Yung Y.L., DeMore W.B. Photochemistry of Planetary Atmospheres
Подождите немного. Документ загружается.

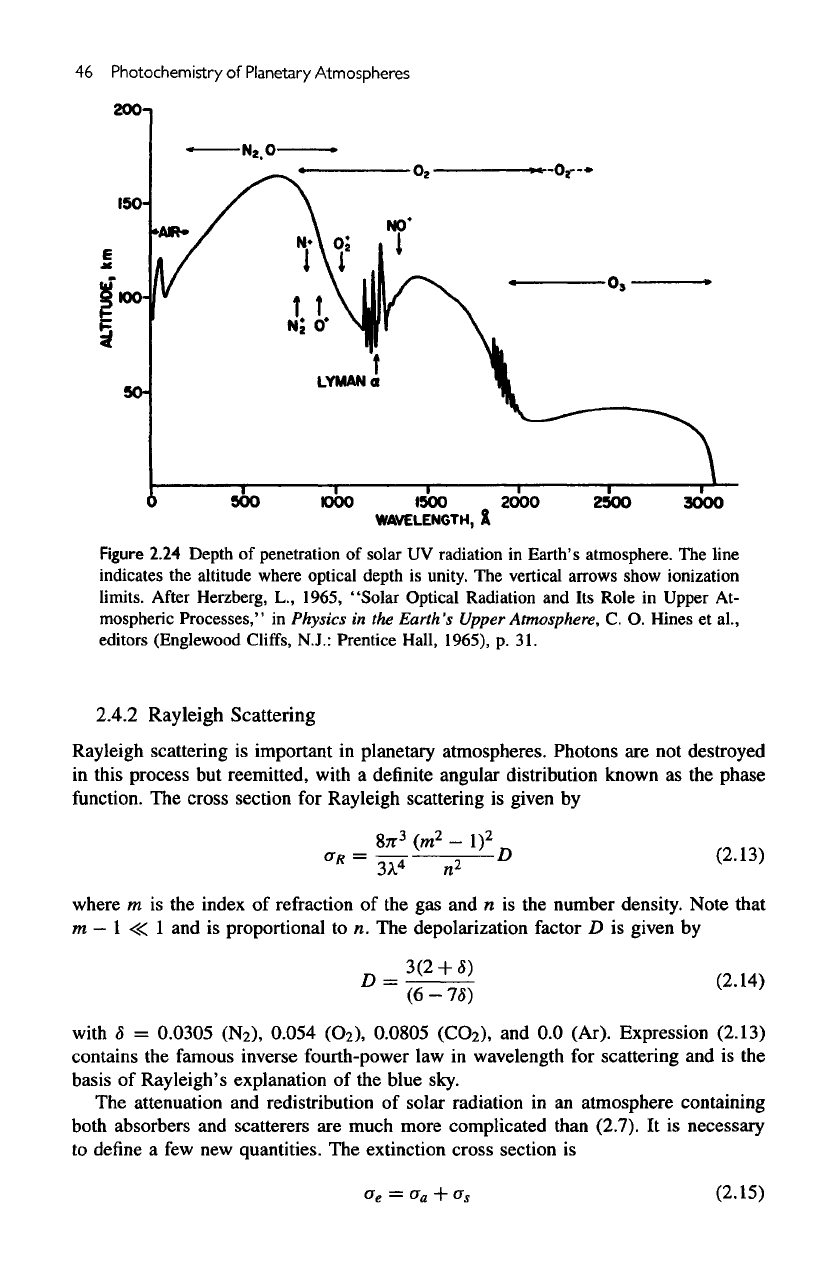
46
Photochemistry
of
Planetary Atmospheres
Figure
2.24
Depth
of
penetration
of
solar
UV
radiation
in
Earth's atmosphere.
The
line
indicates
the
altitude where optical depth
is
unity.
The
vertical arrows show ionization
limits.
After
Herzberg,
L.,
1965,
"Solar
Optical Radiation
and Its
Role
in
Upper
At-
mospheric
Processes,"
in
Physics
in the
Earth's
Upper
Atmosphere,
C. O.
Mines
et
al.,
editors (Englewood
Cliffs,
N.J.:
Prentice
Hall,
1965),
p. 31.
2.4.2 Rayleigh
Scattering
Rayleigh
scattering
is
important
in
planetary atmospheres. Photons
are not
destroyed
in
this process
but
reemitted,
with
a
definite angular distribution known
as the
phase
function.
The
cross
section
for
Rayleigh scattering
is
given
by
where
m is the
index
of
refraction
of the gas and n is the
number density. Note that
m — 1
<SC
1 and is
proportional
to n. The
depolarization factor
D is
given
by
with
S =
0.0305
(N
2
),
0.054
(O
2
),
0.0805
(CO
2
),
and 0.0
(Ar).
Expression (2.13)
contains
the
famous
inverse fourth-power
law in
wavelength
for
scattering
and is the
basis
of
Rayleigh's
explanation
of the
blue
sky.
