Yung Y.L., DeMore W.B. Photochemistry of Planetary Atmospheres
Подождите немного. Документ загружается.


186
Photochemistry
of
Planetary
Atmospheres
5.7
Miscellaneous Topics
5.7.1
AsH
3
andGeH4
Small
concentrations (less
than
1
ppb)
of
arsine
(AsHs)
and
germane
(GeH*)
have
been
detected
in the
atmosphere
of
Jupiter
but not in any
other giant planet.
The
observed abundances
are in
agreement with
thermodynamic
models based
on
cosmic
composition
of the
elements
and
upward mixing
from
the
deep
atmosphere.
5.7.2
Sulfur
Species
Conspicuous
by its
absence
is the
detection
of any
sulfur
species
in the
giant planets.
A
serious search
was
made
for H2S in the
atmosphere
of
Jupiter, yielding
an
upper
limit
that
is
about
300
times less
than
the
cosmic abundance. There
is no
reason
to
expect such
a
large depletion
of
sulfur
in the
giant
planets during formation.
We
believe
that
most
of the
H^S
is
sequestered
in
NUjSH
clouds.
5.7.3
Isotopomers
Deuterated hydrogen (HD)
and
deuterated methane
(CHjD)
have been detected
in
all
the
giant planets. This provides valuable information
on the
D/H
ratios
in
these
planets.
The
D/H
ratios
in the
atmospheres
of
Jupiter
and
Saturn
are
close
to the
cosmic abundance value
of 1.6 x
10~
5
.
The
D/H
ratios
in
Uranus
and
Neptune show
a
significant
enhancement over
the
cosmic abundance value. This could
be
explained
as
follows.
There
were
two
distinct reservoirs
of
deuterium
in the
solar nebula: gaseous
HD and
ices such
as HDO or
CHsD.
The
former
is
characterized
by a
D/H
ratio given
by
cosmic abundance.
The
latter
has a
higher
D/H
ratio, caused
by a
hitherto uniden-
tified
mechanism.
The
hydrogen
and
deuterium
in the
giant planets
are
derived
from
a
combination
of
these
two
reservoirs.
In
Jupiter
and
Saturn
the
gaseous
contribution
dominates;
in
Uranus
and
Neptune
the
ices
are an
important component
of the
total
budget.
See
section
4.10.1
for
comparison
of
D/H
values
in the
solar system
and a
discussion
of the
mechanisms
of
isotopic fractionation.
The
13
C
and
15
N
isotopic
species
have been detected
in the
atmosphere
of
Jupiter.
The
derived isotopic ratios,
13
C/
12
C
and
15
N/
14
N,
appear
to be
close
to the
cosmic
abundance
values, except
for
that inferred
from
13
C2H2-
This last anomaly
may be
due
to the
exceedingly
low
concentration
of
€2^2
in the
atmosphere
of
Jupiter.
In the
other
giant
planets,
only
13
CH4
has
been
detected
in the
atmosphere
of
Saturn,
and
the
inferred
13
C/
12
C
appears
to be
close
to
cosmic abundance.
5.7.4 Unsolved
Problems
The
vitality
of a field is
measured
not
only
by the
number
of
solved problems
but
also
by the
number
of new
questions that
are
raised
in the
investigations
and the
problems that remain
to be
solved.
The
following list
of
unsolved problems
is
given
as
a
challenge
to
future
investigators. Unless otherwise stated,
the
problems refer
to
all
four
giant
planets.

Jovian Planets
187
(a)
Energetics
and
Dynamics
1.
What
is the
energy source
for
maintaining
the
high temperatures
in the
thermo-
spheres?
2.
What
is the
excitation mechanism
for the
excess ultraviolet emissions that cannot
be
accounted
for by
resonance
fluorescence of
sunlight?
3. Why are the
internal heat
fluxes of
Uranus
and
Neptune
so
different
for two
planets
that
are so
similar?
4. Why is the
upper atmosphere
of
Uranus
so
quiescent, whereas
the
upper atmosphere
of
Neptune
is
vigorously mixed?
(b)
Atmospheric Composition
5.
What
is the
mechanism
for
producing
the
multiple layering
in the
lower ionosphere?
6.
What
is the
reason
for an
apparent excess
of He
over
the
cosmic abundance value
in
Neptune?
7. Why are
oxygen
and
sulfur
compounds apparently deficient?
8.
What
are the
precursor molecules
of the
Axel-Danielson
dust
in the
stratosphere?
9.
What
is the
chemical nature
of the
chromophores
in the
atmosphere?
10.
Does
ion
chemistry driven
by
magnetospheric
particles
affect
the
global chemical
composition?
