Yung Y.L., DeMore W.B. Photochemistry of Planetary Atmospheres
Подождите немного. Документ загружается.

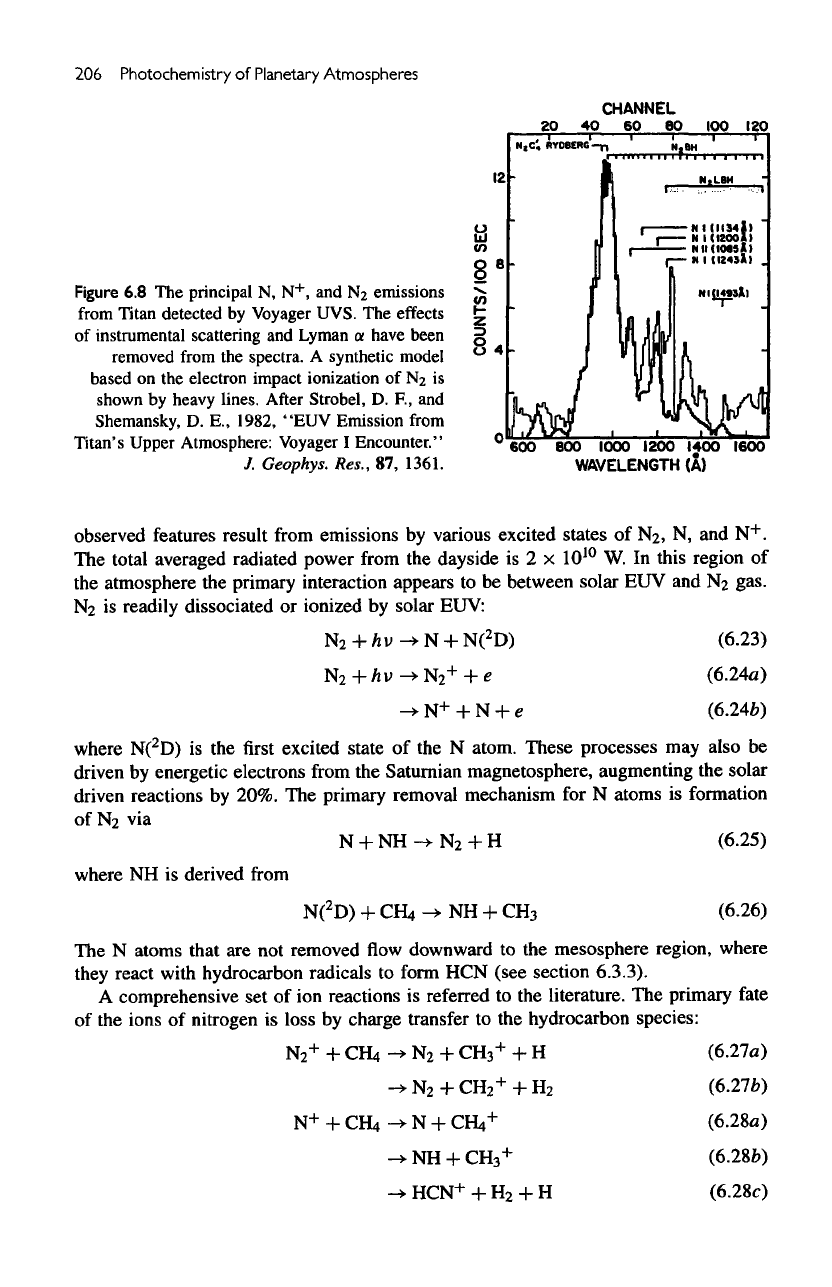
206
Photochem
istry
of
Planetary
Atmospheres
Figure
6.8 The
principal
N,
N
+
,
and
N
2
emissions
from
Titan detected
by
Voyager UVS.
The
effects
of
instrumental scattering
and
Lyman
a
have been
removed
from
the
spectra.
A
synthetic model
based
on the
electron impact ionization
of N2 is
shown
by
heavy
lines.
