Yung Y.L., DeMore W.B. Photochemistry of Planetary Atmospheres
Подождите немного. Документ загружается.


26
Photochemistry
of
Planetary
Atmospheres
On
planets
with
intrinsic
magnetic
fields, the
solar wind
is
repelled
at
distances
many
planetary radii away
from
the
planet.
For
example,
the
solar
wind
at
Earth
is
stopped
~10
Earth radii away,
and at
Jupiter this occurs
at ~60
Jovian radii.
The
exact
distance varies
with
solar activity.
The
solar wind
in
fact
defines
the
outer limits
of
planetary magnetospheres. Thus,
the
total solar wind energy
flux
that
is
intercepted
by
a
magnetic planet
far
exceeds that
of a
nonmagnetic planet.
The
solar wind
is a
major
energy source
for
Earth's
magnetosphere.
The
precipitation
of
energetic particles
from
the
magnetosphere into
the
upper atmospheres
of
planets (usually
in the
polar regions)
gives rise
to
spectacular displays known
as
aurorae.
The
associated energy
fluxes are
highly
variable. Representative values
are
given
in
table
2.2.
The
solar system
is
bathed
in
starlight—we
can
observe
stars
at
night.
In the
inner
solar
system
the
intensity
of
this radiation
is
trivial compared with that
of the
sun,
but in the
outer solar system starlight
is not
negligible. Starlight
is
essentially
isotropic (there
is
little diurnal
or
latitude
effect).
The
spectrum
is
known
and can
be
approximated
by a
superposition
of
several blackbody emissions,
as
shown
in
Figure
2.6 The
interstellar radiation
field at
galactocentric
distances
DC
= 5 and
10
kpc
(kiloparsecs),
respectively. Curves labeled
/ =
1,
2, 3, 4
relate
to
contributions
of
four
stellar components
to the
interstellar
radiation
field. The sun is in the
vicinity
of
DC
— 10
kpc.
Tbb
=
black body temperature.
After
Mathis,
J. S.,
Mezger,
P. G.,
and
Panagia,
N.,
1983,
"Interstellar
Radiation
Field
and
Dust
Tem-
peratures
in the
Diffuse
Interstellar Matter
and in
Giant Molecular
Clouds."
Astron.
Astwphys.
128, 212.
Solar
Flux
and
Molecular
Absorption
27
figure
2.6.
The
hotter blackbody emits more ultraviolet radiation,
which
could
be an
important
source
of
ionization
for
Earth's atmosphere
at
night
or in the
polar night.
The
integrated intensity
of
radiation
from
the
interstellar radiation
field is
2.17
x
1(T
5
W
irr
2
.
The sun is a
potent source
of
hydrogen
Lyman
a
emission. Part
of it
reaches
the
planets
directly,
but a
smaller fraction
can
reach
the
planets indirectly
after
being
scattered
by
hydrogen atoms
in the
interstellar medium.
The
backscattered Lyman
a
is
not
important
for the
inner
solar
system
but
becomes comparable
to the
direct solar
Lyman
a at
Neptune.
The
solar system
is
bombarded
by
galactic cosmic rays,
the
majority
of
which
are
deflected
by the
solar magnetic
field and the
solar wind. However,
the
most energetic
particles
can
penetrate into
the
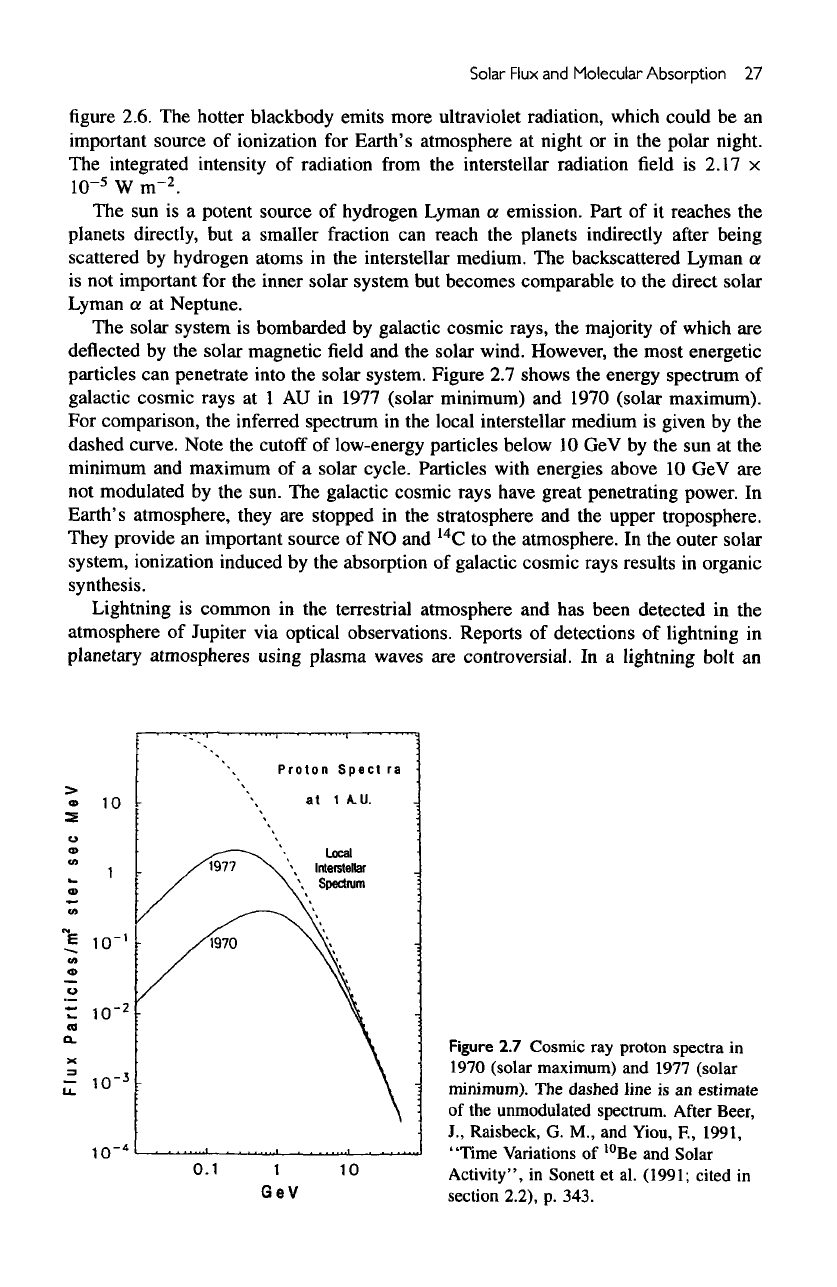
solar system. Figure
2.7
shows
the
energy spectrum
of
galactic cosmic rays
at 1 AU in
1977 (solar minimum)
and
1970 (solar maximum).