11.
What
is the
cause
of the
Lyman
a
bulge
in
Jupiter?
(c)
Photochemistry
and
Kinetics
These
two
areas desperately need
new
input.
In
photochemistry
we
need measurements
of
the
branching ratios
and
dissociation products
as a
function
of
wavelength.
The
measurements
are
more important near
the
thresholds
of
dissociation, where
the
cross
sections
are
smaller
but the
solar
flux is
larger.
In
chemical kinetics
a
whole
new
class
of
experiments needs
to be
done
at low
pressure
(1
mtorr
to 1
torr)
and low
temperature
(50-200
K).
12.
What
are the
photodissociation products
of
CRj
at
Lyman
a
(1216
A) and at
longer
wavelengths
(1450
A)?
13.
What
are the
quantum yields
and
branching ratios
of the
photodissociation
of
C
2
H
2
from
Lyman
a to
threshold (around 2200
A)?
14.
What
is the
fate
of
excited
C
2
H
2
in the
atmosphere?
Does
it
take part
in the
poly-
merization
Of
C2H2?
15.
What
are the
cross sections
and
branching ratios
of
photodissociation
of
C4H2
and
higher
polyynes?
16.
What
are the
rate coefficients
of the
reactions forming higher polyynes
at low
tem-
perature?
The
reactions include
R181:
C
4
H
+
C
2
H
2
and
R190:
C
6
H
+
C
2
H
2
(see
table
5.11).
17.
What
are the
low-temperature rate coefficients
for the CH
insertion reactions:
R120:
CH
+
CH
4
,
R121:
CH +
C
2
H
2
,
R122:
CH +
C
2
H4,
R123:
CH +
C
2
H
6
?
18.
What
are the
photodissociation
cross
sections, branching ratios,
and
dissociation
products
for
methyl acetylene
(CH
3
C
2
H),
allene
(CH
2
CCH
2
),
and
propene
from
Lyman
a to
threshold?

188
Photochemistry
of
Planetary
Atmospheres
19.
What
are the
low-temperature rate coefficients
for the
following reactions that
are
important
in the
synthesis
of
higher hydrocarbons: R135:
CH
3
+CH
3
,
R138:
CH
3
+
C
2
H
3
,
R141:
CH
3
+C
3
H
3
,
R143:
CH
3
+C
3
H
5
,
R167:
C
2
H
3
+
C
2
H
3
,
R171:
C
2
H
3
+
C
2
H
5
?
20.
What
are the
low-temperature rate
coefficients
for the
following reactions that scav-
enge
H
atoms: R84:
H +
C
2
H
2
,
R109:
H +
C
4
H
2>
R164:
C
2
H
3
+
H
2
?
21.
What
are the
low-temperature rate coefficients
for
cracking
of
higher hydrocarbons
by
H
atoms: R94:
H +
CH
3
C
2
H,
Rl
10:
C
4
H
3
+ H?
22.
What
is the
role
of
heterogeneous chemistry
on the
surface
of the
Axel-Danielson
dust?
23.
What
are the
optical properties
of
hydrazine
after
exposure
to
ultraviolet radiation?
24.
What
is the
combined chemistry
of CP and PN,
which
are
chemical analogs
of CN?
25.
What
are the
photochemical products
of
AsH
3
and
GeH4
photochemistry?

Satellites
and
Pluto
6.1
Introduction
The
presence
of an
atmosphere
on a
small planetary body
the
size
of the
Moon
is
surprising. Loss
of
material
by
escape would have depleted
the
atmosphere over
the
age of the
solar system. Since these objects
are not
large enough
to
possess,
or to
sustain
for
long,
a
molten core, continued outgassing
from
the
interior
is not
expected.
However,
it is now
known that
four
small bodies
in the
outer solar system
possess
substantial
atmospheres:
lo,
Titan, Triton,
and
Pluto. These atmospheres range
from
the
very tenuous
on
lo
(of the
order
of a
nanobar)
to the
very massive
on
Titan
(of
the
order
of a
bar).
The
atmospheric pressures
on
Triton
and
Pluto
are of the
order
of
10
fjLbar.
Perhaps
the
most interesting questions about these atmospheres concern
their
unusual origin
and
their chemical evolution.
lo
is the
innermost
of the
four
Galilean
satellites
of
Jupiter,
the
other three being
Ganymede, Europa,
and
Callisto.
All the
Galilean moons
are
comparable
in
size,
but
there
is no
appreciable atmosphere
on the
other moons.