After
Strobel,
D. F., and
Shemansky,
D.
E.,
1982,
"EUV
Emission
from
Titan's Upper Atmosphere: Voyager
I
Encounter."
J.
Geophys.
Res.,
87,
1361.
observed features result
from
emissions
by
various excited states
of
N
2
,
N, and
N
+
.
The
total averaged radiated power
from
the
dayside
is 2 x
10
10
W. In
this region
of
the
atmosphere
the
primary interaction appears
to be
between solar
EUV and
N
2
gas.
N
2
is
readily dissociated
or
ionized
by
solar EUV:
where
N(
2
D)
is the first
excited state
of the N
atom. These
processes
may
also
be
driven
by
energetic electrons
from
the
Saturnian
magnetosphere, augmenting
the
solar
driven
reactions
by
20%.
The
primary removal mechanism
for N
atoms
is
formation
of
N
2
via
where
NH is
derived
from
The N
atoms that
are not
removed
flow
downward
to the
mesosphere region, where
they
react
with
hydrocarbon radicals
to
form
HCN
(see section
6.3.3).
A
comprehensive
set of ion
reactions
is
referred
to the
literature.
The
primary
fate
of
the
ions
of
nitrogen
is
loss
by
charge transfer
to the
hydrocarbon species:
Satellites
and
Pluto
207
More complex hydrocarbon ions
can be
formed,
as in the
following examples:
Further reactions lead
to the
production
of the
terminal
ion
H
2
CN
+
by
As
shown below,
H
2
CN
+
is the
most abundant
ion in the
ionosphere
of
Titan.
A
schematic diagram summarizing
the
principal pathways that result
in the
production
of
H
2
CN
+
,
including most
of the
above reactions,
is
given
in
figure
6.9a.
Complex
hydrocarbon ions
may be
produced
as
follows:
A
schematic diagram showing
the
principal pathways
for the
production
of
complex
hydrocarbon ions
is
given
in
figure
6.9b.
All
ions containing more than three car-
bon
atoms
are
labeled