The
attenuation
and
redistribution
of
solar radiation
in an
atmosphere containing
both
absorbers
and
scatterers
are
much more complicated
than
(2.7).
It is
necessary
to
define
a few new
quantities.
The
extinction cross section
is
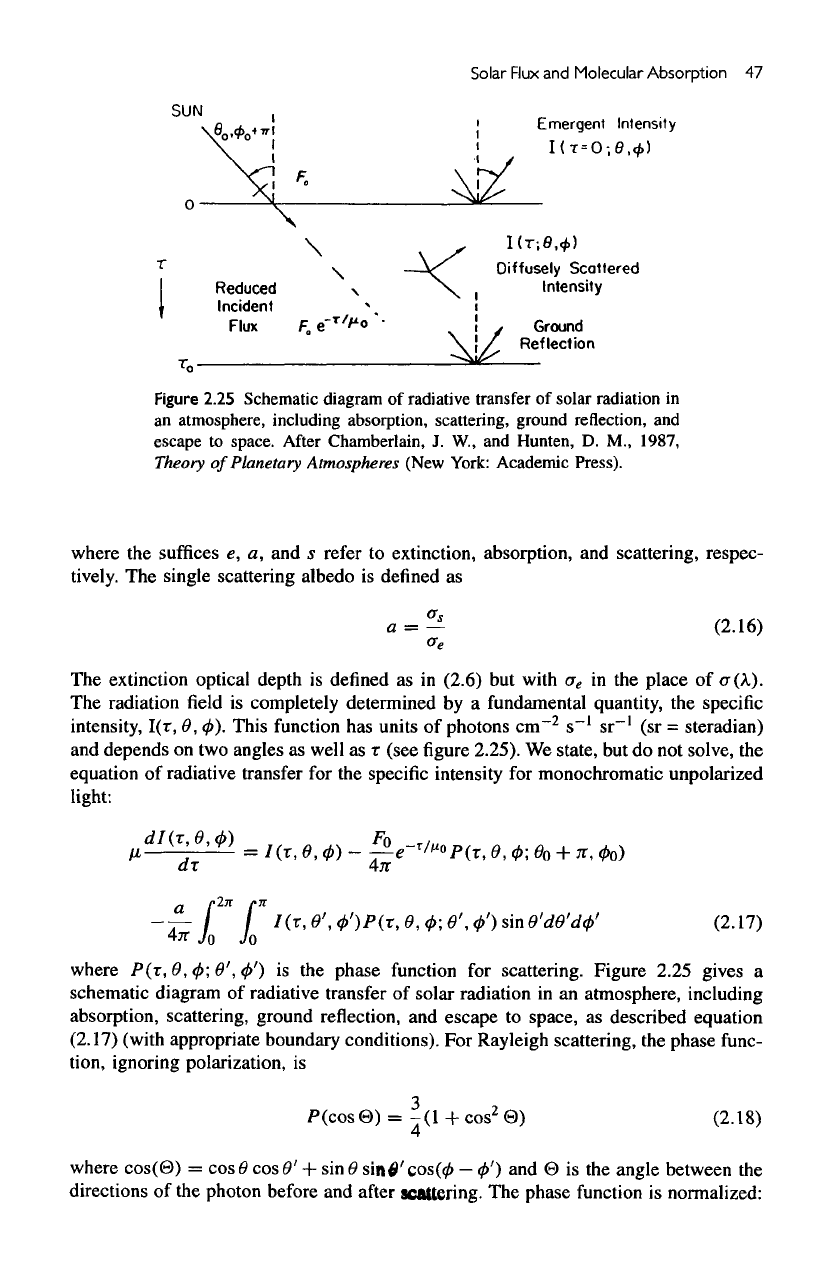
Solar
Flux
and
Molecular Absorption
47
Figure
2.25 Schematic diagram
of
radiative transfer
of
solar radiation
in
an
atmosphere, including
absorption,
scattering, ground reflection,
and
escape
to
space.
After Chamberlain,
J. W., and
Hunten,
D. M.,
1987,
Theory
of
Planetary Atmospheres (New York:
Academic
Press).
where
the
suffices
e, a, and s
refer
to
extinction, absorption,
and
scattering, respec-
tively.
The
single scattering albedo
is
defined
as
The
extinction optical depth
is
defined
as in
(2.6)
but
with
a
e
in the
place
of
The
radiation
field is
completely determined
by a
fundamental quantity,
the
specific
intensity,
I(r,
6,
4>).
This
function
has
units
of
photons
cm~
2
s"
1
sr~'
(sr =
steradian)
and
depends
on two
angles
as
well
as
r
(see
figure
2.25).
We
state,
but do not
solve,
the
equation
of
radiative transfer
for the
specific intensity
for
monochromatic
unpolarized
light:
where P(T,
0,
<t>\
0',
<j>')
is the
phase
function
for
scattering. Figure 2.25 gives
a
schematic
diagram
of
radiative transfer
of
solar radiation
in an
atmosphere, including
absorption,
scattering, ground
reflection,
and
escape
to
space,
as
described equation
(2.17)
(with
appropriate boundary conditions).