The first
indications
that
lo
possesses
an
atmosphere came
in
1974
with
the
discovery
of
sodium atoms
surrounding
the
satellite
and the
detection
of a
well-developed ionosphere
from
the
Pioneer
10
radio occultation experiment.
The
Voyager encounter
in
1979
established
the
existence
of
active volcanoes
as
well
as
SOa
gas.
These
are the
only extraterrestrial
active
volcanoes discovered
to
date,
and
they
owe
their existence
to a
curious tidal
heating
mechanism associated with
the 2:1
resonance between
the
orbits
of
lo
and
Europa.
This tidal heating generates
a
total power
of
10
13
to
10
14
W, a
value
that
may
be
compared
to the
total
geothermal
heat
flux of
Earth,
3 x
10
13
W.
lo's
heat
189
6

190
Photochemistry
of
Planetary
Atmospheres
is
released over
a
surface area that
is
about
12
times smaller than that
of the
Earth.
Hence,
lo's
internal heating
per
unit
area
is an
order
of
magnitude larger
than
that
of
Earth.
The
atmosphere
of
lo
is
continuously being eroded
by
bombardment
by the en-
ergetic particles
in the
Jovian
magnetosphere,
but the
atmosphere
is
resupplied
by
material
of
volcanic origin.
The
sputtered products
escape
into
the
magnetosphere
of
Jupiter
and
create
an
extended cloud
of
neutrals around
lo
and a
torus
of
heavy
ions
in the
orbit
of
lo.
The
major ions
are
oxygen
and
sulfur
ions derived
from
the
dissociation
products
of
SC>2.
In
this chapter,
we
examine
the
bound atmosphere
of
lo:
SO2
photochemistry,
the
ionosphere, atmospheric sputtering,
and the
torus.
Titan,
the
largest satellite
of
Saturn,
is
known
to
have
an
atmosphere, since
CH-4
lines
were spectroscopically identified
in its
spectrum
in
1944. Very little
was
known
about
Titan's atmosphere
until
the
Voyager encounters, which revealed that Titan
has a
massive
N2
atmosphere,
with
surface
pressure
equal
to 1.5
bar.
A
rich variety
of
organic molecules
and
extensive
aerosol
layers were
found
to be
present
in the
atmosphere. Titan
is
believed
to
have formed
in the
Saturnian subnebula
at the
time
of
the
formation
of
Saturn.
Due to the
lower temperatures
in
this region
of the
solar
nebula,
ices were common,
and
Titan could have accreted material that
is
rich
in
ices.
As the
atmospheric constituents
are
photochemically
processed
and
converted
into
condensible
material,
the
ices
on the
surface
or
outgassing
from
the
interior must
maintain
the
supply
of gas to the
atmosphere.
The
emphasis
of
this chapter
is on
the
organic chemistry
in
Titan's atmosphere
and its
implications
for
evolution.
The
hydrocarbon chemistry
on
Titan
provides
an
interesting comparison with that
of the
giant
planets. Organic synthesis
is
greatly facilitated
in
this atmosphere
due to the
lower amount
of the
reducing
gas H2.
This, together with
the
smaller
size
of the
planetary
body, results
in a
higher rate
of
chemical evolution.
In
bulk composition
the
atmosphere
of
Titan
is
mildly reducing, with
an
oxidation
state intermediate between that
of the
giant planets
and the
terrestrial planets. This
chemical
environment
may be
similar
to
that
of
Earth
at the
time
of
formation,
an
environment
that
is
conducive
to the
synthesis
of
complex organic compounds that
may
lead
to the
spontaneous generation
of
life.
Since there
are no
preserved
records
of
the
early chemical environment
of the
Earth, Titan
offers
an
exciting analog
of the
prebiological Earth.
Triton,
the
largest satellite
of
Neptune,
is
believed
to
have formed
from
the icy
debris
in the
outer part
of the
solar nebula.
It was
subsequently captured
by
Neptune,
and
this accounts
for its
unusual retrograde orbit.
The
bulk composition
of
Triton
is
interesting because
it
provides
a
sampling
of the
condensible
species
in the
solar
nebula
such
as
CH
4
,
CO,
CO2,
NHs,
N
2
,
and
HaO.
These
species
are
also present
in
molecular clouds, precursors
of the
solar nebula.
Any
major difference
in the
inventory
of
the
major
volatiles between
the
solar nebula
and the
molecular clouds would yield
valuable
clues
on
physical
and
chemical processing that must have occurred during
the
formation
of the
solar system.
The
atmosphere
of
Triton consists primarily
of
N2,
with
trace concentrations
of
CH.4.
CO and CO2
have been detected
in the ice
on
the
surface.
There
is an
upper limit
of 1% for CO in the
atmosphere
based
on a
combination
of
observation
and
modeling.
