Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.

small at room temperature, the degree of solid-solution strengthening is limited. The
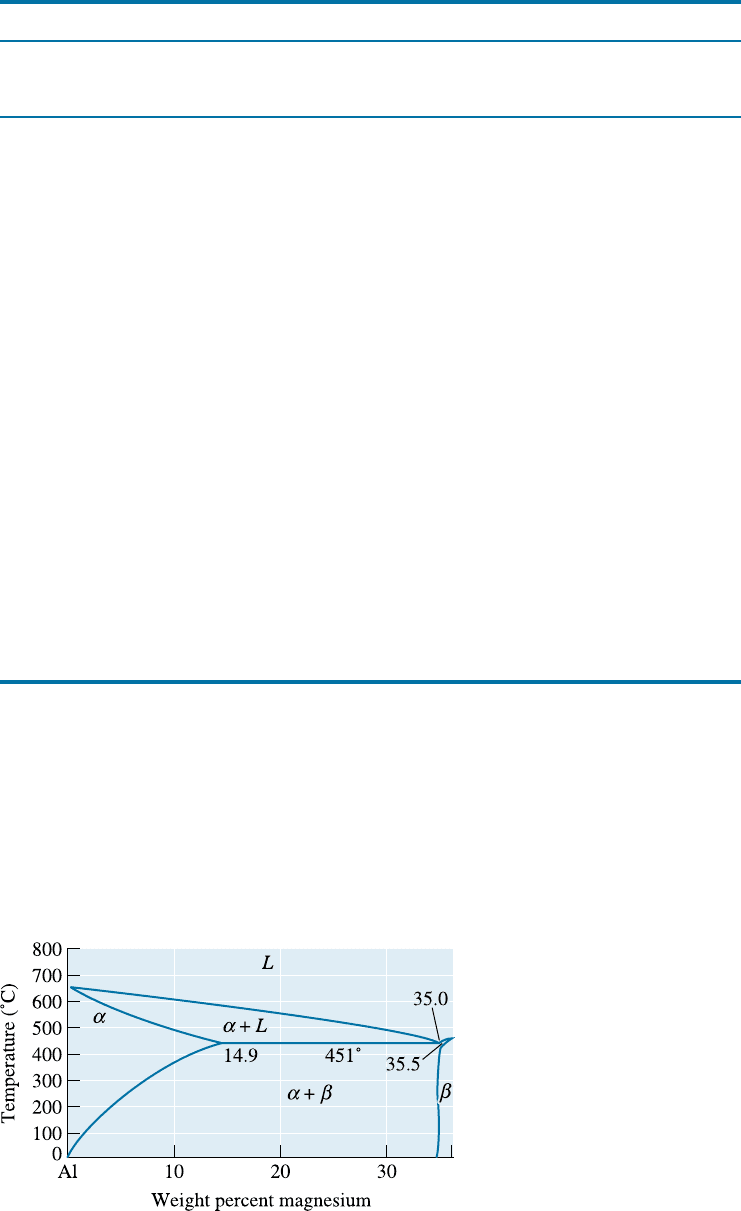
5xxx alloys contain two phases at room temperature—a , a solid solution of magnesium
in aluminum, and Mg
2
Al
3
, a har d, brittle intermetallic compound (Figure 14-2). The
aluminum-magnesium alloys are strengthened by a fine dispersion of Mg
2
Al
3
, as well
as by strain hardening, solid-solution strengthening, and grain-size control. However,
TABLE 14-4 9 Temper designations for aluminum alloys
F As-fabricated (hot-worked, forged, cast, etc.)
O Annealed (in the softest possible condition)
H Cold-worked
H1x—cold-worked only. (x refers to the amount of cold work and strengthening.)
H12—cold work that gives a tensile strength midway between the O and H14 tempers.
H14—cold work that gives a tensile strength midway between the O and H18 tempers.
H16—cold work that gives a tensile strength midway between the H14 and H18 tempers.
H18—cold work that gives about 75% reduction.
H19—cold work that gives a tensile strength greater than 14 MPa of that obtained by the H18
temper.
H2x—cold-worked and partly annealed.
H3x—cold-worked and stabilized at a low temperature to prevent age hardening of the structure.
W Solution-treated
T Age-hardened
T1—cooled from the fabrication temperature and naturally aged.
T2—cooled from the fabrication temperature, cold-worked, and naturally aged.
T3—solution-treated, cold-worked, and naturally aged.
T4—solution-treated and naturally aged.
T5—cooled from the fabrication temperature and artificially aged.
T6—solution-treated and artificially aged.
T7—solution-treated and stabilized by overaging.
T8—solution-treated, cold-worked, and artificially aged.
T9—solution-treated, artificially aged, and cold-worked.
T10—cooled from the fabrication temperature, cold-worked, and artificially aged.