For
Rayleigh scattering,
the
phase
func-
tion,
ignoring polarization,
is
where
cos(0)
=
cos0
cos0'
+ sin 0
sin0'cos(</>
-
<£')
and © is the
angle between
the
directions
of the
photon before
and
after
scattering.
The
phase
function
is
normalized:
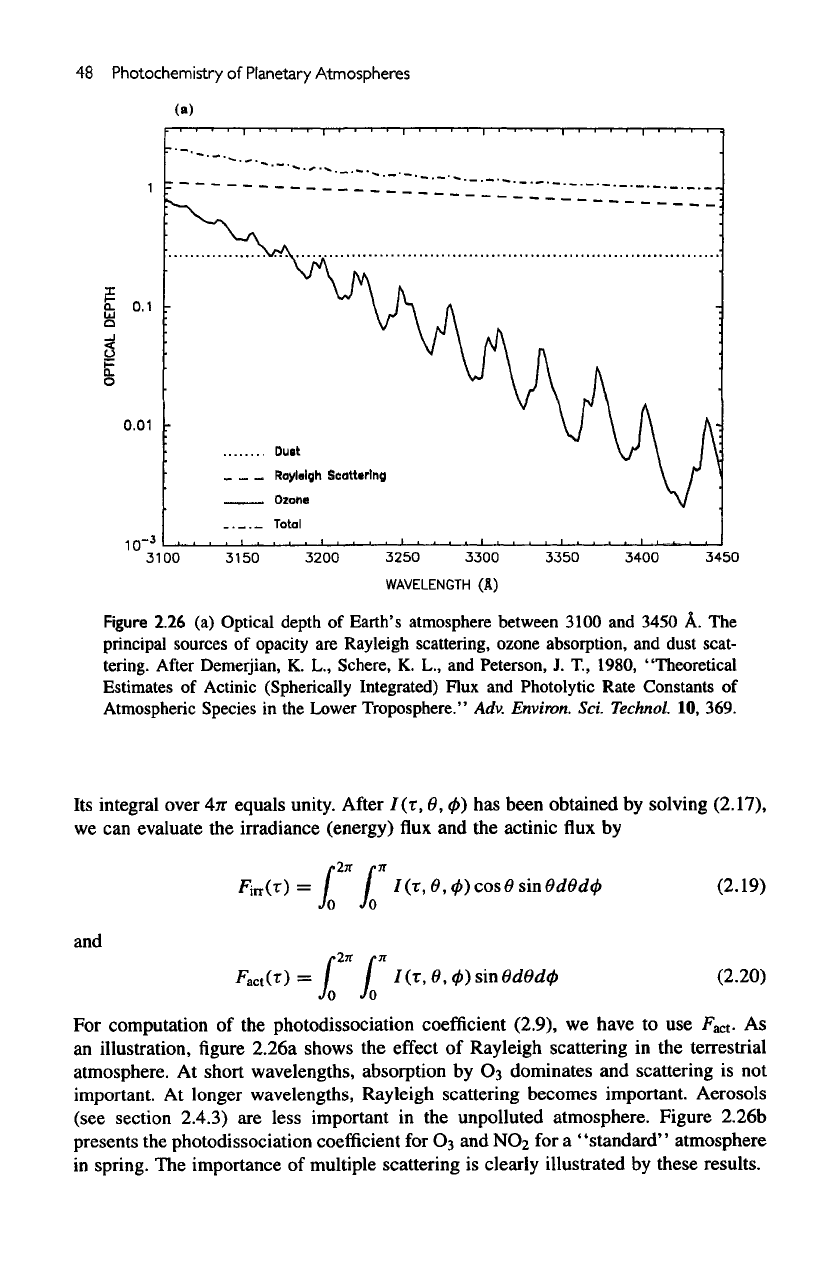
48
Photochemistry
of
Planetary Atmospheres
(a)
Figure
2.26
(a)
Optical depth
of
Earth's atmosphere between 3100
and
3450
A. The
principal
sources
of
opacity
are
Rayleigh scattering, ozone absorption,
and
dust scat-
tering.
After
Demerjian,
K.
L.,
Schere,
K. L., and
Peterson,
J. T.,
1980,
"Theoretical
Estimates
of
Actinic (Spherically Integrated) Flux
and
Photolytic Rate Constants
of
Atmospheric
Species
in the
Lower Troposphere." Adv.
Environ.
Sci.
Technol.
10,
369.
Its
integral
over
4n
equals
unity. After
/(T,
0,
</>)
has
been
obtained
by
solving
(2.17),
we
can
evaluate
the
irradiance
(energy)
flux and the
actinic
flux by
and
For
computation
of the
photodissociation coefficient
(2.9),
we
have
to use
F,^.
As
an
illustration,
figure
2.26a
shows
the
effect
of
Rayleigh scattering
in the
terrestrial
atmosphere.