The
vapor pressure
of CO2 is
sufficiently
low
that
the gas is
negligible
in the
atmosphere.
The
photochemistry
of N2 and
CFLj
in
the
atmosphere
of
Triton
is
similar
to
that
of
Titan, except that
the
temperature
is

Satellites
and
Pluto
191
much
lower
and the
surface pressure
of
Triton corresponds
to
that
of the
mesosphere
of
Titan.
Pluto,
the
ninth
planet
of the
solar system,
has
little
in
common
with
the
other eight
planets
but
seems
to
closely resemble Triton. Both
are
believed
to be icy
planetesimals
formed
between
30 and 50 AU in the
solar
nebula.
We
know much
less
about Pluto than
Triton.
The
bulk atmosphere
is
believed
to be
composed
of
Na,
in
vapor equilibrium
with
N2 ice on the
surface.
CH*
and CO
ices
have been identified
in the
reflection
spectrum
of
Pluto,
and we
expect
trace
amounts
of
these gases
to be
present
in the
atmosphere.
The
photochemistry
of the
atmosphere
of
Pluto
is
expected
to be
similar
to
that
of
Triton, given
our
current lack
of
knowledge
of
Pluto.
An
important
aspect
of the
atmospheres
of the
small
bodies
is
their physical extent.
In
our
experience with
the
terrestrial planets
and the
giant planets,
the
altitude
of the
exobase
of the
atmosphere
is
usually small compared
with
the
planetary radius. Thus,
the
plane parallel approximation
is
valid. However,
in the
case
of the
small
bodies,
this
approximation
is
invalid.
The
extension
of the
atmosphere
is
comparable
to the
radius
of the
solid body.
The
equations
of
continuity must
be
formulated
in
spherical
coordinates.
6.2
lo
6.2.1
Neutral
Atmosphere
Pre-Voyager
ground-based infrared reflectance measurements have shown
the
presence
of
SOa
frost
on the
surface
of
lo.
Gaseous
SO2 has
been
definitively
identified
on
lo
by
the
Voyager infrared radiometer, interferometer
and
spectrometer (IRIS) instrument.
The
original report
of
these data interpreted
the
observed
gas as
vapor
in
equilibrium
with
SC>2
frost
on the
surface
at 130 K.
However,
the
Voyager observations
may be
associated
with
volcanic plumes. Therefore, exactly
how
much
SC>2
the
atmosphere
contains
is an
unresolved issue.
The
problem
can
best
be
appreciated
by
referring
to
figure
6.
la,
which shows
the
pressure dependence
of
862
on
temperature.
In
regions
of
active volcanic activity
the
temperature
may
exceed
300 K; the
corresponding
SC>2
vapor
is
exceedingly high.
At the
subsolar
point,
the
temperature could
be as
high
as
130
K and the
vapor pressure
of
SC>2
exceeds
10~
7
bar. However,
in the
polar regions
and
on the
night side,
the
temperatures drop below
90 K and the
vapor
pressure
of
SC>2
is
less
than
10~
12
bar,
which
is
close
to the
lower
limit
of a
collisional atmosphere.
The
simplest picture
is
that
lo's
atmosphere
may be of a
transient nature: thick
and
dense atmosphere near
the
volcanic plumes
and at the
subsolar
point,
gradually
decaying
away toward
the
poles
and the
nightside.
The
best observational evidence
in
support
of
this
picture
is the
microwave observation.
The
experiment reported detection
of
4-35
x
10~
9
bar of
SC>2
covering 3-15%
of the
surface
of
lo,
a
result consistent
with
SC>2
being
in
equilibrium
with
the
surface
frost.
There
is
circumstantial evidence
for
the
presence
of
about
20 x
10~
9
bar of a
noncondensible gas, such
as
C>2
or SO, in
the
atmosphere.
02 has
been postulated
to
impede
the
lateral
flow of SO2
away
from
the
subsolar
point
and to
account
for the
warm exosphere
of
lo.
There
is no
direct
observation
to
support
this
hypothesis.
Two
models
of
lo's
atmosphere,
a
high-density
and
a
low-density model,
are
shown
in figure
6.1b.