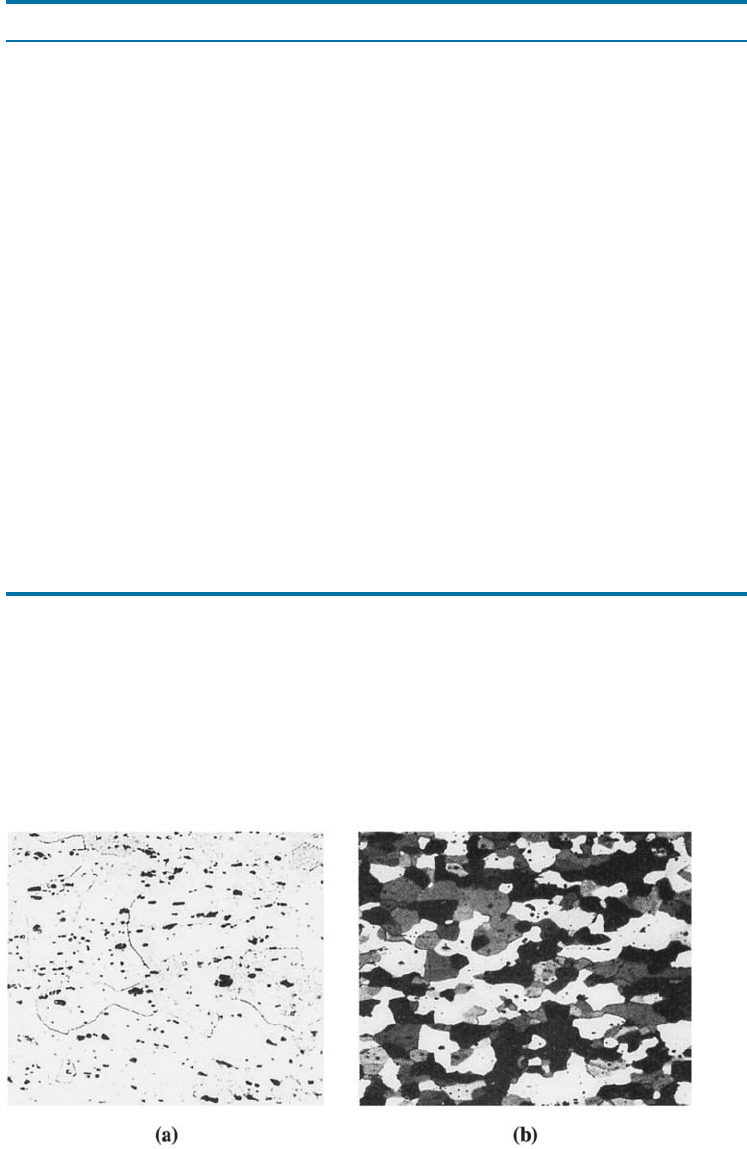
Figure 14-1 (a) FeAl
3
inclusions in annealed 1100 aluminum (350). (b) Mg
2
Si precipitates
in annealed 5457 aluminum alloy (75). (From ASM Handbook, Vol. 7, (1972), ASM
International, Materials Park, OH 44073.)
CHAPTER 14 Nonferrous Alloys440
because Mg
2
Al
3
is not coherent, age-hardening treatments are not possible. The 4xxx
series alloys also contain two phases, a and nearly pure silicon, b (Chapter 11).
Alloys that contain both silicon and magnesium can be age hardened by permitting
Mg
2
Si to precipitate. The 2xxx, 6xxx, and 7xxx alloys are age-hardenable alloys.
Although excellent specific strengths are obtained for these alloys, the amount of pre-
cipitate that can form is limited. In addition, they cannot be used at temp eratures
TABLE 14-5 9 Properties of typical aluminum alloys
Tensile
Strength
Yield
Strength %
Alloy (MPa) (MPa) Elongation Applications
Non heat-treatable wrought alloys:
1100-O
1100-H18
3004-O
3004-H18
4043-0
4043-H18
5182-O
5182-H19
>99% Al
1.2% Mn-1.0% Mg
5.2% Si
4.5% Mg
90
166
179
283
145
283
290
421
35
152
69
248
69
269
131
393
40
10
25
9
22
1
25
4
Electrical components, foil,
food processing,
beverage can bodies,
architectural uses,
filler metal for welding,
beverage can tops, and
marine components
Heat-treatable wrought alloys:
2024-T4
2090-T6
4032-T6
6061-T6
7075-T6
4.4% Cu
2.4% Li-2.7% Cu
12% Si-1% Mg
1% Mg-0.6% Si
5.6% Zn-2.5% Mg
469
552
379
310
572