At
short wavelengths, absorption
by
Os
dominates
and
scattering
is not
important.
At
longer wavelengths, Rayleigh scattering becomes important. Aerosols
(see section
2.4.3)
are
less important
in the
unpolluted atmosphere. Figure
2.26b
presents
the
photodissociation
coefficient
for 03 and
NC>2
for a
"standard"
atmosphere
in
spring.
The
importance
of
multiple scattering
is
clearly illustrated
by
these results.
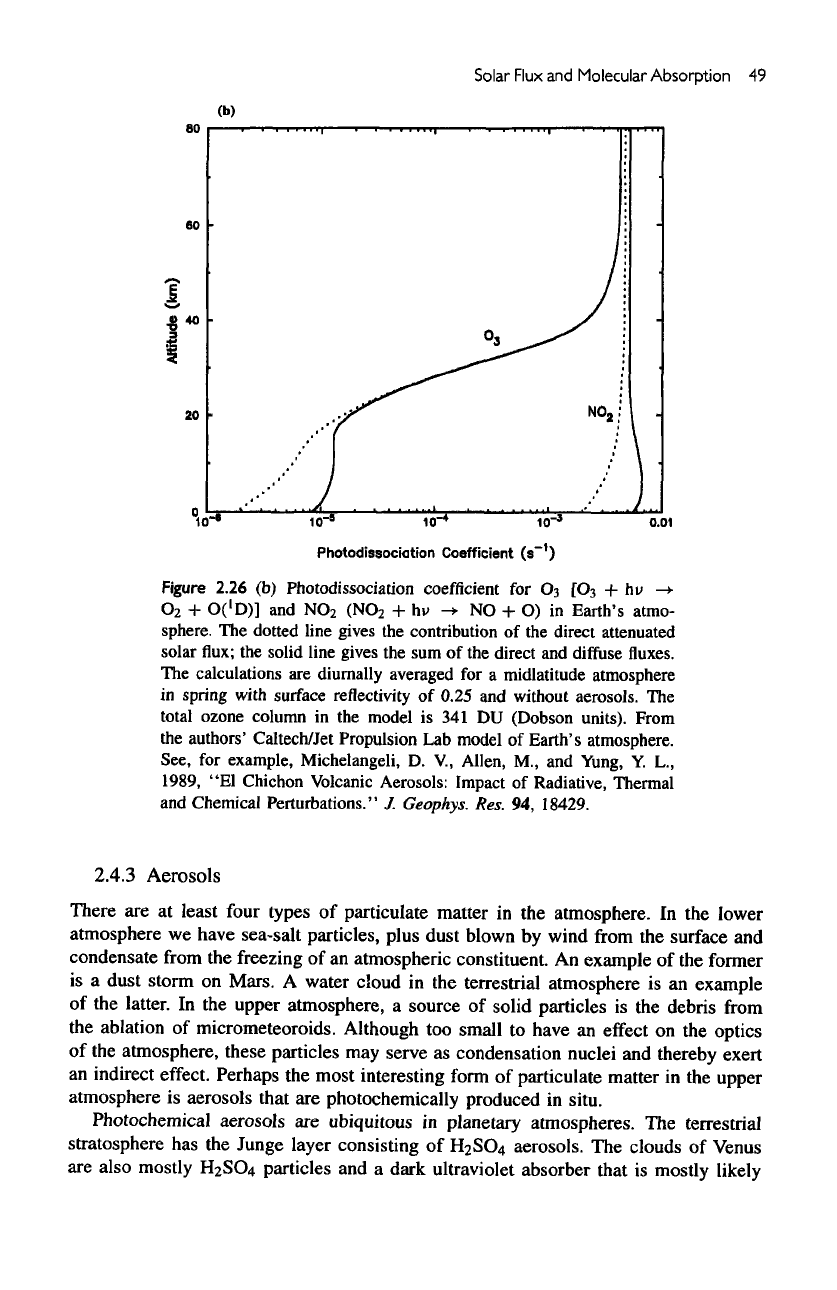
Solar
Flux
and
Molecular
Absorption
49
(b)
Figure
2.26
(b)
Photodissociation
coefficient
for
Oi
[O$
+
hu
->•
O
2
+
O('D)]
and
NO
2
(NO
2
+
hi>
-+
NO + O) in
Earth's atmo-
sphere.
The
dotted line gives
the
contribution
of the
direct attenuated
solar
flux; the
solid line gives
the sum of the
direct
and
diffuse
fluxes.
The
calculations
are
diurnally
averaged
for a
midlatitude
atmosphere
in
spring with surface reflectivity
of
0.25
and
without
aerosols.
The
total
ozone column
in the
model
is 341 DU
(Dobson units). From
the
authors'
Caltech/Jet
Propulsion
Lab
model
of
Earth's atmosphere.
See,
for
example, Michelangeli,
D. V,
Allen,
M., and
Yung,
Y. L.,
1989,
"El
Chichon
Volcanic Aerosols: Impact
of
Radiative, Thermal
and
Chemical
Perturbations."