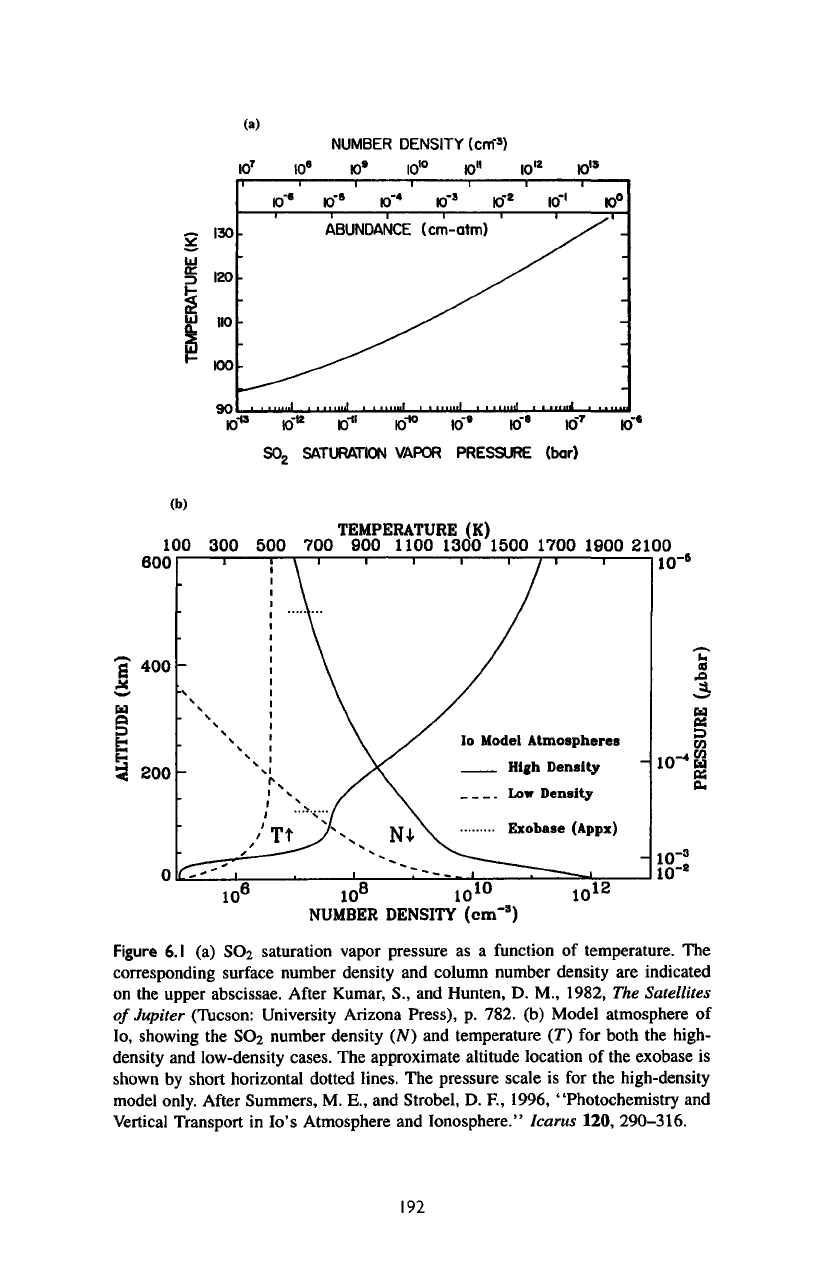
Figure
6.1
(a)
SOi
saturation vapor pressure
as a
function
of
temperature.
The
corresponding surface number density
and
column number density
are
indicated
on
the
upper
abscissae.
After
Kumar,
S., and
Hunten,
D. M.,
1982,
The
Satellites
of
Jupiter (Tucson: University Arizona
Press),
p.
782.
(b)
Model atmosphere
of
Io,
showing
the
SC>2
number density
(N) and
temperature
(T) for
both
the
high-
density
and
low-density
cases.
The
approximate altitude location
of the
exobase
is
shown
by
short horizontal dotted lines.
The
pressure
scale
is for the
high-density
model only.
After
Summers,
M. E., and
Strobel,
D.
P.,
1996,
"Photochemistry
and
Vertical
Transport
in
lo's
Atmosphere
and
Ionosphere."
Icarus
120,
290-316.
192
Satellites
and
Pluto
193
The
photochemistry
of SO2 in the
atmosphere
is
initiated
by the
photolysis
of
SO
2
:
where
the
thresholds
of
reactions
(6. la) and
(6.1b)
are
2170
and
2084
A,
respectively.
The
products
can
undergo
further
dissociation:
Some
of
these atoms
can
diffuse
to the
exosphere, where they
are
lost
due to
sputtering
by
the
magnetospheric energetic particles. Recombination
of the
dissociation products
can
also occur:
where
the
third body
(M) is
either
the
ambient atmosphere
of
SO
2
or the
surface. For-
mation
of
more complex compounds
can
readily occur,
as in the
following
examples:
But
these compounds
are
unstable
in the
atmosphere
of
lo
and are
readily removed
by
reactions such
as
A
simple reaction
set
describing
the
photochemistry
of
SO
2
is
given
in
table 6.1.