324
517
317
276
503
20
6
9
15
11
Truck wheels, aircraft skins,
pistons, canoes, railroad
cars, and aircraft frames
Casting alloys:
201-T6
319-F
356-T6
380-F
390-F
443-F
4.5% Cu
6% Si-3.5% Cu
7% Si-0.3% Mg
8.5% Si-3.5% Cu
17% Si-4.5% Cu
5.2% Si (sand cast)
(permanent mold)
(die cast)
483
186
228
317
283
131
159
228
434
124
166
159
241
55
62
110
7
2
3
3
1
8
10
9
Transmission housings,
general purpose castings,
aircraft fittings, motor
housings, automotive
engines, food-handling
equipment, and marine
fittings
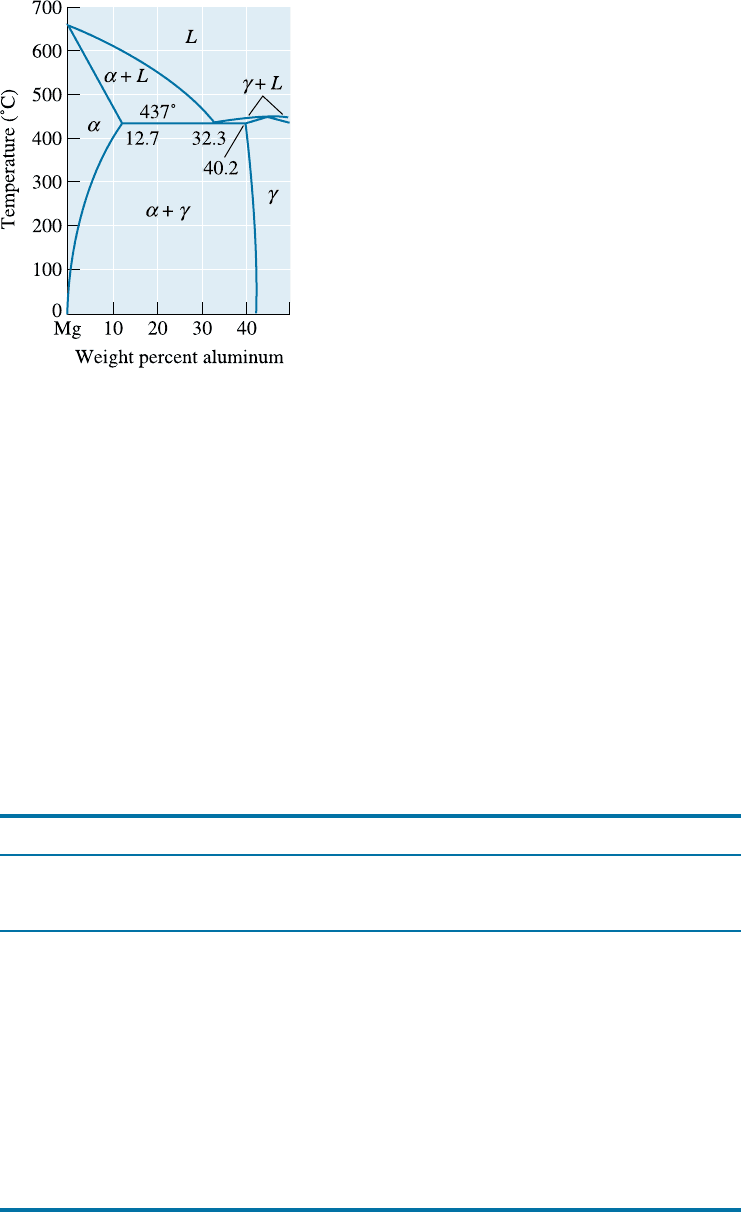
Figure 14-2
Portion of the aluminum-
magnesium phase diagram.
14-1 Aluminum Alloys 441
above approximately 175
C in the aged condition. The most widely used aircraft
aluminum alloy is 2024. There is also an interest in the development of precipitation-
hardened Al-Li alloys due to their high Young’s modulus and low density. However,
high-processing costs, anisotropic properties, and lower fracture toughness have proved
to be limiting factors. Al-Li alloys are used to make space shuttle fuel tanks.
Casting Alloys Many of the common aluminum casting alloys shown in Table 14-5
contain enough silicon to cause the eutectic reaction, giving the alloys low melting
points, good fluidity, and good castability. Fluidity is the ability of the liquid metal to
flow through a mold without prematurely solidifying, and castability refers to the ease
with which a good casting can be made from the alloy.