J.
Geophys. Res.
94,
18429.
2.4.3
Aerosols
There
are at
least
four
types
of
particulate
matter
in the
atmosphere.
In the
lower
atmosphere
we
have sea-salt particles, plus dust blown
by
wind
from the
surface
and
condensate
from the freezing of an
atmospheric constituent.
An
example
of the
former
is
a
dust storm
on
Mars.
A
water
cloud
in the
terrestrial atmosphere
is an
example
of
the
latter.
In the
upper atmosphere,
a
source
of
solid particles
is the
debris
from
the
ablation
of
micrometeoroids. Although
too
small
to
have
an
effect
on the
optics
of
the
atmosphere, these particles
may
serve
as
condensation nuclei
and
thereby exert
an
indirect
effect.
Perhaps
the
most interesting
form
of
particulate matter
in the
upper
atmosphere
is
aerosols
that
are
photochemically produced
in
situ.
Photochemical
aerosols
are
ubiquitous
in
planetary
atmospheres.
The
terrestrial
stratosphere
has the
Junge layer consisting
of
H2SO
4
aerosols.
The
clouds
of
Venus
are
also
mostly
H
2
SC>4
particles
and a
dark ultraviolet absorber that
is
mostly
likely

50
Photochemistry
of
Planetary
Atmospheres
polysulfur.
The
origin
of
these aerosols
is
photochemical, with
the
"parent
molecules"
such
as COS and
SCh
supplied from
the
surface.
In the
outer solar system
the
presence
of
a
dark hydrocarbon
aerosol
seems
to be
well established
in the
stratospheres
of the
giant
planets
and
Titan.
The
ultimate source
of
this material, known
as
Axel-Danielson
dust,
is
CH4.
The
presence
of
aerosols
in the
upper atmosphere profoundly affects
the
radiation
field. The
theory
of the
interaction between
an
electromagnetic
field and an
aerosol
has
been well developed only
for
spherical particles,
and our
discussion
is
restricted
to
this theory, known
as Mie
theory.
For a
sphere
of
radius
r
made
of
material
with
index
of
refraction
m (a
complex number),
the
cross
sections
are
given
by
where
£?<>,
Q
a
,
and
Q
s
denote
efficiency
of
extinction,
absorption,
and
scattering,
respectively.
Mie
theory provides exact expressions
for the
efficiency
factors. Note
that
Q
e
=
Q
a
+
Q
s
,
and in the
case
of a
nonabsorbing material
(m - a
real number),
Q
a
= 0.
Figure
2.27
shows
the
values
of
Q
e
for m =
1.33
(water)
+
\k,
where
k
=
the
imaginary part
of the
index
of
refraction
and is
plotted against
the
size parameter
x —
27rr/A..
It is
clear that
for
small particles
(x
-C
1), the
extinction
efficiency
is
small.
In
fact
it
will
approach
the
limit
of
Rayleigh scattering
and we
will
recover
the
formula
in
(2.13).
For
large particles
x
»
1, the
extinction
efficiency
approaches
an
asymptotic value
of 2.
This
is
surprising because
we
expect
the
limit
to be 1
from
geometric optics.
The
reason
is
that
Mie
theory includes
the
extremely narrow forward
diffraction
peak, which
is not
accounted
for in
geometric optics.
The
high-frequency
oscillations
are
caused
by
wave interference
in the
sphere
and
will
be filtered
when
we
increase
the
imaginary part
of the
index
of
refraction (k).
The
wave interference
features
are
also suppressed when
we
carry
out an
averaging over spheres
of
various
sizes.
The
single scattering albedo
can be
defined using
(2.16),
with
the
appropriate cross
sections given
by
(2.21)-(2.23).
The
phase
function
can
also
be
obtained
from
Mie
theory
in
closed form.
For
small particles,
the Mie
phase
function
is the
same
as the
Rayleigh
phase
function
(2.18).
For
large dielectric particles,
the Mie
phase
function
is
strongly peaked
in the
forward direction
(the
diffraction
peak).
The
asymmetry
parameter
(g)
provides
a
measure
of
this behavior:
For
isotropic
and
Rayleigh scattering,
g
~
0; g is
positive
or
negative
as the
particle
scatters more
or
less energy
into
the
forward
or
backward direction.
The
equation
of
radiative
transfer
(2.17)
may now be
solved,
and the
relevant quantities,
the
irradiance
and
actinic
fluxes,
may be
derived
from
the
solution.
It
can be
shown that
for a
given amount
of
material,
the
greatest opacity
is
obtained
if
the
material
is
used
to
form aerosols
with
scattering parameter
x
equal
to
about
1.
That
is, the
particle size
is
roughly
the
same
as the
wavelength
of the
incident
light.

Figure
2.27 Extinction
efficiency
for a
dielectric
sphere
as a
function
of the
size parameter (x-axis).