For
comparison,
the
inferred spectrum
in the
local interstellar medium
is
given
by the
dashed curve. Note
the
cutoff
of
low-energy particles below
10 GeV by the sun at the
minimum
and
maximum
of a
solar cycle. Particles
with
energies above
10 GeV are
not
modulated
by the
sun.
The
galactic cosmic rays have great penetrating power.
In
Earth's atmosphere, they
are
stopped
in the
stratosphere
and the
upper troposphere.
They provide
an
important source
of NO and
14
C
to the
atmosphere.
In the
outer solar
system, ionization induced
by the
absorption
of
galactic cosmic rays results
in
organic
synthesis.
Lightning
is
common
in the
terrestrial atmosphere
and has
been detected
in the
atmosphere
of
Jupiter
via
optical observations. Reports
of
detections
of
lightning
in
planetary
atmospheres
using
plasma
waves
are
controversial.
In a
lightning bolt
an
Figure
2.7
Cosmic
ray
proton spectra
in
1970 (solar maximum)
and
1977 (solar
minimum).
The
dashed
line
is an
estimate
of
the
unmodulated spectrum.
After
Beer,
J.,
Raisbeck,
G. M., and
Yiou,
F,
1991,
'
Time
Variations
of
10
Be
and
Solar
Activity",
in
Sonett
et
al.
(1991;
cited
in
section 2.2),
p.
343.
28
Photochemistry
of
Planetary
Atmospheres
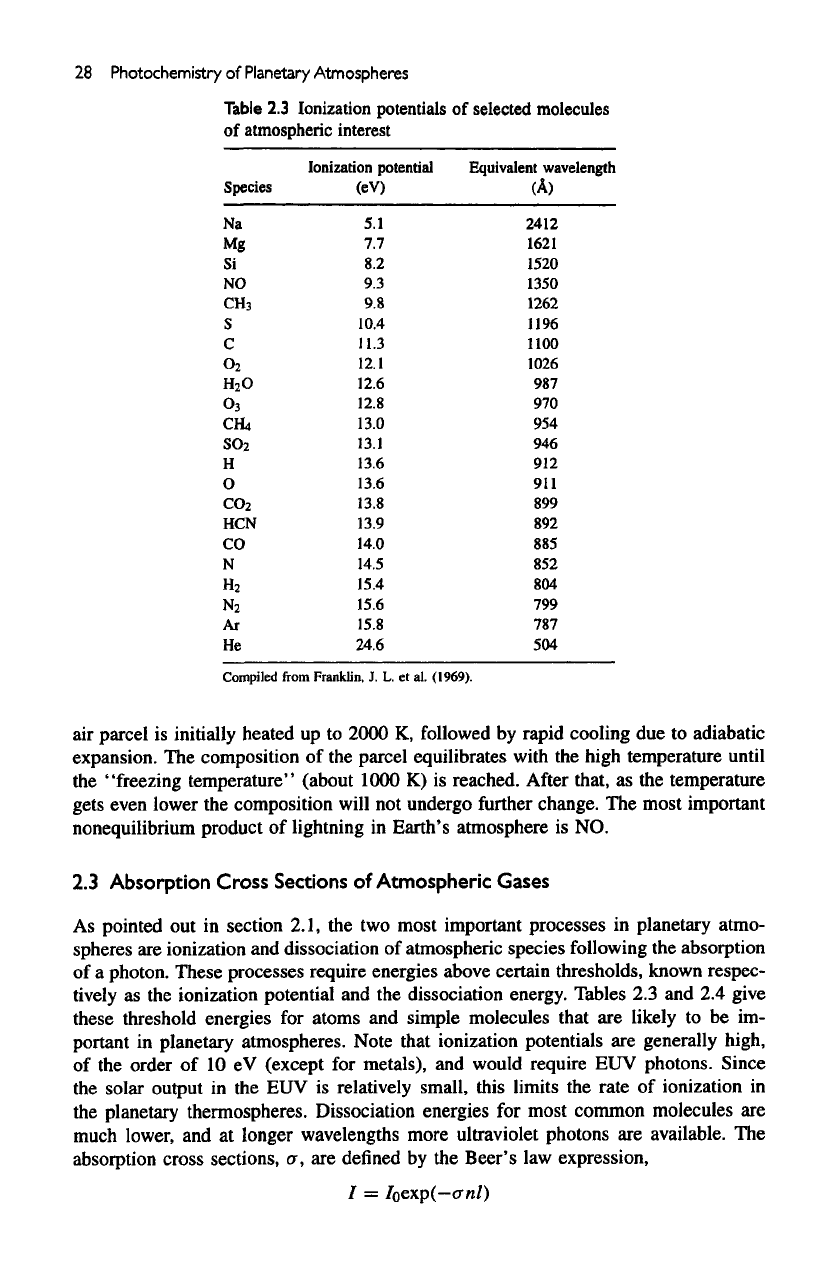
Table
2.3
lonization
potentials
of
selected
molecules
of
atmospheric
interest
Species
Na
Mg
Si
NO
CH
3
S
C
0
2
H
2
O
0
3
CH4
S0
2
H
o
C0
2
HCN
CO
N
H
2
N
2
Ar
He
lonization potential
(eV)
5.1
7.7
8.2
9.3
9.8
10.4
11.3
12.1
12.6
12.8
13.0
13.1
13.6
13.6
13.8
13.9
14.0
14.5
15.4
15.6
15.8
24.6
Equivalent
wavelength
(A)
2412
1621
1520
1350
1262
1196
1100
1026
987
970
954
946
912
911
899
892
885
852
804
799
787
504
Compiled
from
Franklin,
J. L. et
al.