The properties of the aluminum-silicon alloys are controlled by solid-solution
strengthening of the a aluminum matrix, dispersion strengthening by the b phase, and
solidification, which controls the primary grain size and shape as well as the nature
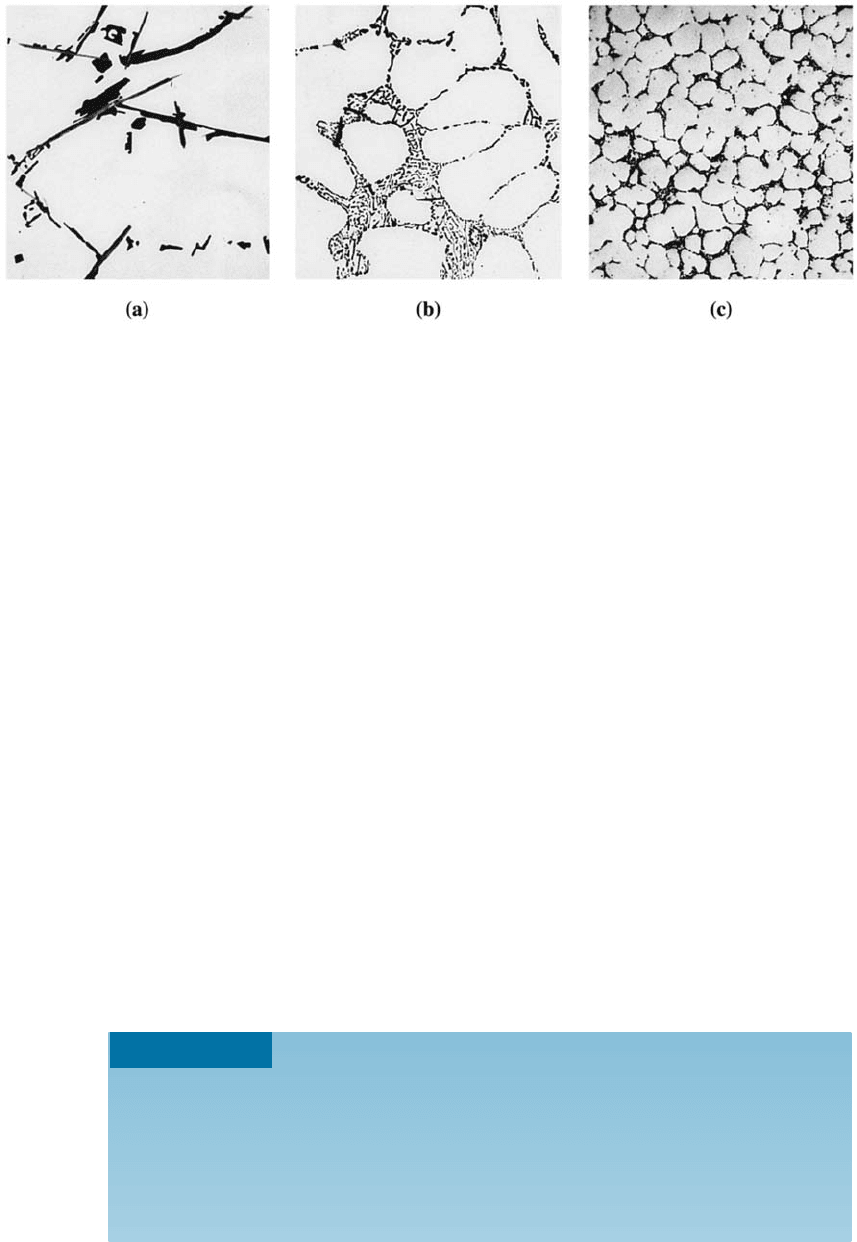
of the eutectic microconstituent. Fast cooling obtained in die casting or permanent-
mold casting (Chapter 9) increases strength by refining grain size and the eutectic
microconstituent (Figure 14-3). Grain refinement using boron and titanium additions,
modification using sodium or strontium to change the eutectic structure, and hardening
with phosphorus to refine the primary silicon (Chapter 9) are all done in certain alloys
to improve the microstructure and, thus, the degree of dispersion strengthening. Many
alloys also contain copper, magnesium, or zinc, thus permitting age hardening. The
following examples illustrate applications of aluminum alloys.
Figure 14-3 (a) Sand-cast 443 aluminum alloy containing coarse silicon and inclusions.
(b) Permanent-mold 443 alloy containing fine dendrite cells and fine silicon due to faster
cooling. (c) Die-cast 443 alloy with a still finer microstructure (350). (From ASM Handbook,
Vol. 7, (1972), ASM International, Materials Park, OH 44073.)
EXAMPLE 14-1
Strength-to-Weight Ratio in Design
A steel cable 1.25 cm in diameter has a yield strength of 483 MPa. The density
of steel is about 7.87 g/cm
3
. Based on the data in Table 14-5, determine (a) the
maximum load that the steel cable can support, (b) the diameter of a cold-
worked aluminum-manganese alloy (3004-H 18) required to support the same
load as the steel, and (c) the weight per meter of the steel cable versus the alu-
minum alloy cable.
