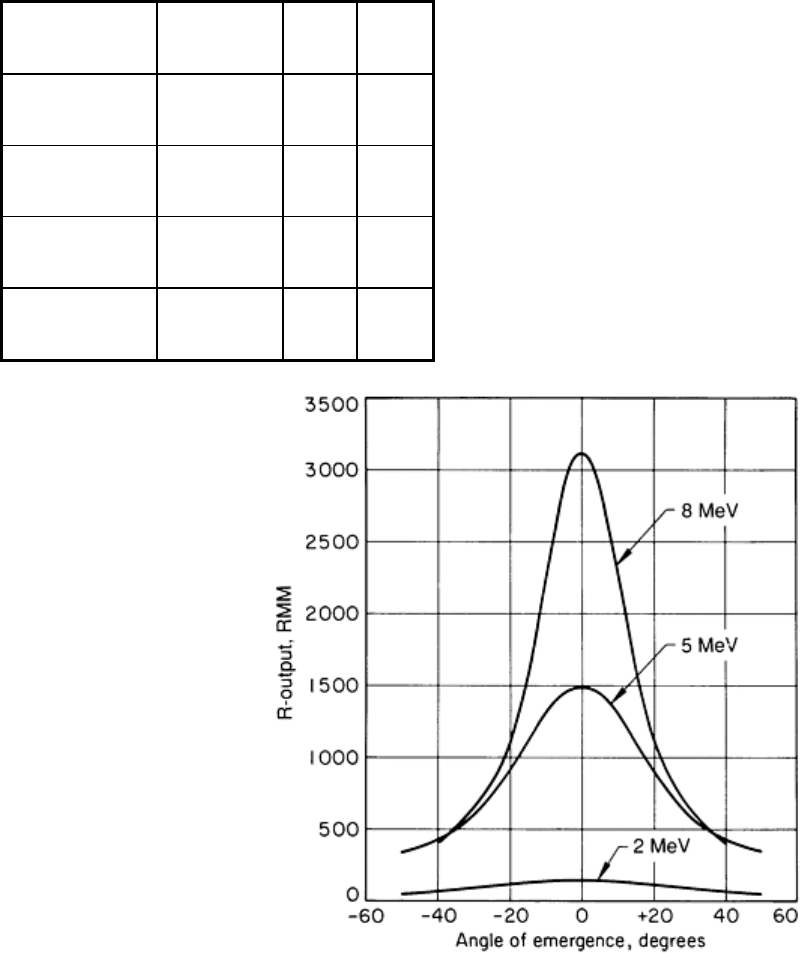
extremely pronounced at energies exceeding 2 MeV. The variation of R-output with angle of emergence, shown in Fig. 20
for three values of electron energy, is of considerable practical importance because this effect can severely limit the area
of coverage, especially for short source-to-detector distances. The calculated angles between the central beam and the
focus of points of half intensity (that is, where the intensity is one-half that of the central beam), as well as the
corresponding field diameters at 1 m (3.3 ft), are given in Table 3 for several values of electron energy, assuming normal
incidence of the electrons on the target.
Table 3 Half intensity and field diameter at various electron energy values
Field diameter at
1 m (3.3 ft)
Electron energy,
MeV
Angle to half
intensity,
degrees
mm
in.
5 11 380
15.0
10 7 244
9.6
18 5 174
6.9
31 3.4 119
4.7
50 1.9 66 2.6
Fig. 20 Variation of R-output with angle of emergence relative to the central beam of a high-
energy source.
Curves were calculated for x-
rays emitted at three levels of electron energy from back side of tungsten target,
assuming electron impingement normal to target surface.
As Table 3 indicates, the useful field produced by a parallel stream of electrons with energy above 10 MeV is
inconveniently small for most applications. Fortunately, many high-energy sources employ some form of device to focus
the electron beam on the target so that a converging beam rather than a parallel beam of electrons strikes the target.
Originally intended to achieve a reduced focal-spot size, beam convergence has the secondary effect of increasing the
angle to half intensity, thus increasing the effective field at any focal distance. For most high-energy sources, the effect of
electron beam convergence is to at least double the field diameter.
In addition to electron beam convergence, almost all high-energy sources are equipped with a field compensator, which
further increases the effective field size. A field compensator is a metallic filter that is thicker in the center than it is at the
edges. By progressively absorbing less radiation with increasing angle of emergence relative to the central beam, a field
compensator can almost double the effective field size. The intensity of the entire beam of radiation is reduced by a field
compensator, but even a reduction in central beam intensity of about 35% does not seriously increase film exposure times
for most applications. In fact, the increase in effective field size usually more than compensates for the loss of intensity.