(1969).
air
parcel
is
initially heated
up to
2000
K,
followed
by
rapid cooling
due to
adiabatic
expansion.
The
composition
of the
parcel equilibrates with
the
high temperature until
the
"freezing
temperature"
(about 1000
K) is
reached.
After
that,
as the
temperature
gets even lower
the
composition will
not
undergo
further
change.
The
most important
nonequilibrium
product
of
lightning
in
Earth's atmosphere
is NO.
2.3
Absorption
Cross Sections
of
Atmospheric
Gases
As
pointed
out in
section 2.1,
the two
most important
processes
in
planetary atmo-
spheres
are
ionization
and
dissociation
of
atmospheric species
following
the
absorption
of
a
photon. These
processes
require energies above certain thresholds, known
respec-
tively
as the
ionization potential
and the
dissociation energy. Tables
2.3 and 2.4
give
these threshold energies
for
atoms
and
simple molecules that
are
likely
to be im-
portant
in
planetary atmospheres. Note that ionization potentials
are
generally high,
of
the
order
of 10 eV
(except
for
metals),
and
would require
EUV
photons. Since
the
solar output
in the EUV is
relatively small, this limits
the
rate
of
ionization
in
the
planetary
thermospheres.
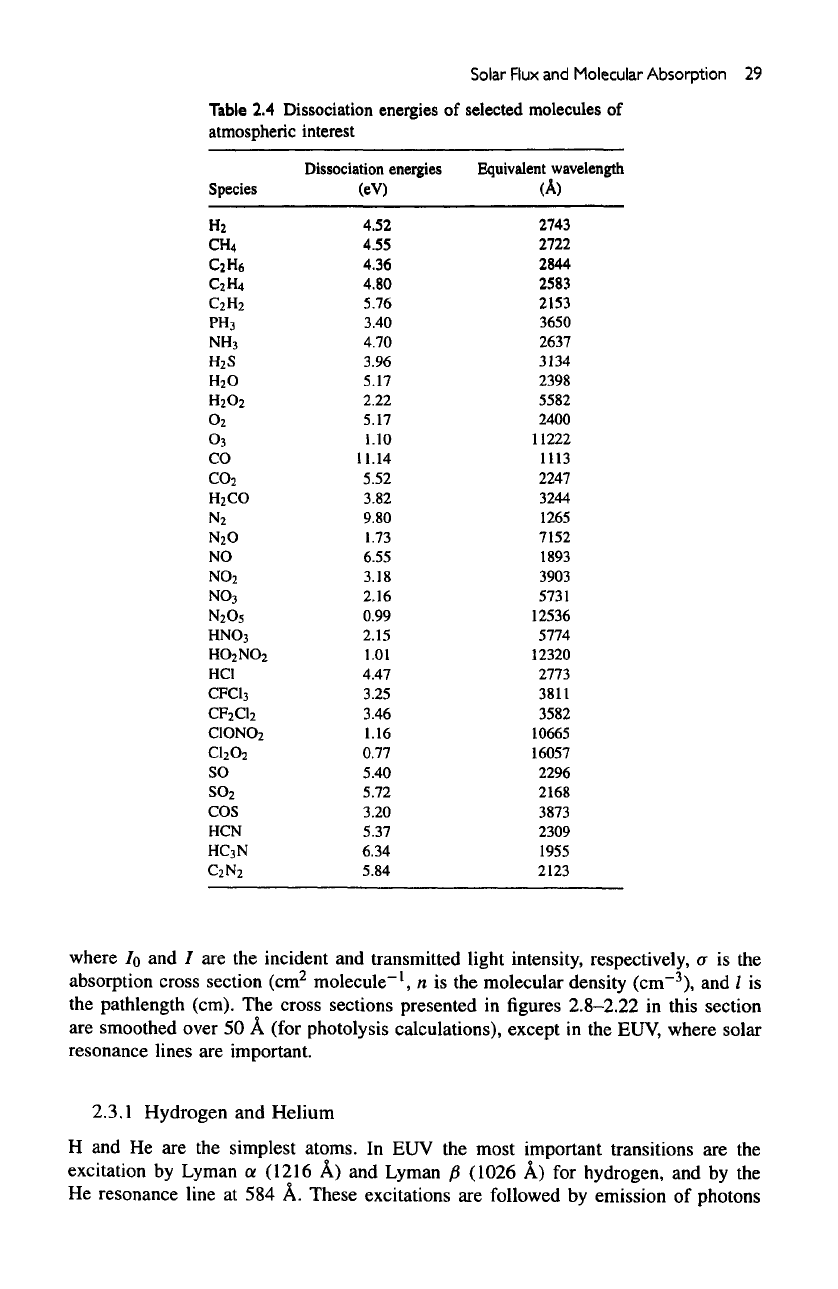
Dissociation energies
for
most common molecules
are
much
lower,
and at
longer wavelengths more ultraviolet photons
are
available.