CHAPTER 14 Nonferrous Alloys442
SOLUTION
a. Load ¼ F ¼ðs
y
AÞ¼483 MPa
p
4
ð1:25 cmÞ
2
¼ 59;297 N
b. The yield strength of the aluminum alloy is 248 MPa. Thus:
A ¼
p
4
d
2
¼
F
s
y
¼
59;297 N
248 MPa
¼ 2:39 cm
2
9 d ¼ 1:744 cm
c. Density of steel ¼ r ¼ 7:87 g=cm
3
Density of aluminum ¼ r ¼ 2:70 g=cm
3
Weight of steel ¼ Alr ¼
p
4
ð1:25 cmÞ
2
ð100Þð7:87Þ¼966:183 g=m
Weight of aluminum ¼ Alr ¼
p
4
ð1:744Þ
2
ð100Þð2:7Þ¼645:24 g=m
Although the yield strength of the aluminum alloy is lower than that of the
steel and the cable must be larger in diameter, the aluminum alloy cable weighs
only about half as much as the steel cable. When comparing materials, a
proper factor-of-safety should also be included during design.
EXAMPLE 14-2
Design of an Aluminum Recycling Process
Design a method for recycling aluminum alloys used for beverage cans.
SOLUTION
Recycling aluminum is advantageous because only a fraction (about 5%) of the
energy required to produce aluminum from Al
2
O
3
is required. However, re-
cycling beverage cans does present several di‰culties.
The beverage cans are made of two aluminum alloys (3004 for the main
body, and 5182 for the lids) having di¤erent compositions (Table 14-5). The
3004 alloy has the exceptional formability needed to perform the deep-drawing
process; the 5182 alloy is harder and permits the pull-tops to function properly.
When the cans are remelted, the resulting alloy contains both Mg and Mn and
is not suitable for either application.
One approach to recycling the cans is to separate the two alloys from the
cans. The cans are shredded, then heated to remove the lacquer that helps
protect the cans during use. We could then further shred the material at a
temperature where the 5182 alloy begins to melt. The 5182 alloy has a wider
freezing range than the 3004 alloy and breaks into very small pieces; the more
ductile 3004 alloy remains in larger pieces. The small pieces of 5182 can there-
fore be separated by passing the material through a screen. The two separated
alloys can then be melted, cast, and rolled into new can stock.
An alternative method would be to simply remelt the cans. Once the cans
have been remelted, we could bubble chlorine gas through the liquid alloy. The
chlorine reacts selectively with the magnesium, removing it as a chloride. The
remaining liquid can then be adjusted to the proper composition and be
recycled as 3004 alloy.
14-1 Aluminum Alloys 443

EXAMPLE 14-3 Design/Materials Selection for a Cryogenic Tank
Design the material to be used to contain liquid hydrogen fuel for the space
shuttle.
SOLUTION
Liquid hydrogen is stored below 253
C; therefore, this tank must have good
cryogenic properties. The tank is subjected to high stresses, particularly when
the space shuttle enters into orbit, and it should have good fracture toughness
to minimize the chances of catastrophic failure. Finally, it should be light in
weight to permit higher payloads or less fuel consumption.
A lightweight aluminum alloy would appear to be a good choice. Alumi-
num does not show a ductile to brittle transition. Because of its good ductility,
we expect aluminum to also have good fracture toughness, particularly when
the alloy is in the annealed condition.
One of the most common cryogenic aluminum alloys is 5083-O.
Aluminum-lithium alloys are also being considered for low-temperature appli-
cations to take advantage of their even lower density.
14-2 Magnesium and Beryllium Alloys
Magnesium, which is often extracted electrolytically from concentrated magnesium
chloride in seawater, is lighter than aluminum with a density of 1.74 g/cm
3
, and it melts
at a slightly lower temperature than aluminum. In many environments, the corrosion
resistance of magnesium approaches that of aluminum; however, exposure to salts, such
as that near a marine environment, can cause rapid deterioration. Alloys of magnesium
that are resistant to corrosion have been developed. Although magnesium alloys are not
as strong as aluminum alloys, their specific strengths are comparable. Consequently,
magnesium alloys are used in aerospace applications, high-speed machinery, and
transportation and materials handling equipment.
Magnesium, however, has a low modulus of elasticity (44:8 10
3
MPa) and poor
resistance to fatigue, creep, and wear. Magnesium also poses a hazard during casting
and machining, since it combines easily with oxygen and burns. Finally, the response of
magnesium to strengthening mechanisms is relatively poor.
Structure and Properties Magnesium, which has the HCP structure, is less ductile
than aluminum. However, the alloys do have some ductility because alloying increases
the number of active slip planes. Some deformation and strain hardening can be
accomplished at room temperature, and the alloys can be readily deformed at elevated
temperatures. Strain hardening produces a relatively small e¤ect in pure magnesium
because of the low strain-hardening coe‰cient (Chapters 6 and 8).
As in aluminum alloys, the solubility of alloying elements in magnesium at room
temperature is limited, causing only a small degree of solid-solution strengthening.
However the solubility of many alloying elements increases with temperature, as shown
in the Mg-Al phase diagram (Figure 14-4). Therefore, alloys may be strengthened
CHAPTER 14 Nonferrous Alloys444
by either dispersion strengthening or age hardening. Some age-hardened magnesium
alloys, such as those containing Zr, Th, Ag, or Ce, have good resistance to overaging at
temperatures as high as 300
C. Alloys containing up to 9% Li have exceptionally light
weight. Properties of typical magnesium alloys are listed in Table 14-6.