There is one type of application in which the inherent variation in intensity with angle of emergence is beneficial. A solid
sphere or solid cylinder varies continuously in thickness across a diametral plane. When incident radiation from a
uniform-intensity source penetrates a solid sphere or cylinder parallel to a diametral plane, there is a progressive variation
in transmitted intensity, because of thickness variations, which leads to underexposure of the central area of the
radiograph and overexposure along the edges, often resulting in unsatisfactory definition. With a nonuniform radiation
field, however, inherent variations in penetrated thickness are at least partly counter-balanced by variations in incident
intensity across the radiation field, which results in a much better resolution of testpiece detail.
Radiographic Inspection
Revised by the ASM Committee on Radiographic Inspection
*
Gamma-Ray Sources
Gamma rays are high-energy electromagnetic waves of relatively short wavelength that are emitted during the radioactive
decay of both naturally occurring and artificially produced unstable isotopes. In all respects other than their origin, γ-rays
and x-rays are identical. Unlike the broad-spectrum radiation produced by an x-ray tube, γ-ray sources emit one or more
discrete wavelengths of radiation, each having its own characteristic photon energy.
The two most common radioactive isotopes used in radiography are iridium-192 and cobalt-60. Ytterbium-169 has also
gained a measure of acceptability in the radiography of thin materials and small tubes, especially boiler tubes in power
plants. Cesium-137, although not used in radiography, has been useful in radiometry and computed tomography.
Radiation Characteristics of -Ray Sources. Because gamma radiation is produced by the radioactive decay of
unstable atomic nuclei, there is a continuous reduction in the intensity of emitted radiation with time as more and more
unstable nuclei transform to stable nuclei. This reduction follows a logarithmic law, and each radioactive isotope has a
characteristic half-life, or amount of time that it takes for the intensity of emitted radiation to be reduced by one-half. The
term half-life should not be misinterpreted as meaning that the intensity of emitted radiation will be zero at the end of the
second half-life. Rather, the intensity remaining at the end of a second half-life will be one-half the intensity at the end of
the first half-life, or one-fourth of the initial intensity. The intensity at the end of the third half-life will be one-eighth of
the initial intensity, and so on.
As described in the section "Radiation Sources" in this article, the strength of γ-ray sources is the source activity given in
the units of gigabecquerel or curies. If the source activity is known at any given time, a table of radioactive-decay factors
for the specific radioactive isotope (or the corresponding logarithmic decay formula) can be used to calculate source
activity at any subsequent time. Then, radiation intensity, which is usually measured in roentgens per hour, can be found
by multiplying source activity by the specific -ray constant given in roentgens per hour per gigabecquerel (or curie).
Although radiation output is constant for a given isotope, large-size sources emit slightly less radiation per gigabecquerel
(curie) than small-size sources; that is, large-size sources have somewhat lower effective output. The difference between
calculated radiation output and effective radiation output is the result of self-absorption within the radioactive mass, in
which -rays resulting from decay of atoms in the center of the mass are partly absorbed by the mass itself before they
can escape. Most source manufacturers measure source activity in units of effective gigabecquerel (or curies), which is
the net of self-absorption.
Specific activity, commonly expressed as gigabecquerels (curies) per gram or gigabecquerels (curies) per cubic
centimeter, is a characteristic of radioactive sources that expresses their degree of concentration. A source that has a high
specific activity will be smaller than another source of the same isotope and activity that has a lower specific activity.
Generally, sources of high specific activity are more desirable because they have lower self-absorption and provide less
geometric unsharpness in the radiographs they produce than sources of low specific activity.
The more important characteristics of γ-ray sources are summarized in Table 4. Source activity and specific activity are
not listed, because these characteristics vary according to the physical size of the source, the material and design of its
encapsulation, and the degree of concentration at the time the source was originally produced.