"C
n
H
m
+
."
Note that charge transfer reactions
are
capable
of
producing interesting neutral
molecules
such
as
NH
3
by
although
the
amount that
can be
produced
is
insignificant.
The
ultimate
fate
of
H
2
CN
+
and the
hydrocarbon ions
is
loss
by
dissociative
recombination
in
reactions such
as
where
p + r = n and q + s =
m.
The
number densities
of the
major ions
in the
model
are
presented
figure
6.10a.
H
2
CN
+
is the
most
abundant ion, with peak concentration
in
excess
of
10
3
cm~
3
at
about
1200
km
above
the
surface.
The
second most abundant
ion
is the sum of
complex hydrocarbon ions containing more
than
three carbon atoms
(C
B
H
m
+
).
N
2
+
is a
minor
ion
even though
the
production rate
of
N
2
+
by
(6.24a)
and
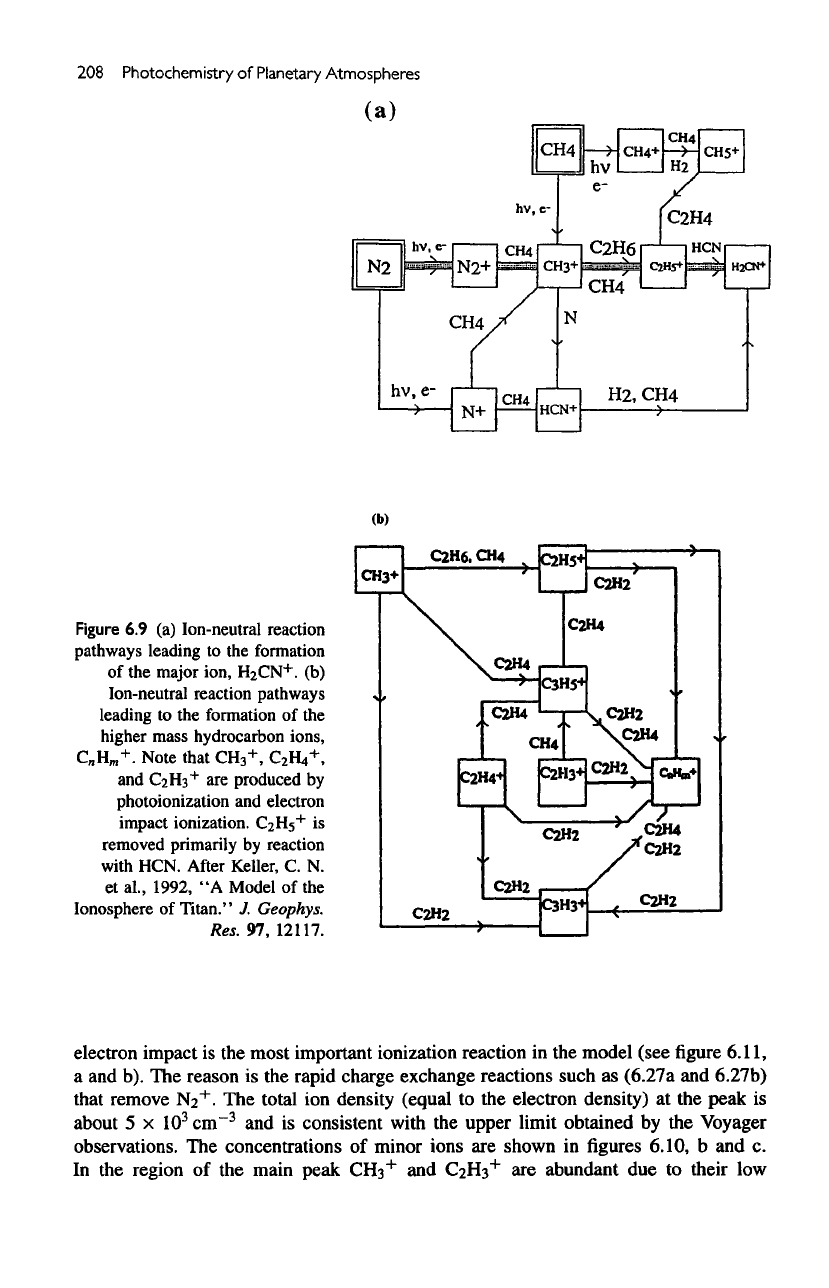
208
Photochemistry
of
Planetary Atmospheres
(a)
(b)
Figure
6.9 (a)
Ion-neutral reaction
pathways
leading
to the
formation
of
the
major
ion,
H
2
CN
+
.
(b)
Ion-neutral reaction pathways
leading
to the
formation
of the
higher
mass hydrocarbon ions,
C
n
H
m
+.
Note that
CH
3
+,
C
2
H4
+
,
and
CaH3
+
are
produced
by
photoionization
and
electron
impact ionization.
C2H5
+
is
removed primarily
by
reaction
with
HCN.
After
Keller,
C. N.
etal.,
1992,
"A
Model
of the
Ionosphere
of
Titan."
J.
Geophys.
Res.
97,
12117.
electron impact
is the
most important ionization reaction
in the
model (see
figure
6.11,
a and b). The
reason
is the
rapid charge exchange reactions such
as
(6.27a
and
6.27b)
that
remove
N2
+
.
The
total
ion
density (equal
to the
electron
density)
at the
peak
is
about
5 x
10
3
cm"
3
and is
consistent with
the
upper limit obtained
by the
Voyager
observations.
The
concentrations
of
minor ions
are
shown
in
figures
6.10,
b and c.