Advanced magnesium alloys include those with very low levels of impurities and
those containing large amounts (>5%) of cerium and other rare earths. These alloys
form a protective MgO film that improves corrosion resistance. Rapid solidification
processing permits larger amounts of alloying elements to be dissolved in the magne-
sium, further improving corrosion resistance. Improvements in strength, particularly
at high temperatures, can be obtained by introducing ceramic particles or fibers such as
silicon carbide into the metal.
Beryllium is lighter than aluminum, with a density of 1.848 g/cm
3
, yet it is sti¤er
than steel, with a modulus of elasticity of 290 10
3
MPa. Beryllium alloys are rather
Figure 14-4
The magnesium-aluminum phase diagram.
TABLE 14-6 9 Properties of typical magnesium alloys
Tensile Yield
Strength Strength %
Alloy Composition (MPa) (MPa) Elongation
Pure Mg:
Annealed 159 90 3–15
Cold-worked 179 117 2–10
Casting alloys:
AM 100-T6 10% Al-0.1% Mn 276 152 1
AZ81A-T4 7.6% Al-0.7% Zn 276 83 15
ZK61A-T6 6% Zn-0.7% Zr 310 193 10
Wrought alloys:
AZ80A-T5 8.5% Al-0.5% Zn 379 276 7
ZK40A-T5 4% Zn-0.45% Zr 276 255 4
HK31A-H24 3% Th-0.6% Zr 262 207 8
14-2 Magnesium and Beryllium Alloys 445
limited. Composites based on Be and Al have yield strengths of 207 to 345 MPa, have
high specific strengths and maintain both strength and sti¤ness to high temperatures.
Instrument grade beryllium is used in inertial guidanc e systems where the elastic de-
formation must be minimal; structural grades are used in aerospace applications; and
nuclear applications take advantage of the transparency of beryllium to electromagnetic
radiation. Unfortunately, beryllium is expensive (Table 14-1), brittle, reactive, and
toxic. Its production is quite complicated and hence the applications of Be alloys are
very limited. Beryllium oxide (BeO), which is also toxic in a powder form, is used to
make high-thermal conductivity ceramics.
EXAMPLE 14-4
Magnesium Alloys for Orthopedic Implant Applications
Currently, titanium alloys, stainless steels, and cobalt chromium alloys are
used as biomedical implants. It has been suggested that magnesium alloys have
mechanical properties similar to those of bones and will make suitable implant
materials. One of the most commonly used magnesium alloy is AZ91, which
has 9 wt.% Al, and 1 wt.% Zn. This material can be prepared by the so-called
squeeze-casting process, which allows for faster cooling and, hence, a finer
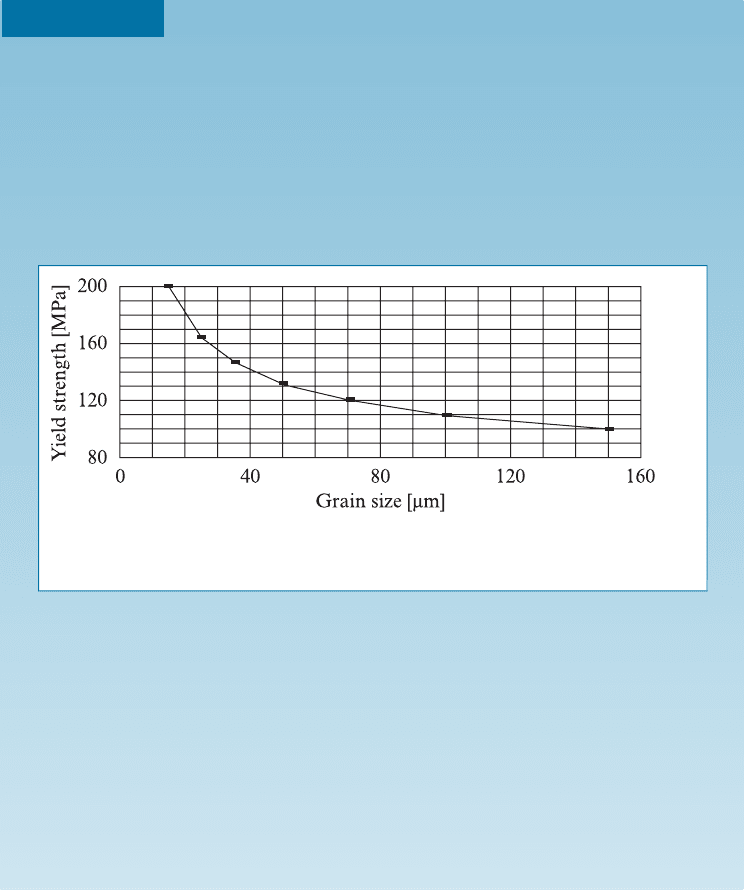
grain size (Chapter 9). The e¤ect of grain size on the strength of A Z91 on the
yield strength of AZ91 alloy is shown in Figure 14-5.