The
main uncertainties
are the
chemical kinetics
at low
temperature near
the
surface
and
the
fate
of the
dissociation products
at the
surface.
We
adopt
the
"reasonable"
assumption
that
S
atoms stick
to the
surface
but O
atoms recombine
to
form
O
2
and
are
released back
to the
atmosphere.
The
results
of a
representative model
are
given
in figure
6.2a
for
neutral
species.
SO
2
is the
most abundant molecule
in the
atmosphere near
the
lower boundary.
At
higher altitudes
O and S
atoms
become
the
dominant
species.
The
photochemical products
SO and
O
2
are
next
in
abundance.
The
abundance
of
O
2
is
small compared with
the
other dissociation products primarily
because
of the
reaction that removes
O
2
rapidly
via
where
the S
atoms
are
derived
from
(6.1b)
and
(6.2).
Since
the
atmosphere
is so
thin,
ternary reactions that require collisional stabi-
lization
are too
slow
in the gas
phase. However,
the
surface
may
serve
as a
good
"sponge
layer"
for the
atoms
to
react
to
form
stable molecules.
The
photochemical
model predicts
a
high
abundance
of SO,
formed mainly
from
SO
2
photolysis
and by
recombination
of S and O
atoms
on the
surface
of
lo.
Since
SO
2
condenses
on the
194
Photochemistry
of
Planetary Atmospheres
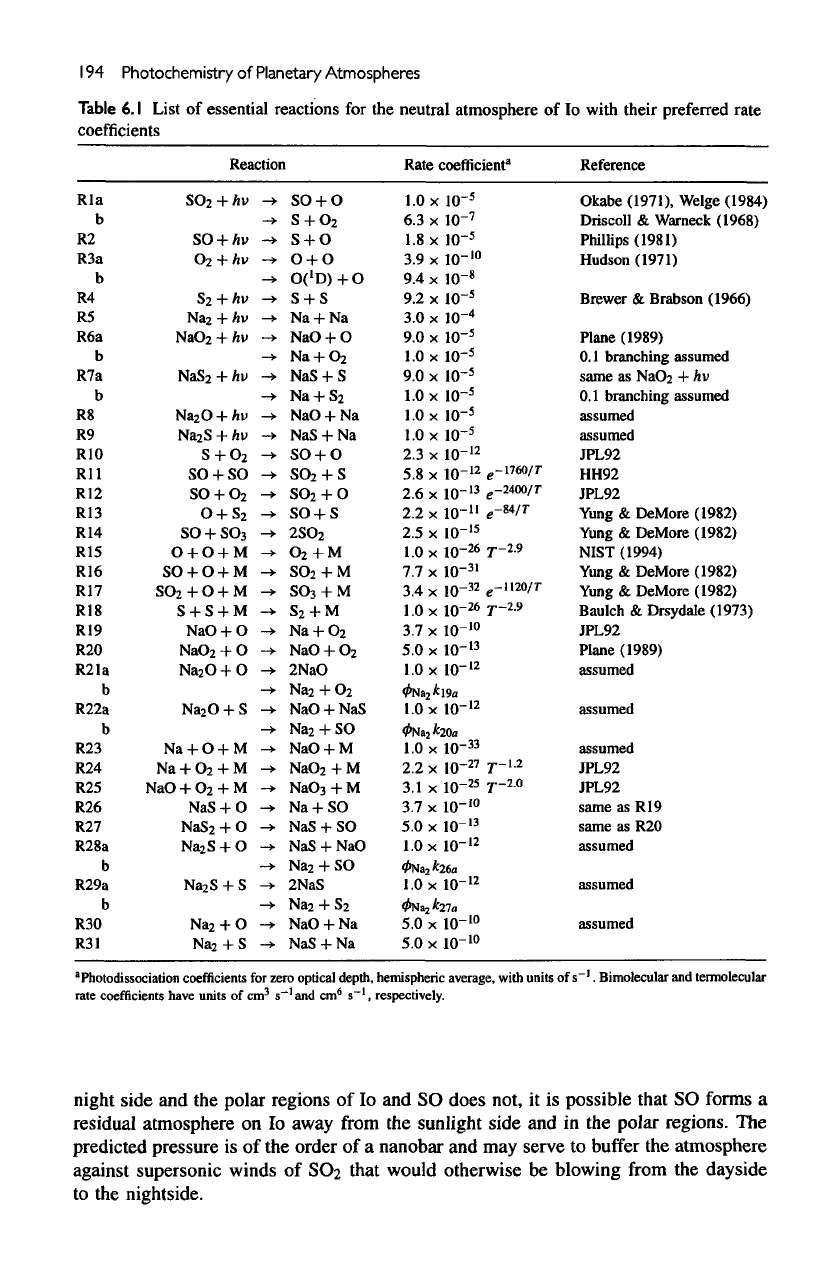
Table
6.1
List
of
essential
reactions
for the
neutral
atmosphere
of
lo
with their preferred
rate
coefficients
Reaction
Rla
b
R2
R3a
b
R4
R5
R6a
b
R7a
b
R8
R9
RIO
Rll
R12
R13
R14
R15
R16
R17
R18
R19
R20
R21a
b
R22a
b
R23
R24
R25
R26
R27
R28a
b
R29a
b
R30
R31
SO
2
+ hv
SO
+
hv
O
2
+ hv
S
2
+ hv
Na
2
+ hv
NaO
2
+ hv
NaS
2
+ hv
Na
2
O
+ hv
Na
2
S
+ hv
S
+
O
2
SO
+ SO
S0
+
0
2
O
+
S
2
SO
+
SO
3
O
+ O + M
SO
+ 0 + M
SO
2
+ O + M
S
+ S + M
NaO
+ O
NaO
2
+ O