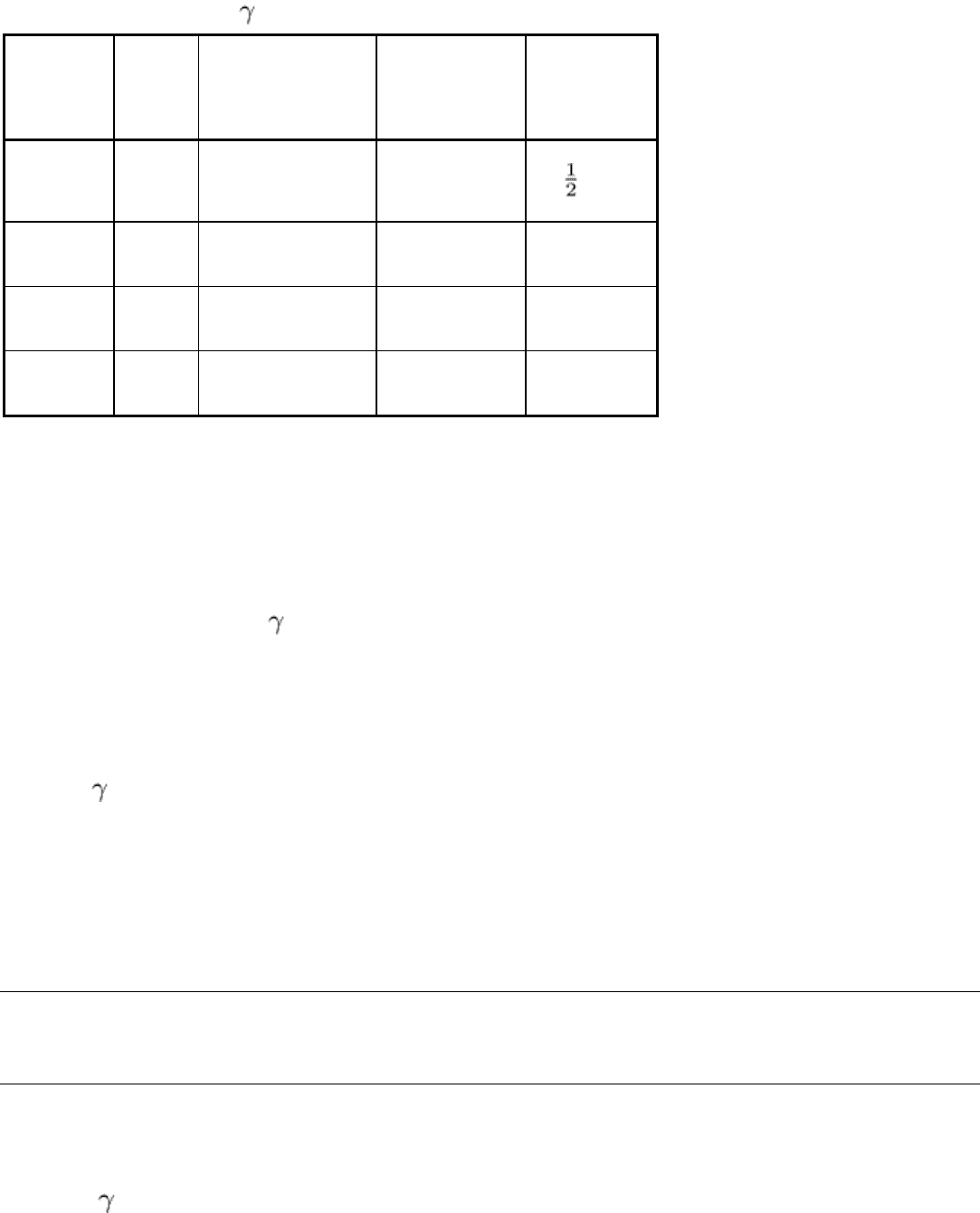
Table 4 Characteristics of -ray sources used in industrial radiography
γ-ray source
Half-life Photon energy, MeV Radiation output,
RHM/Ci
(a)
Penetrating
power, mm (in.)
of steel
Thulium-170
128 days
0.054 and 0.084
(b)
0.003
13 ( )
Iridium-192 74 days 12 rays from 0.21-0.61
0.48
75 (3)
Cesium-137 33 years 0.66 0.32
75 (3)
Cobalt-60 5.3 years
1.17 and 1.33 1.3 230 (9)
(a)
Output for typical unshielded, encapsulated sources; RHM/Ci, roentgens
per hour at 1 m per curie.
(b)
Against strong background of higher-MeV radiation.
Physical Characteristics of -Ray Sources. Radioactive sources, which are usually metallic but may be salts or
gases adsorbed on a block of charcoal, are encapsulated in a protective covering, usually a thin stainless steel sheath or a
thicker sheath of aluminum. Encapsulation prevents abrasion, spillage, or leakage of the radioactive material; reduces the
likelihood of loss or accidental mishandling; and provides a convenient means by which wires, rods, or other handling
devices can be attached to the source.
Gamma-ray sources are housed in protective containers made of lead, depleted uranium, or other dense materials that
absorb the -rays, thus providing protection from exposure to the radiation. Two basic types of containers are generally
used. One type incorporates an inner rotating cylinder that contains the isotope and is rotated toward a conical opening in
the outer cylinder to expose the source and allow the escape of radiation. This type is often referred to as a radioisotope
camera. The second type incorporates a remote-controlled mechanical or pneumatic positioner that moves the
encapsulated radioactive source out of the container and into a predetermined position where it remains until exposure is
completed; the source is then returned to the protective container, again by remote control. This type is more versatile
than the first type and is much more extensively used. Remote control of the positioner allows the operator to remain at a
safe distance from the source while manipulating the capsule out of and into the protective container.