The
absorption
cross
sections,
a,
are
defined
by the
Beer's
law
expression,
7
=
/oexp(—anl)
Solar
Flux
and
Molecular
Absorption
29
Table
2.4
Dissociation energies
of
selected
molecules
of
atmospheric interest
Dissociation
energies
Species
(eV)
H
2
CH»
C
2
H
6
CaKt
C
2
H
2
PH
3
NH
3
H
2
S
H
2
O
H
2
0
2
0
2
0
3
CO
C0
2
H
2
CO
N
2
N
2
0
NO
NO
2
NO
3
N
2
0
5
HNO
3
HO
2
NO
2
HCI
CFC1
3
CF
2
C1
2
C1ONO
2
C1
2
0
2
SO
SO
2
COS
HCN
HC
3
N
C
2
N
2
4.52
4.55
4.36
4.80
5.76
3.40
4.70
3.96
5.17
2.22
5.17
1.10
11.14
5.52
3.82
9.80
1.73
6.55
3.18
2.16
0.99
2.15
1.01
4.47
3.25
3.46
1.16
0.77
5.40
5.72
3.20
5.37
6.34
5.84
Equivalent
wavelength
(A)
2743
2722
2844
2583
2153
3650
2637
3134
2398
5582
2400
11222
1113
2247
3244
1265
7152
1893
3903
5731
12536
5774
12320
2773
3811
3582
10665
16057
2296
2168
3873
2309
1955
2123
where
/o
and 7 are the
incident
and
transmitted
light
intensity, respectively,
a is the
absorption cross section
(cm
2
molecule"
1
,
n is the
molecular density
(cm~
3
),
and
/
is
the
pathlength (cm).
The
cross
sections presented
in figures
2.8-2.22
in
this section
are
smoothed over
50 A
(for photolysis calculations), except
in the
EUV, where solar
resonance lines
are
important.
2.3.1 Hydrogen
and
Helium
H and He are the
simplest atoms.
In EUV the
most important transitions
are the
excitation
by
Lyman
a
(1216
A) and
Lyman
ft
(1026
A) for
hydrogen,
and by the
He
resonance
line
at 584 A.
These excitations
are
followed
by
emission
of
photons
30
Photochemistry
of
Planetary
Atmospheres
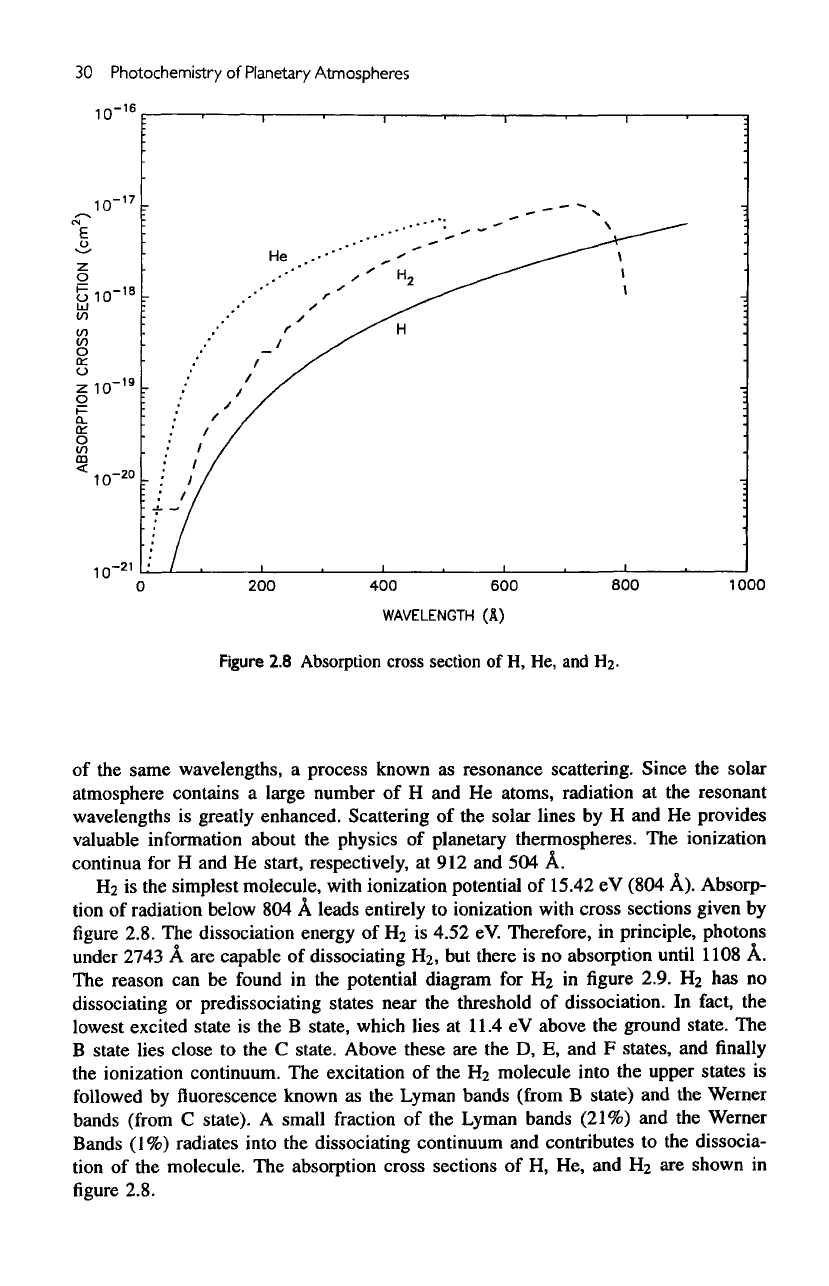
Figure
2.8
Absorption
cross
section
of H, He, and
H2-
of
the
same wavelengths,
a
process
known
as
resonance scattering. Since
the
solar
atmosphere contains
a
large number
of H and He
atoms, radiation
at the
resonant
wavelengths
is
greatly enhanced. Scattering
of the
solar lines
by H and He
provides
valuable
information about
the
physics
of
planetary
thermospheres.