In
the
region
of the
main peak
CHj
+
and
C2^
+
are
abundant
due to
their
low
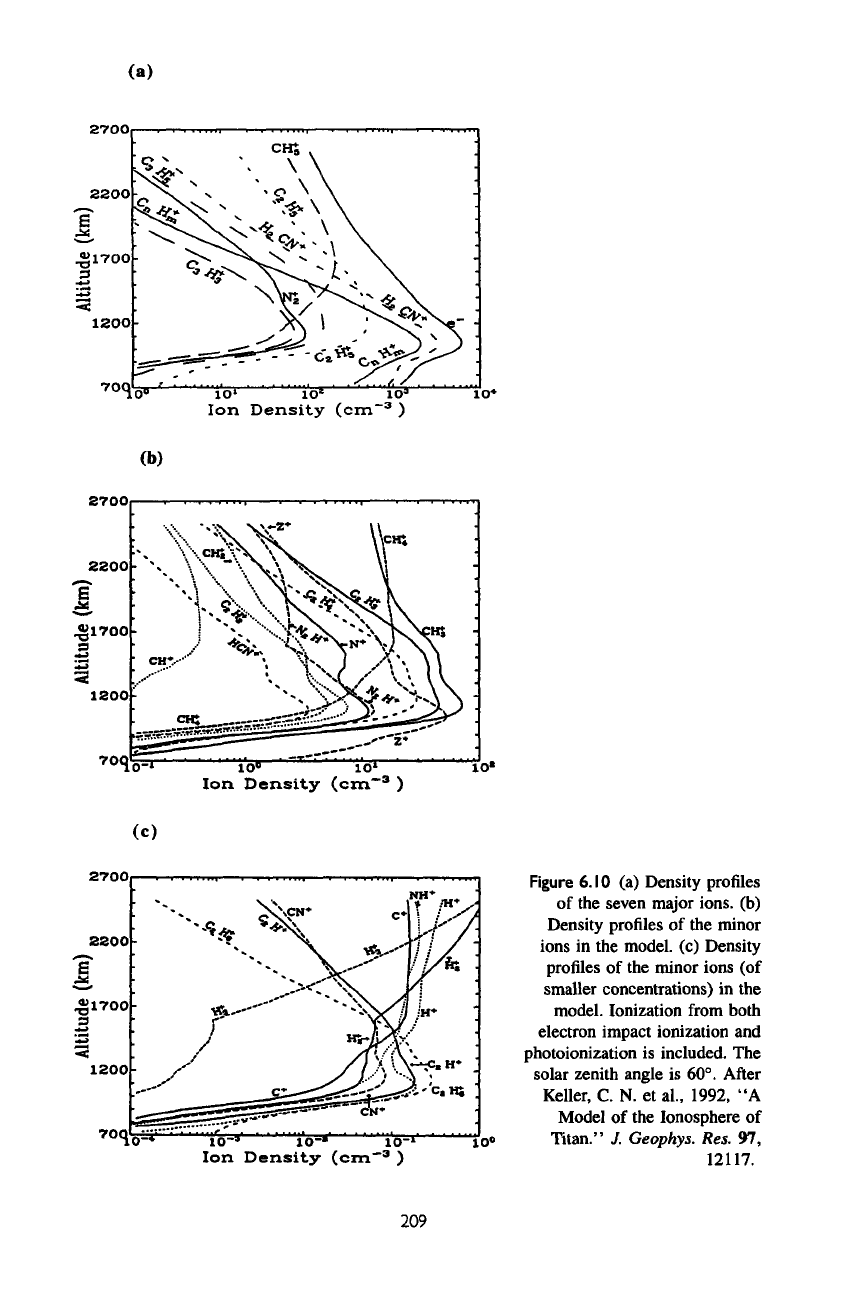
Figure
6.10
(a)
Density profiles
of
the
seven
major
ions,
(b)
Density
profiles
of the
minor
ions
in the
model,
(c)
Density
profiles
of the
minor ions
(of
smaller concentrations)
in the
model.