The
real part
of the
index
of
refraction
is
1.33,
and
the
imaginary part
of the
index
of
refraction
(k) is
given
in the figure.
Each
curve
(except
the
bottom one)
has
been shifted
up by 1
unit relative
to the
one
beneath
it.
After
Bohren,
C.
F.,
and
Huffman,
D. R.,
1983, Absorption
and
Scattering
of
Light
by
Small Particles (New York: Wiley).
51

52
Photochemistry
of
Planetary Atmospheres
The
reason
is as
follows:
When
the
particle sizes
are
small,
in the
Rayleigh
limit,
the
extinction
efficiency
is too
small.
For
large particles,
the
extinction
cross
section
varies
as r
2
, but the
volume varies
as r
3
.
Therefore, large aerosols
are not
efficient
in
generating
atmospheric opacities.
The
aerosols
in the
upper atmospheres
of
planets have scattering parameters
of the
order
of
unity,
and
hence
are
extremely
effective
in
using
a
limited amount
of
material
for
altering
the
radiation
field. The
total fractional abundance
of
material
is
often
in
the
range
of
10~
7
to
10~
9
,
and yet in the
form
of
these
aerosols
they have
a
profound
optical
effect.
The
H2SO4 particles
in the
atmospheres
of
Venus
and
Earth
are
white
(conservative)
scatterers,
and
their
first-order
effect
is to
redistribute radiation.
The
Axel-Danielson
dust
in the
atmospheres
of the
outer solar system
is
dark
and is a
good absorber
of
solar radiation.
2.4.4
Radiative
Transfer
in
Planetary
Atmospheres
The
general problem
of
radiative transfer
in
planetary atmospheres involves
the
com-
bined
interaction
of
absorption, Rayleigh scattering,
and
absorption
and
scattering
by
aerosols.
The
combined problem
is a
straightforward extension
of
what
has
been
discussed above.
The
total optical depth
is the sum of the
optical depths
for
each pro-
cess.
The
overall albedo
and
phase
function
are the
weighted average
of the
individual
processes.
The
interaction between solar radiation
and the
atmosphere gives rise
to a
number
of
interesting feedbacks, which
can
profoundly alter
the
composition
and the
structure
of
the
atmosphere.
An
example
is the
absorption
of
ultraviolet radiation,
A.
<
2400
A,
by
O2.
This results
in the
dissociation
of
C>2
and
production
of
Os,
which
now
absorbs
all
ultraviolet radiation
to
3000
A.
Heating
of the
atmosphere
by 03
maintains
the
thermal inversion
in the
stratosphere, which inhibits exchange
of air
masses
between
the
troposphere
and the
stratosphere. Thus,
the
absorption
of
solar ultraviolet radiation
by
O2
initiates
a
sequence
of
events that ultimately controls
the
thermal structure
and
dynamics
of the
middle atmosphere. Another example
is the
absorption
of far
UV
radiation
(A.
<
1450
A) by CH4 in the
atmospheres
of the
outer solar system, leading
to the
production
of
C2H2-
The
latter
can
absorb solar radiation
to
A.
<
2000
A, and its
subsequent
photochemistry results
in the
production
of the
Axel-Danielson dust.
These
dark
aerosols
then
become
the
major absorber
of
solar energy
in the
upper atmosphere,
creating
a
thermal inversion,
a
stratosphere. Note that
the
dynamical stability
of the
stratosphere (caused
by the
thermal inversion)
is in
turn
important
for
trapping
the
Axel-Danielson
dust
in the
upper atmosphere.
In
most modeling studies
of
planetary atmospheres,
the
interactions outlined above
between
radiation, chemistry,
and
dynamics
are
conveniently decoupled. Chemical
studies
are
carried
out by
using
an
observed temperature profile
and
empirical trans-
port
coefficients.
Thermal modeling
is
based
on
observed
chemical composition.
A
new
kind
of
modeling that includes
the
coupled effects
of
radiation, chemistry,
and
dynamics
is
needed.
An
analogy
is the ab
initio
calculation
in
quantum chemistry.
We
may
ask why
this kind
of
modeling
is
interesting
at
all.
After
all,
the
atmospheres
have
been observed,
and
even
the
most sophisticated models have
to
satisfy
the ob-
servational constraints.
The
answer
is
that
the
coupled model allows
us to
study
the
stability
of
planetary atmospheres.