The
ionization
continua
for H and He
start, respectively,
at 912 and 504 A.
H2
is the
simplest molecule, with ionization potential
of
15.42
eV
(804
A).
Absorp-
tion
of
radiation below
804 A
leads entirely
to
ionization
with
cross
sections given
by
figure
2.8.
The
dissociation energy
of
H
2
is
4.52
eV.
Therefore,
in
principle, photons
under
2743
A are
capable
of
dissociating
H2, but
there
is no
absorption until
1108
A.
The
reason
can be
found
in the
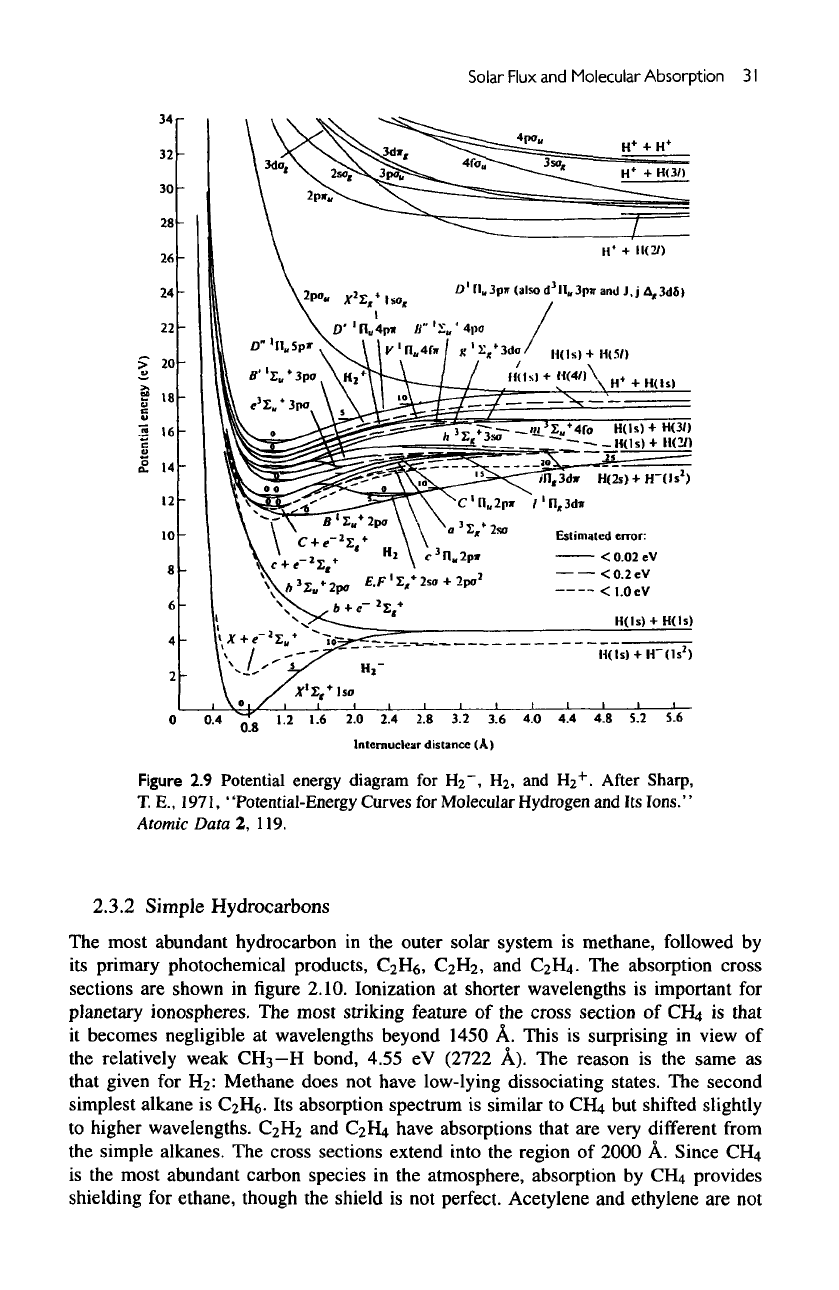
potential diagram
for
H
2
in figure
2.9.
H
2
has no
dissociating
or
predissociating states near
the
threshold
of
dissociation.
In
fact,
the
lowest excited
state
is the B
state, which lies
at
11.4
eV
above
the
ground state.
The
B
state lies close
to the C
state. Above these
are the D, E, and F
states,
and finally
the
ionization continuum.