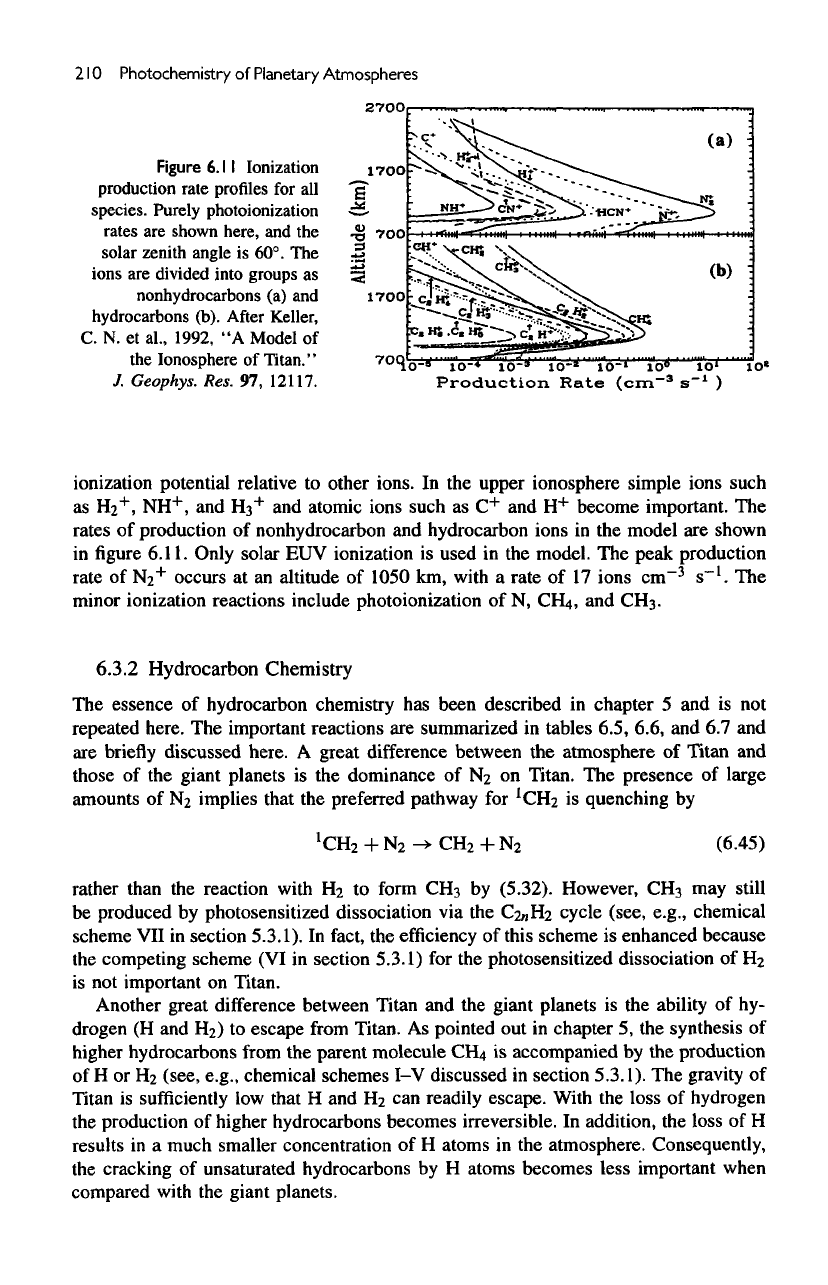
lonization
from
both
electron
impact ionization
and
photoionization
is
included.
The
solar zenith angle
is
60°.
After
Keller,
C. N. et
al.,
1992,
"A
Model
of the
Ionosphere
of
Titan."
J.
Geophys.
Res.
97,
12117.