Na
2
O+O
Na
2
O
+ S
Na
+ O + M
Na
+
O
2
+ M
NaO
+
O
2
+ M
NaS
+ O
NaS
2
+ O
Na
2
S
+ O
Na
2
S
+ S
Na
2
+ O
Na
2
+S
—
>•
-».
—
>•
_»
_»
-»
->.
—
>
-».
—
»
-»
—
»
—
>
—
>•
->.
_».
-».
—
>•
->
->
->.
->
-»
_*.
->
->
—
>•
-».
_».
_».
—
>
—
»
—
»•
—
>
—>
-»
->
—
*
—
»
so + o
S+0
2
s + o
o + o
O('D)
+ O
s + s
Na
+ Na
NaO
+ O
Na
+
Ch
NaS
+ S
Na
+
S
2
NaO
+ Na
NaS
+ Na
SO
+ O
SO
2
+ S
S0
2
+ 0
so + s
2SO
2
Oi
+ M
S0
2
+ M
SO
3
+ M
S
2
+ M
Na
+
O
2
NaO
+
O
2
2NaO
Na
2
+
O
2
NaO
+ NaS
Na
2
+ SO
NaO
+ M
NaO
2
+ M
NaO
3
+ M
Na
+ SO
NaS
+ SO
NaS
+ NaO
Na
2
+ SO
2NaS
Na
2
+
S
2
NaO
+ Na
NaS
+ Na
Rate
coefficient
2
1.0
6.3
1.8
3.9
9.4
9.2
3.0
9.0
1.0
9.0
1.0
1.0
1.0
2.3
5.8
2.6
2.2
2.5
1.0
7.7
3.4
1.0
3.7
5.0
1.0
0Na
1.0
#Na
1.0
2.2
3.1
3.7
5.0
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
2*
X
l
k
X
X
X
X
X
l.Ox
#Na
1.0
0Na
5.0
5.0
X
^
X
X
io-
5
io-
7
IO-
5
10
-.o
io-
8
io-
5
10-"
io-
5
io-
5
io-
5
io-
5
io-
5
io-
5
io-
12
IO-
12
e-
17
*/
7
'
10
-i3
e
-
2
4<x>/r
10-"
e-**/
T
io-
15
IO-
26
T-
2
-
9
io-
31
io-
32
e
-
im
/
T
10
-26
j-2.9
10
-io
10-
13
10-
12
190
io-
12
20n
io-
33
,
0
-27
r
-1.2
,0-25
J--2.0
,0-io
io-
13
io-
12
26n
10-
12
27o
io-
10
10
-io
Reference
Okabe
(1971),
Welge
(1984)
Driscoll
&
Warneck
(1968)
Phillips (1981)
Hudson
(1971)
Brewer
&
Brabson
(1966)
Plane (1989)
0.1
branching assumed
same
as
NaO
2
+ hv
0.1
branching assumed
assumed
assumed
JPL92
HH92
JPL92
Yung
&
DeMore (1982)
Yung
&
DeMore (1982)
NIST
(1994)
Yung
&
DeMore (1982)
Yung
&
DeMore (1982)
Baulch
&
Drsydale
(1973)
JPL92
Plane
(1989)
assumed
assumed
assumed
JPL92
JPL92
same
as R19
same
as R20
assumed
assumed
assumed
a
Photodissocialion
coefficients
for
zero
optical depth, hemispheric
average,
with units
of s
'.