Are the
present
states
stable? What
is the
range
Solar Flux
and
Molecular
Absorption
53
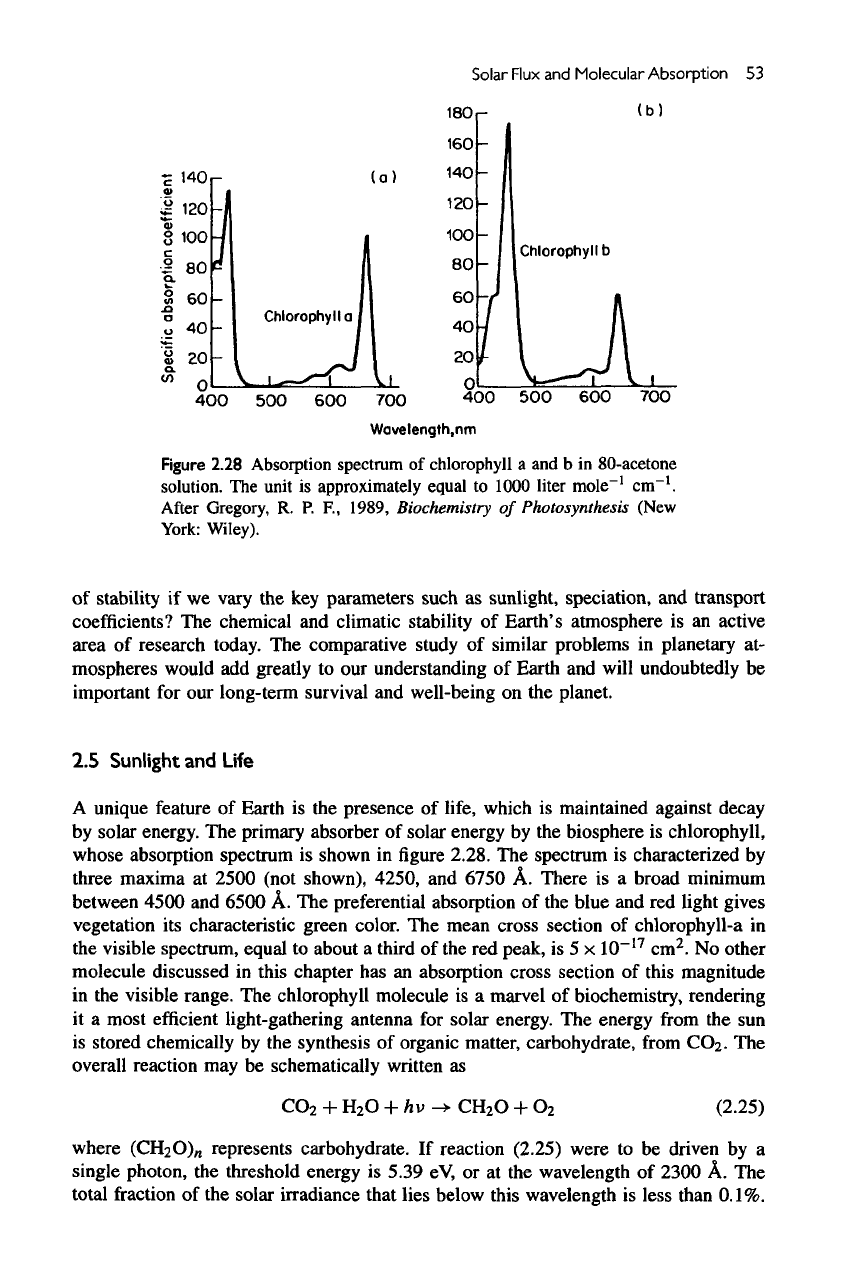
Figure
2.28 Absorption spectrum
of
chlorophyll
a and b in
80-acetone
„-!
solution.
The
unit
is
approximately equal
to
1000 liter mole
cm
After
Gregory,
R. P.
R,
1989, Biochemistry
of
Photosynthesis (New
York:
Wiley).
of
stability
if we
vary
the key
parameters
such
as
sunlight,
speciation,
and
transport
coefficients?
The
chemical
and
climatic stability
of
Earth's
atmosphere
is an
active
area
of
research today.
The
comparative study
of
similar problems
in
planetary
at-
mospheres would
add
greatly
to our
understanding
of
Earth
and
will
undoubtedly
be
important
for our
long-term survival
and
well-being
on the
planet.
2.5
Sunlight
and
Life
A
unique feature
of
Earth
is the
presence
of
life,
which
is
maintained against decay
by
solar energy.
The
primary absorber
of
solar energy
by the
biosphere
is
chlorophyll,
whose absorption spectrum
is
shown
in
figure
2.28.
The
spectrum
is
characterized
by
three maxima
at
2500
(not shown),
4250,
and
6750
A.
There
is a
broad minimum
between
4500
and
6500
A. The
preferential absorption
of the
blue
and red
light gives
vegetation
its
characteristic green color.
The
mean
cross
section
of
chlorophyll-a
in
the
visible spectrum, equal
to
about
a
third
of the red
peak,
is 5 x
10~
17
cm
2
.
No
other
molecule discussed
in
this chapter
has an
absorption cross section
of
this magnitude
in
the
visible
range.
The
chlorophyll
molecule
is a
marvel
of
biochemistry,
rendering
it
a
most
efficient
light-gathering antenna
for
solar energy.
The
energy
from
the sun
is
stored chemically
by the
synthesis
of
organic matter, carbohydrate,
from
CC>2.
The
overall reaction
may be
schematically written
as
where
(CH2O)
n
represents carbohydrate.