209
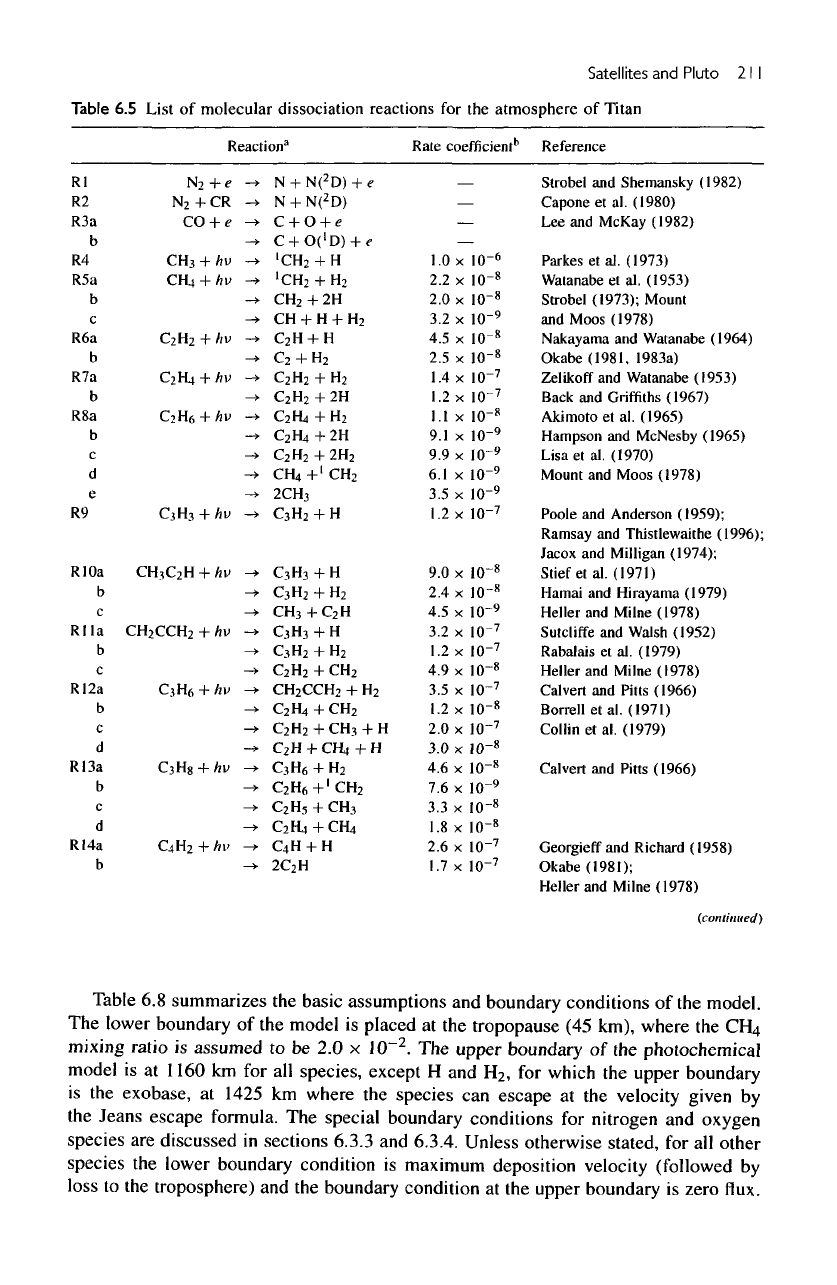
210
Photochemistry
of
Planetary
Atmospheres
Figure
6.11
lonization
production
rate
profiles
for all
species. Purely photoionization
rates
are
shown here,
and the
solar
zenith
angle
is
60°.
The
ions
are
divided into groups
as
nonhydrocarbons
(a) and
hydrocarbons
(b).
After
Keller,
C. N. et
al.,
1992,
"A
Model
of
the
Ionosphere
of
Titan."
J.
Geophys.
Res.
97,
12117.
ionization
potential relative
to
other ions.
In the
upper ionosphere simple ions such
as
H2
+
,
NH
+
,
and
Hj
+
and
atomic ions such
as
C
+
and
H
+
become
important.
The
rates
of
production
of
nonhydrocarbon
and
hydrocarbon ions
in the
model
are
shown
in
figure
6.11.
Only solar
EUV
ionization
is
used
in the
model.
The
peak production
rate
of
N2
+
occurs
at an
altitude
of
1050
km,
with
a
rate
of 17
ions
cm~
3
s~'.
The
minor
ionization reactions include photoionization
of N,
CH(,
and
6.3.2
Hydrocarbon
Chemistry
The
essence
of
hydrocarbon chemistry
has
been described
in
chapter
5 and is not
repeated
here.