Radiographic Inspection
Revised by the ASM Committee on Radiographic Inspection
*
Attenuation of Electromagnetic Radiation
X-rays and -rays interact with any substance, even gases such as air, as the rays pass through the substance. It is this
interaction that enables parts to be inspected by differential attenuation of radiation and that enables differences in the
intensity of radiation to be detected and recorded. Both these effects are essential to the radiographic process. The
attenuation characteristics of materials vary with the type, intensity, and energy of the radiation and with the density and
atomic structure of the material.

The attenuation of electromagnetic radiation is a complex process. Because of their electromagnetic properties, x-rays and
-rays are affected by the electric fields surrounding atoms and their nuclei. It is chiefly interaction with these electric
fields that causes the intensity of electromagnetic radiation to be reduced as it passes through any material.
The intensity of radiation varies exponentially with the thickness of homogeneous material through which it passes. This
behavior is expressed as:
I = I
0
exp (- t)
(Eq 6)
where I is the intensity of the emergent radiation, I
0
is the initial intensity, t is the thickness of homogeneous material, and
is a characteristic of the material known as the linear absorption coefficient. The coefficient μ is constant for a given
situation, but varies with the material and with the photon energy of the radiation. The units of μ are reciprocal length (for
example, cm
-1
).
The absorption coefficient of a material is sometimes expressed as a mass absorption coefficient (μ/ρ), where ρ is the
density of the material. Alternatively, the absorption coefficient can be expressed as an effective absorbing area of a
single atom; this property, called the atomic absorption coefficient (μ
a
) or cross section, is equal to the linear absorption
coefficient divided by the number of atoms per unit volume. The cross section, usually expressed in barns (1 barn = 10
-24
cm
2
), indicates the probability that a photon of radiation will interact with a given atom.
Atomic Attenuation Processes
Theoretically, there are four possible interactions between a photon (quantum) of electromagnetic radiation and material.
Also, there are three possible results that an interaction can have on the photon. Thus, there are 12 possible combinations
of interaction and result. However, only four of these have a high enough probability of occurrence to be important in the
attenuation of x-rays or γ-rays. These four combinations are photoelectric effect, Rayleigh scattering, Compton scattering,
and pair production.
The photoelectric effect is an interaction with orbital electrons in which a photon of electromagnetic radiation is
consumed in breaking the bond between an orbital electron and its atom. Energy in excess of the bond strength imparts
kinetic energy to the electron.
The photoelectric effect generally decreases with increasing photon energy, E, as E
-3.5
, except that, at energies
corresponding to the binding energies of electrons to the various orbital shells in the atom, there are abrupt increases in
absorption. These abrupt increases are called absorption edges and are given letter designations corresponding to the
electron shells with which they are associated. At photon energies exceeding an absorption edge, the photoelectric effect
again diminishes with increasing energy.
For elements of low atomic number, the photoelectric effect is negligible at photon energies exceeding about 100 keV.
However, the photoelectric effect varies with the fourth to fifth power of atomic number; thus, for elements of high
atomic number, the effect accounts for an appreciable portion of total absorption at photon energies up to about 2 MeV.
Rayleigh scattering, also known as coherent scattering, is a form of direct interaction between an incident photon and
orbital electrons of an atom in which the photon is deflected without any change in either the kinetic energy of the photon
or the internal energy of the atom. In addition, no electrons are released from the atom. The angle between the path of the
deflected photon and that of the incident radiation varies inversely with photon energy, being high for low-energy photons
and low for high-energy photons. There is a characteristic photon energy, which varies with atomic number, above which
Rayleigh scattering is entirely in the forward direction and no attenuation of the incident beam can be detected.
Rayleigh scattering is most important for elements of high atomic number and low photon energies. However, Rayleigh
scattering never accounts for more than about 20% of the total attenuation.
Compton scattering is a form of direct interaction between an incident photon and an orbital electron in which the
electron is ejected from the atom and only a portion of the kinetic energy of the photon is consumed. The photon is
scattered incoherently, emerging in a direction that is different from the direction of incident radiation and emerging with
reduced energy and a correspondingly lower wavelength. The relationship of the intensity of the scattered beam to the
intensity of the incident beam, scattering angle, and photon energy in the incident beam is complex, yet is amenable to