The
excitation
of the
H
2
molecule into
the
upper
states
is
followed
by fluorescence
known
as the
Lyman
bands
(from
B
state)
and the
Werner
bands
(from
C
state).
A
small
fraction
of the
Lyman bands
(21%)
and the
Werner
Bands
(1%)
radiates into
the
dissociating continuum
and
contributes
to the
dissocia-
tion
of the
molecule.
The
absorption cross sections
of H, He, and
H
2
are
shown
in
figure
2.8.
Solar
Flux
and
Molecular
Absorption
31
Figure
2.9
Potential
energy
diagram
for
HI",
Hj,
and
HI
+
.
After
Sharp,
T.
E.,
1971, "Potential-Energy Curves
for
Molecular
Hydrogen
and Its
Ions."
Atomic
Data
2,
119.
2.3.2
Simple
Hydrocarbons
The
most abundant hydrocarbon
in the
outer
solar
system
is
methane, followed
by
its
primary photochemical products,
€2^,
€2^2,
and
CzH*.
The
absorption
cross
sections
are
shown
in figure
2.10.
lonization
at
shorter wavelengths
is
important
for
planetary
ionospheres.
The
most
striking
feature
of the
cross
section
of
CHt
is
that
it
becomes negligible
at
wavelengths beyond 1450
A.
This
is
surprising
in
view
of
the
relatively weak
CH
3
-H
bond, 4.55
eV
(2722
A). The
reason
is the
same
as
that
given
for H2:
Methane
does
not
have low-lying dissociating states.
The
second
simplest alkane
is
C2He.
Its
absorption spectrum
is
similar
to
CRt
but
shifted
slightly
to
higher wavelengths.
C2H2
and
CaHi
have absorptions that
are
very
different
from
the
simple alkanes.
The
cross sections extend into
the
region
of
2000
A.
Since
CH
4
is
the
most abundant carbon species
in the
atmosphere, absorption
by
CFU
provides
shielding
for
ethane, though
the
shield
is not
perfect. Acetylene
and
ethylene
are not

32
Photochemistry
of
Planetary
Atmospheres
Figure
2.10
Absorption
cross
section
of
CUt,
CaHg,
C2H4,
and
shielded
at all in the
long-wavelength region, where
the
solar
flux
is
rapidly increasing.
Thus,
the
initial absorption
of
photons under 1450
A by CH4
leads
to the
production
of
simple photochemical products, which
can
then absorb
at
higher wavelengths.
The
latter
process
is
particularly interesting
for the
case
of
C2H2,
since
the
photolysis
of
C2H2
initiates
new
photochemical reactions that
can
result
in the
breaking
up of the
CH4
molecule:
This
process
is
known
as
photosensitized dissociation
of CH4 and
could,
in
principle,
be
more important than
the
primary photolytic
process
for
reasons
discussed
in the
previous
paragraph.
In the
laboratory,
a
classic example
is the
photosensitized
disso-
ciation
of CH4 at
2537
A,
catalyzed
by
mercury atoms.
At
this wavelength,
CH4
is
transparent
but Hg has a
resonance
line.
The
excited
Hg
atom transfers
its
energy
to
CH4,
resulting
in its
dissociation
into
CH
3
+ H.
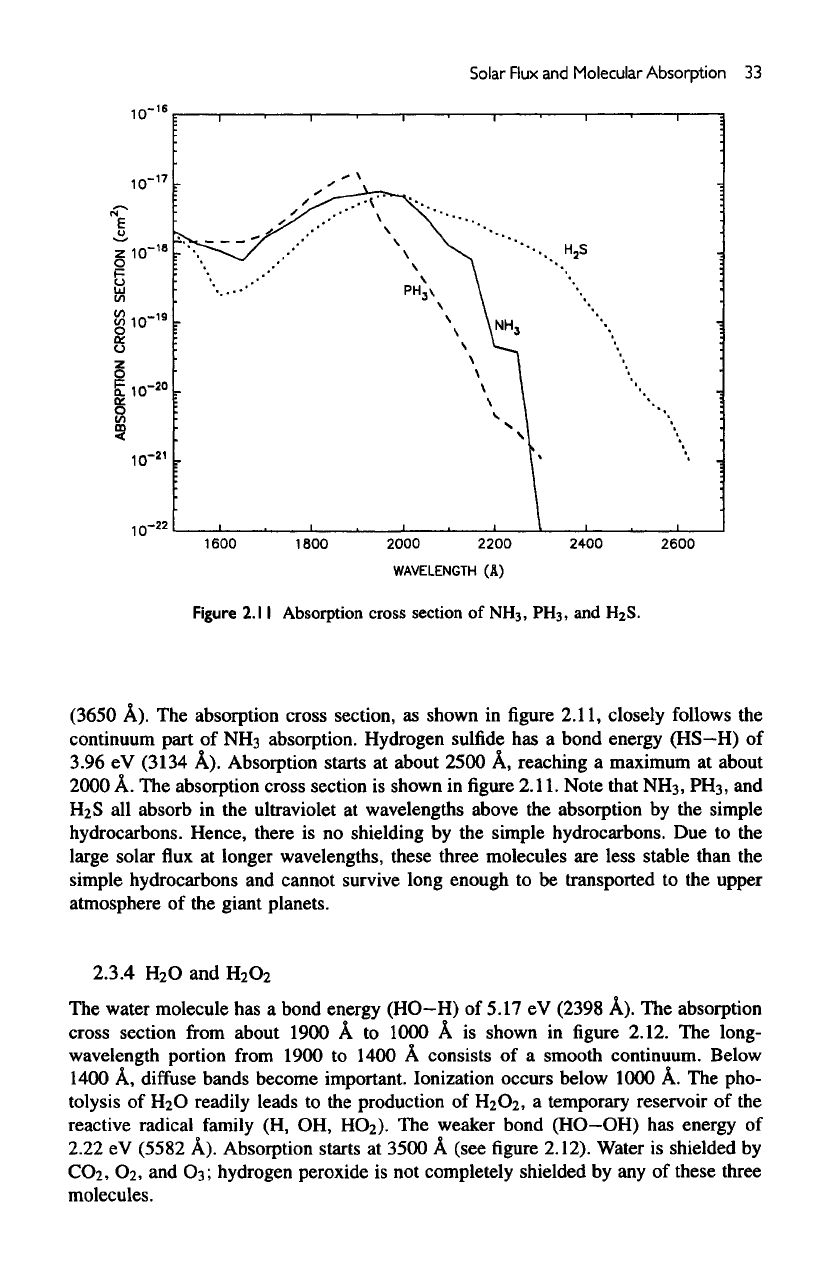
2.3.3
NH
3
,
PH
3
,
and
H
2
S
The
bond energy
for
ammonia
(NH2—H)
is
4.70
eV
(2637
A).