Bimolecular
and
termolecular
rate coefficients have units
of
cm
3
s"'and
cm"
s~',
respectively.
night
side
and the
polar
regions
of Io and SO
does
not,
it is
possible that
SO
forms
a
residual
atmosphere
on Io
away from
the
sunlight
side
and in the
polar
regions.
The
predicted
pressure
is of the
order
of a
nanobar
and may
serve
to
buffer
the
atmosphere
against supersonic winds
of SO2
that would
otherwise
be
blowing from
the
dayside
to the
nightside.
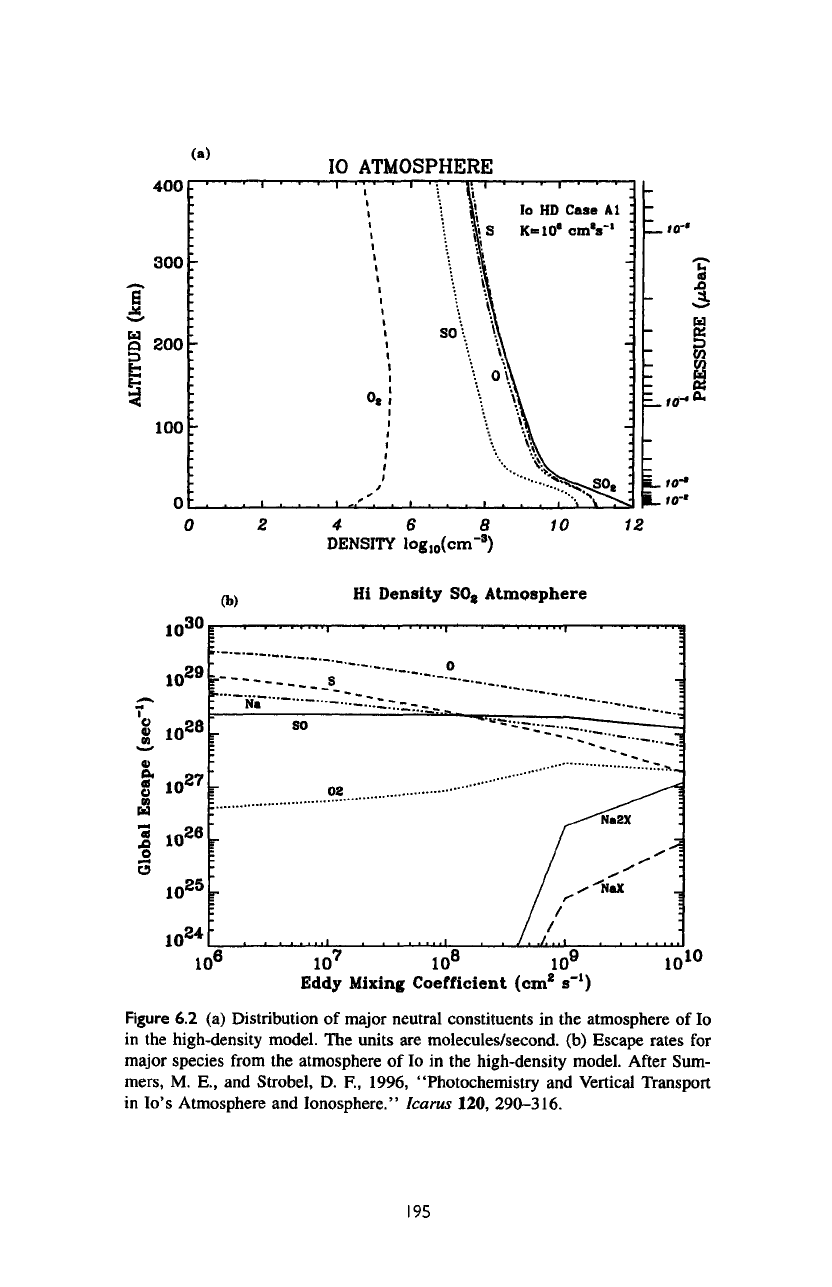
Figure
6.2 (a)
Distribution
of
major
neutral constituents
in the
atmosphere
of
lo
in
the
high-density model.
The
units
are
molecules/second,
(b)
Escape
rates
for
major
species
from
the
atmosphere
of
lo
in the
high-density model.
After
Sum-
mers,
M.
E.,
and
Strobel,
D. E,
1996, "Photochemistry
and
Vertical Transport
in
lo's
Atmosphere
and
Ionosphere."
Icarus 120,
290-316.
195