The
important reactions
are
summarized
in
tables
6.5, 6.6,
and 6.7 and
are
briefly
discussed
here.
A
great
difference between
the
atmosphere
of
Titan
and
those
of the
giant planets
is the
dominance
of N2 on
Titan.
The
presence
of
large
amounts
of N2
implies that
the
preferred pathway
for
'CH2
is
quenching
by
rather than
the
reaction with
H
2
to
form
CHb
by
(5.32).
However,
CHs
may
still
be
produced
by
photosensitized dissociation
via the
C2«H2
cycle
(see, e.g.,
chemical
scheme
VII in
section
5.3.1).
In
fact,
the
efficiency
of
this scheme
is
enhanced because
the
competing
scheme
(VI in
section
5.3.1)
for the
photosensitized dissociation
of H2
is
not
important
on
Titan.
Another
great
difference
between Titan
and the
giant planets
is the
ability
of hy-
drogen
(H and H2) to
escape
from
Titan.
As
pointed
out in
chapter
5, the
synthesis
of
higher hydrocarbons from
the
parent
molecule
CH4 is
accompanied
by the
production
of
H or H2
(see, e.g.,
chemical schemes
I-V
discussed
in
section
5.3.1).
The
gravity
of
Titan
is
sufficiently
low
that
H and H2 can
readily
escape.
With
the
loss
of
hydrogen
the
production
of
higher hydrocarbons becomes irreversible.
In
addition,
the
loss
of H
results
in a
much smaller concentration
of H
atoms
in the
atmosphere. Consequently,
the
cracking
of
unsaturated hydrocarbons
by H
atoms becomes
less
important when
compared
with
the
giant planets.
Satellites
and
Pluto
21
I
Table
6.5
List
of
molecular
dissociation
reactions
for the
atmosphere
of
Titan
Rl
R2
R3a
b
R4
R5a
b
c
R6a
b
R7a
b
R8a
b
c
d
e
R9
RlOa
b
c
Rlla
b
c
R12a
b
c
d
R13a
b
c
d
R14a
b
Reaction
3
N,
+e
_>
N +
N(
2
D)
+
f
N
2
+ CR
->
N +
N(
2
D)
CO
+ e
->•
C +
O
+
e
->
C +
O('D)
+
?
CH
3
+ hv
-+
'CH
2
+ H
CHi
+
hv
->
'CH
2
+
H
2
->
CH
2
+ 2H
->
CH + H +
H
2
C
2
H
2
+
Au
-*
C
2
H
+ H
->
C
2
+
H
2
C
2
H4
+ hv
->
C
2
H
2
+
H
2
->
C
2
H
2
+ 2H
CjHe
+
Au
-*
C
2
H4
+
H
2
-»•
C
2
H4
+ 2H
->
C
2
H
2
+
2H
2
-»
CH
4
+'CH
2
->
2CH
3
C
3
H
3
+
/ii>
-»
C
3
H
2
+ H
CH
3
C
2
H
+ hv
-»
C
3
H
3
+ H
-»•
C
3
H
2
+
H
2
->
CH
3
+
C
2
H
CH
2
CCH
2
+ hv
->
C
3
H
3
+ H
->
C
3
H
2
+
H
2
->
C
2
H
2
+
CH
2
C
3
H
6
+
An
->
CH
2
CCH
2
+
H
2
->
C
2
H
4
+
CH
2
->
C
2
H
2
+
CH
3
+ H
->
C
2
H
+
CH
4
+ H
CjHt
+
hv
->•
C
3
H
6
+
H
2
->•
C
2
H
6
+'CH
2
->
C
2
H
5
+
CH
3
->•
C
2
H
4
+CH
4
C
4
H
2
+
AK
-f
C
4
H
+ H
->•
2C
2
H
Rate
coefficient
11
_
—
—
—
1.0
x
10~
6
2.2
x
10~
8
2.0 x
10~
8
3.2
x
10-
9
4.5 x
10-
8
2.5 x
10~
8
1.4
x
10~
7
1.2
x
10^
7
1.1
x
10~
8
9.1
x
10~
9
9.9 x
10^
9
6.1
x
10~
9
3.5 x
10~
9
1.2
x
10-
7
9.0 x
10~
8
2.4 x
10~
8
4.5 x
10~
9
3.2 x
1Q-
7
1.2
x
I0~
7
4.9 x
10~
8
3.5 x
10~
7
1.2
x
10~
8
2.0 x
10~
7
3.0 x
10~
8
4.6 x
10~
8
7.6
x
10~
9
3.3 x
10~
8
1.8
x
10~
8
2.6 x
10~
7
1.7
x
10~
7
Reference
Strobel
and
Shemansky
(1982)
Caponeet
al.