Absorption becomes
important
under
2300
A,
reaching peak values near
2000
A, as
shown
in figure
2.11.
The
diffuse
bands superposed
on the
continuum
are due to
out-of-plane
vibration
of
the
excited molecule. Phosphine
has a
smaller bond energy
(PH2—H)
of 3.4 eV
Solar
Flux
and
Molecular
Absorption
33
Figure
2.
1
1
Absorption cross section of NH, PH , andH S.
(3650
A). The
absorption cross section,
as
shown
in
figure
2.11,
closely follows
the
continuum
part
of
NHs
absorption. Hydrogen
sulfide
has a
bond energy
(HS—H)
of
3.96
eV
(3134
A).
Absorption
starts
at
about
2500
A,
reaching
a
maximum
at
about
2000
A. The
absorption
cross
section
is
shown
in
figure
2.11.
Note that
NHs,
PHs,
and
H
2
S
all
absorb
in the
ultraviolet
at
wavelengths above
the
absorption
by the
simple
hydrocarbons. Hence, there
is no
shielding
by the
simple hydrocarbons.
Due to the
large solar
flux at
longer wavelengths, these three molecules
are
less stable
than
the
simple hydrocarbons
and
cannot
survive
long enough
to be
transported
to the
upper
atmosphere
of the
giant planets.
2.3.4
H
2
O
and
H
2
O
2
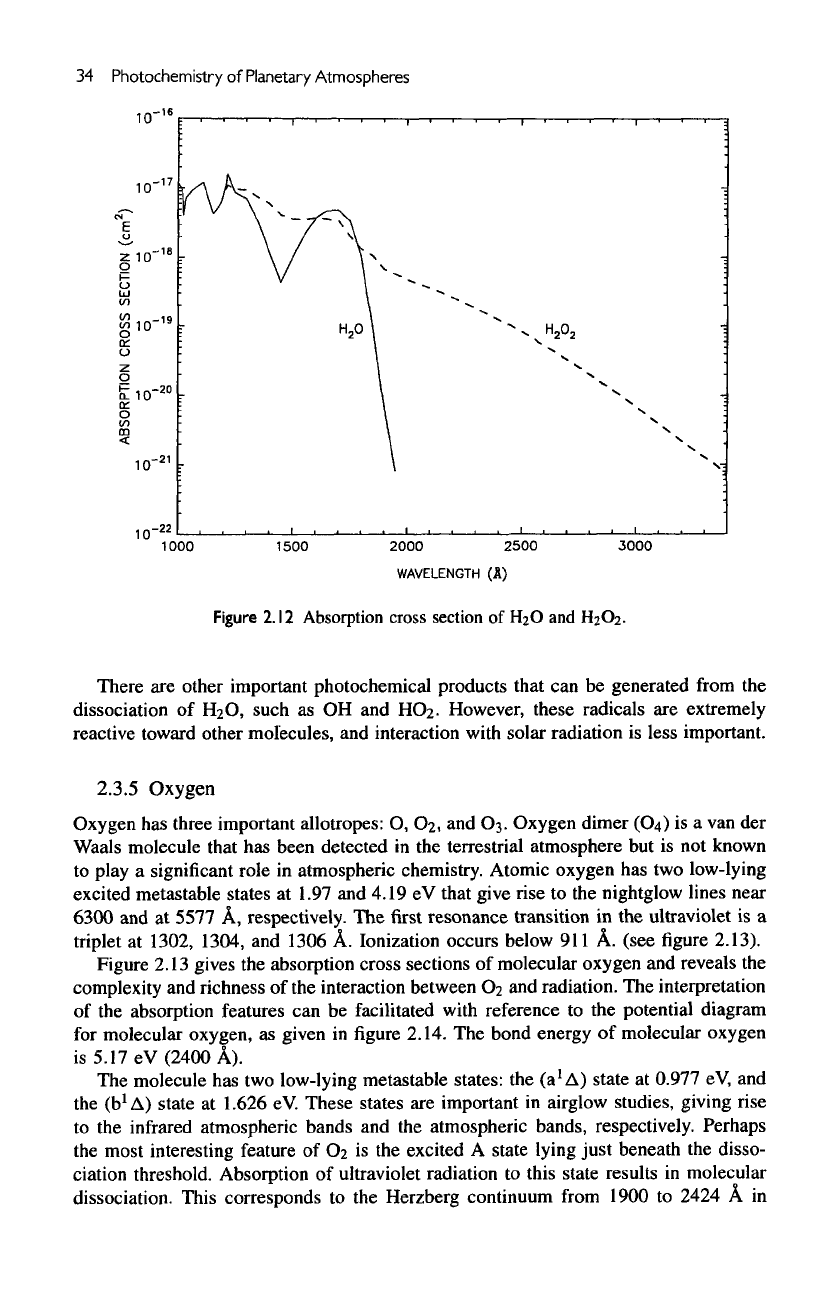
The
water molecule
has a
bond energy
(HO-H)
of
5.17
eV
(2398
A). The
absorption
cross
section
from
about 1900
A to
1000
A is
shown
in figure
2.12.