Assume that these data will fit the Hall-Petch equation (Chapter 4) and
calculate the value of constants s
0
and K for this alloy. What will be the yield
strength of the 10 mm alloy?
SOLUTION
The Hall-Petch equation (Chapter 4) is
s ¼ s
0
þ Kd
1=2
We use a spreadsheet program (in this case Excel
TM
) to fit these data to a
straight line. To do this, we plot the yield stress as a function of inverse of the
square root of the grain size. These data are shown in the following table.
Figure 14-5 The dependence of yield strength of Mg alloy AZ91 with grain size.
(Source: Data from Kainer, K.U. and Benzler, T.U., Magnesium Alloys and
Technology, Ed. K.U. Kainer, 2003, p. 60.)
CHAPTER 14 Nonferrous Alloys446

Grain Size
(d) Micrometers
Inverse of Grain
Size Square Root
(Micrometers)
1/2
Yield Stress (MPa)
15 0.25819889 200
25 0.2 165
35 0.169030851 150
50 0.141421356 130
70 0.119522861 120
100 0.1 110
150 0.081649658 100
When the yield stress ðsÞ and d
1=2
values are fitted to a straight line
equation, we get the slope (K) as 567.53 MPa(mm)
1=2
and the intercept ðs
0
Þ¼
52:54 MPa. Thus, in this case, the Hall-Petch equation is
s ¼ 52:54 MPa þ 567:53ð MPa mm
1=2
Þd
1=2
From this, for a grain size of 10 mm, the predicted yi eld strength for the Mg
alloy will be
s ¼ 52:54 MPa þ 567:53ðMPa mm
1=2
Þð10Þ
1=2
Therefore, the predicted yield strength of an Mg-alloy casting with a 10 mm
grain size will be 232 MPa.
14-3 Copper Alloys
Copper occurs in nature as sulfides and also as elemental copper. Copper was extracted
successfully from rock long before iron, since the relatively lower temperatures required
for copper extraction could be achie ved more easily. Copper is typically produced by a
pyrometallurgical (high-temperature) process. The copper ore containing high-sulfur
contents is concentrated, then converted into a molten immiscible liquid containing
copper sulfide-iron sulfide and is known as a copper matte. This is done in a flash
smelter. In a separate reactor, known as a copper converter, oxygen introduced to the
matte converts the iron sulfide to iron oxide and the copper sulfide to an impure copper
called blister copper, which is then purified electrolytically. Other methods for copper
extraction include leaching copper from low-sulfur ores with an acid, then electrolyti-
cally extracting the copper from the solution.
Copper-based alloys have higher densities than that for steels. Although the yield
strength of some alloys is high, their specific strength is typically less than that of alu-
minum or magnesium alloys. These alloys have better resistance to fatigue, creep, and
wear than the lightweight aluminum and magnesium alloys. Many of these alloys have
excellent ductility, corrosion resistance, electrical and thermal conductivity, and most
can easily be joined or fabricated into useful shapes. Applications for copper-based
alloys include electrical components (such as wire), pumps, valves, and plumbing parts,
where these properties are used to advantage.
Copper alloys are also unusual in that they may be selected to produce an appro-
priate decorative color. Pure copper is red; zinc additions produce a yellow color, and
nickel produces a silver color. Copper can corrode easily; forming a basic copper sulfate
14-3 Copper Alloys 447
(CuSO
4
3Cu(OH)
2
). This is a green compound that is insoluble in water (but soluble
in acids). This green patina provides an attractive finish for many applications. The
Statue of Liberty is green because of the green patina of the oxidized coppe r skin that
covers the steel structure.
The wide variety of copper-based alloys take advantage of all of the strengthening
mechanisms that we have discussed. The e¤ects of these strengthening mechanisms on
the mechanical properties are summarized in Table 14-7.
Copper containing less than 0.1% impurities is used for electrical and micro-
electronics applications. Small amounts of cadmium, silver, and Al
2
O
3
improve their
hardness without significantly impairing conductivity. The single-phase copper alloys
are strengthened by cold working. Examples of this e¤ect are shown in Table 14-7. The
FCC structure of copper provides for excellent ductility and a high strain-hardening
coe‰cient.