If
reaction (2.25) were
to be
driven
by a
single photon,
the
threshold energy
is
5.39
eV, or at the
wavelength
of
2300
A. The
total
fraction
of the
solar
irradiance
that lies below this wavelength
is
less
than
0.1%.

54
Photochemistry
of
Planetary
Atmospheres
However,
the
plant
is
able
to
carry
out
this
synthesis using eight photons
of
much
lower energy
so as to
take advantage
of a
greater fraction
of the
sun's
energy that
is
in
the
visible part
of the
spectrum.
How
this
is
achieved
need
not
concern
us
here.
The key
step
is the
temporary storage
of
solar energy
in
complex molecules
known
as
adenosine triphosphate (ATP)
and
nicotinamide
adenine dinucleotide (NADP). These
molecules
can
later deliver
the
necessary energy
to the
reaction center where
the
final
synthesis
takes
place,
with
the
absorption
of
more photons.
The
beauty
of the
scheme
is
threefold. First,
all the
energy required
to
drive
the
reaction does
not
need
to
be
absorbed
at
once
at the
reaction center. Second,
the
energy
efficiency
of the
multiquantum
process
is as
high
as
33%. Third, since eight photons must
be
used
for
the
synthesis
of
carbohydrate,
the
product
is
extremely stable against photolytic
destruction
(a
single-photon
process)
by the
same photons.
No
manmade chemical
scheme
has
been able
to
duplicate this
truly
elegant solar energy collector
of
Nature,
the
ultimate source
of
almost
all
life
on
this planet.
The
only exceptions
are
probably
the
bacteria that
use the
Earth's internal heat (see table 2.2)
and
chemical energy
in
underwater thermal vents.
In
section 2.4.4
we
point
out the
curious consequence
of
photochemistry
in
plane-
tary
atmospheres—the
synthesis
of
chemicals that absorb
at
increasingly longer wave-
lengths (where there
is
more solar energy). Chlorophyll
seems
an
extreme extension
of
this concept.
It is a
biochemical triumph
to be
able
to tap
into
the
visible part
of
the
sun's radiation
and
turn
its
enormous energy
flux
into chemical energy.
What
is
life
but an
excited state
of
matter that
can
extend itself
in
space
(by
growth)
and
continue itself
in
time
(by
reproduction)?
Since
life
is an
excited state
it is out
of
equilibrium
with
its
natural surroundings. Coming
into
an
equilibrium would mean
decay (the
opposite
of
growth)
and
death (the opposite
of
reproduction).
How do we
measure
life
quantitatively? Here
are
some possibilities:
1.
number
of
carbon
bonds
made
2.
amount
of
solar
energy
converted
into
chemical
energy
3.
amount
of
negative
entropy
needed
4.
number
of
cells reproduced
(equal
to
number
of
deaths
in
steady state)
5.
number
of
genetic
mutations
Sunlight
is the
ultimate quantitative limit
of
life.
But
electromagnetic energy
is
hard
to
store, transport,
and
use.
The
synthesis
of
carbohydrates converts
an
elusive
form
of
energy into
a
more suitable form
for
living organisms.
We may
take this
as a
good planetary
definition
of the
biosphere. Here
is the
beauty
of
it—as
an
extension
of
photochemistry.
If
we
regard
the
biosphere
as a
planetary disequilibrium phenomenon driven
by the
sun,
it
would
be of
interest
to
compare
it
with
other similarly driven planetary phenom-
ena:
the
ionosphere,
the
ozone layer,
and the
Hadley circulation
of the
troposphere.
In
a
generalized sense,
the
biosphere
may be
regarded
as a
global photochemical
extension.

Chemical
Kinetics
3.1
Introduction
It
is
convenient
to
distinguish
two
types
of
chemistry
in the
solar system.
The first
is
thermochemical
chemistry driven
by the
thermal energy
of the
atmosphere. This
type
of
chemistry
is
important,
for
example,
in the
interior
of the
giant planets where
pressures
and
temperatures exceed
1000
bar and
1000
K,
respectively.
The
second
type
is
disequilibrium chemistry, driven
by an
external energy source,
of
which solar
radiation
is the
most important
in the
solar
system.
Chemical
reactions between stable molecules
are
very slow
at
pressures less than
1
bar in
planetary atmospheres. Sunlight
is the
ultimate source
of
greater chemical
activity
in the
middle
and
upper atmospheres
of
planets.
As
shown
in
chapter
2, the
absorption
of
solar ultraviolet radiation
by
atmospheric
gases
leads
to the
production
of
radical species
(e.g.,
atoms, ions, excited molecules) that
are
extremely reactive.
The
bulk
of
atmospheric chemistry involves
the
reaction between
the
radicals themselves
and
between
the
radicals
and
stable molecules.
In
this
chapter
we
briefly
survey
the
chemical kinetics that
are
important
for un-
derstanding
the
chemistry
of
planetary atmospheres.
All
atmospheric reactions
may
be
classified
into
three types:
1.
Uni
molecular
reactions
55
3