The
long-
wavelength
portion
from
1900
to
1400
A
consists
of a
smooth continuum. Below
1400
A,
diffuse
bands become important.
lonization
occurs below 1000
A. The
pho-
tolysis
of
H
2
O
readily leads
to the
production
of
H
2
O
2
,
a
temporary reservoir
of the
reactive radical
family
(H, OH,
HC>2).
The
weaker bond
(HO—OH)
has
energy
of
2.22
eV
(5582
A).
Absorption starts
at
3500
A
(see
figure
2.12).
Water
is
shielded
by
CO
2
,
O
2
,
and
03;
hydrogen peroxide
is not
completely shielded
by any of
these three
molecules.
34
Photochemistry
of
Planetary
Atmospheres
Figure
2.12
Absorption
cross
section of H2O and H2O2.
There
are
other important photochemical products that
can be
generated
from
the
dissociation
of
H2<D,
such
as OH and
HO2-
However, these radicals
are
extremely
reactive toward other molecules,
and
interaction
with
solar radiation
is
less
important.
2.3.5 Oxygen
Oxygen
has
three important allotropes:
O, O2, and
03.
Oxygen
dimer
(04)
is a van der
Waals
molecule that
has
been detected
in the
terrestrial atmosphere
but is not
known
to
play
a
significant
role
in
atmospheric chemistry. Atomic oxygen
has two
low-lying
excited
metastable states
at
1.97
and
4.19
eV
that give
rise
to the
nightglow lines near
6300
and at
5577
A,
respectively.
The first
resonance
transition
in the
ultraviolet
is a
triplet
at
1302, 1304,
and
1306
A.
lonization
occurs below
911
A.
(see
figure
2.13).
Figure
2.13
gives
the
absorption
cross
sections
of
molecular oxygen
and
reveals
the
complexity
and
richness
of the
interaction between
02 and
radiation.
The
interpretation
of
the
absorption features
can be
facilitated with reference
to the
potential diagram
for
molecular oxygen,
as
given
in figure
2.14.
The
bond energy
of
molecular oxygen
is
5.17
eV
(2400
A).
The
molecule
has two
low-lying metastable states:
the
(a
1
A)
state
at
0.977
eV, and
the
(b'A)
state
at
1.626
eV.
These
states
are
important
in
airglow studies, giving rise
to the
infrared
atmospheric bands
and the
atmospheric bands, respectively. Perhaps
the
most interesting feature
of O2 is the
excited
A
state
lying
just beneath
the
disso-
ciation threshold. Absorption
of
ultraviolet radiation
to
this state results
in
molecular
dissociation.
This corresponds
to the
Herzberg continuum
from
1900
to
2424
A in

Solar Flux
and
Molecular
Absorption
35
Figure
2.13
Absorption
cross
section
of
Oa
and O.
figure
2.13. Between
1900
and
1750
A the
absorption
goes
through
a
series
of
bands
known
as the
Schumann-Runge bands
due to the X-B
transitions
(see
figure
2.14).
Both
of the
oxygen atoms
are
produced
in the
ground state
by
predissociation.
Be-
tween
1750
A and
1300
A the
absorption
goes
through another continuum known
as
the
Schumann-Runge continuum. Here
one of the
oxygen atoms produced
is in the
excited state,
O('D).
Below
1300
A
there
are
numerous Rydberg
states.
lonization
becomes important below
1026
A.
The
bond energy
of
ozone
(O
2
-O)
is
1.10
eV
(11,222
A).
Ozone
has
three
ab-
sorption bands,
as
shown
in figure
2.15a.
The
Chappuis bands start
at
9000
A,
reach
a
peak
at
6000
A, and
fall
off at
4500
A. The
cross
sections
are
small.
The
products
of
dissociation
are O and
62
in the
ground
states.
The
Huggins bands range
from
3000
to
3600
A.
There
is a
strong temperature dependence
in the
cross
sections
of
O
3
in the
Huggins bands,
as
shown
in
high resolution
(1
A) in figure
2.15b.
At
lower
temperatures,
the
valleys
of the
bands become much deeper;
the
peaks remain roughly
constant.
The
products
of
dissociation include
O
2
('A)
and
O('D).
The
O('D)
yield
declines
rapidly above
3080
A. The
primary absorption
of
O
3
is due to the
Hartley
bands
from
2000
A to
3000
A,
with
maximum
cross
section approaching
10~
17
cm
2
.
The
products
of
photolysis
are
mostly
(^('A)
and
O('D),
although some fraction
(5-10%)
of the O is in the
ground state. Note that
the
bulk
of the
ultraviolet shielding
of the
terrestrial biosphere
is via the
Hartley
and the
Huggins bands
of
ozone. That
the
efficiency
of
these bands
for filtering out the
actinic rays
is
high
is
demonstrated
by