Solid-Solution-Strengthened Alloys A number of copper-based alloys contain large
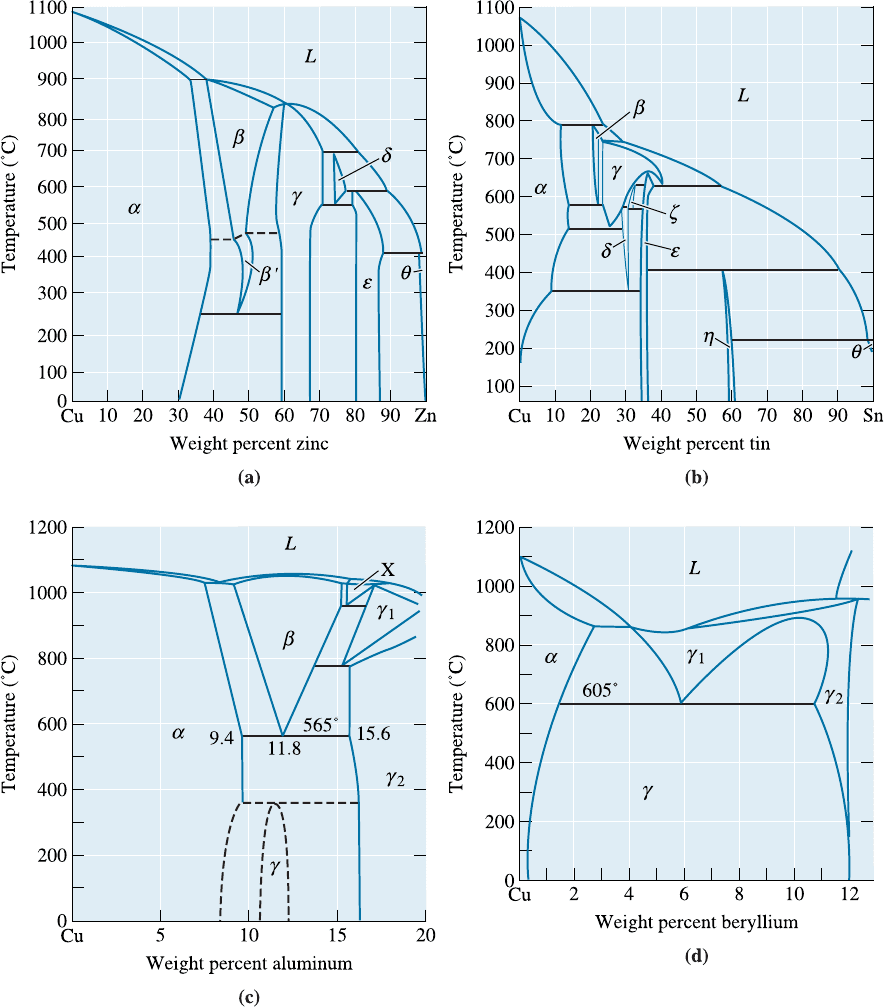
quantities of alloying elements, yet remain single phase. Important binary phase dia-
grams are shown in Figure 14-6. The copper-zinc, or brass, alloys with less than 40% Zn
form single-phase solid solutions of zinc in copper. The mechanical properties—even
elongation—increase as the zinc content increases (Figure 10-8). These alloys can be
cold formed into rather complicated yet corrosion-re sistant components. Bronzes are
alloys of copper containing tin and can certainly contain other elements. Manganese
bronze is a particularly high-strength alloy containing manganese as well as zinc for
solid-solution strengthening.
Tin bronzes, often called phosphor bronzes, may contain up to 10% Sn and remain
single phase. The phase diagram predicts that the alloy will contain the Cu
3
Sn (e)
compound. However, the kinetics of the reaction are so slow that the precipitate par-
ticles often do not form.
Alloys containing less than about 9% Al or less than 3% Si are also single phase.
These aluminum bronzes and silicon bronzes have good forming characteristics and are
often selected for their good strength and excellent toughness.
TABLE 14-7 9 Properties of typical copper alloys obtained by different strengthening mechanisms
Tensile Yield
Strength Strength % Strengthening
Material (MPa) (MPa) Elongation Mechanism
Pure Cu, annealed 209 33 60 None
Commercially pure Cu,
annealed to coarse grain size
221 69 55 Solid solution
Commercially pure Cu,
annealed to fine grain size
234 76 55 Grain size
Commercially pure Cu,
cold-worked 70%
393 365 4 Strain hardening
Annealed Cu-35% Zn 324 103 62 Solid solution
Annealed Cu-10% Sn 455 193 68 Solid solution
Cold-worked Cu-35% Zn 676 434 3 Solid solution þ
strain hardening
Age-hardened Cu-2% Be 1310 1207 4 Age hardening
Quenched and tempered Cu-Al 759 414 5 Martensitic reaction
Cast manganese bronze 490 193 30 Eutectoid reaction
CHAPTER 14 Nonferrous Alloys448
Age-Hardenable Alloys A number of copper-base alloys display an age-hardening
response, including zirconium-coppe r, chromium-copper, and beryllium-copper. The
copper-beryllium alloys are used for their high strength, their high sti¤ness (making
them useful as springs and fine wires), and their nonsparking qualities (making them
useful for tools to be used near flammable gases and liquids).
Figure 14-6 Binary phase diagrams for the (a) copper-zinc, (b) copper-tin, (c) copper-
aluminum, and (d) copper-beryllium systems.
14-3 Copper Alloys 449