(1980)
Lee and
McKay
(1982)
Parkes
et al.
(1973)
Walanabeet
al.
(1953)
Strobel
(1973);
Mount
and
Moos
(1978)
Nakayama
and
Watanabe
(1964)
Okabe
(1981,
1983a)
Zelikoff
and
Watanabe
(1953)
Back
and
Griffiths
(1967)
Akimotoet
al.
(1965)
Hampson
and
McNesby
(1965)
Lisaet
al.
(1970)
Mount
and
Moos
(1978)
Poole
and
Anderson
(1959);
Ramsay
and
Thistlewaithe
(1996);
Jacox
and
Milligan
(1974);
Stief
et al.
(1971)
Hamai
and
Hirayama
(1979)
Heller
and
Milne
(1978)
Sutcliffe
and
Walsh
(1952)
Rabalais
et al.
(1979)
Heller
and
Milne
(1978)
Calvert
and
Pitts
(1966)
Borrell
et al.
(1971)
Collinetal.
(1979)
Calvert
and
Pitts
(1966)
Georgieff
and
Richard
(1958)
Okabe
(1981);
Heller
and
Milne
(1978)
(continued)
Table
6.8
summarizes
the
basic assumptions
and
boundary conditions
of the
model.
The
lower boundary
of the
model
is
placed
at the
tropopause
(45
km), where
the
Cfy
mixing
ratio
is
assumed
to be 2.0 x
10~
2
.
The
upper
boundary
of the
photochemical
model
is at
1160
km for all
species,
except
H and
H
2
,
for
which
the
upper boundary
is
the
exobase,
at
1425
km
where
the
species
can
escape
at the
velocity given
by
the
Jeans
escape
formula.
The
special boundary conditions
for
nitrogen
and
oxygen
species
are
discussed
in
sections 6.3.3
and
6.3.4.
Unless otherwise stated,
for all
other
species
the
lower boundary condition
is
maximum deposition velocity (followed
by
loss
to the
troposphere)
and the
boundary condition
at the
upper boundary
is
zero
flux.
212
Photochemistry
of
Planetary
Atmospheres
Table
6.5
(continued)
Reaction
3
R15a
b
R16a
b
R17
R18
R19
R20a
b
R21a
b
R22a
b
R23
R24
CsH
2
+
hv
C
8
H
2
+hv
HCN
+ hv
HC
3
N
+
/(v
C
2
N
2
+
hv
CO
2
+ hv
H
2
O
+
hv
H
2
CO
+ hv
HCO
+ hv
CH
2
CO
+ hv
-»•
C
6
H
+ H
-*
C
4
H
+
C
2
H
->•
C
6
H
+
C
2
H
->•
2C
4
H
->
H + CN
->
C
2
H
+ CN
->
2CN
->•
CO + O
-»
CO +
O('D)
->•
H + OH
->
H
2
+0('D)
->•
H
2
+ CO
->
H + HCO
-*•
H + CO
->
CH
2
+ CO
Rate
coefficient
b
2.6 x
10~
7
1.7
x
1(T
7
2.6
x
10~
7
1.7
x
10~
7
l.l
x
10~
7
4.0 x
10~
7
2.7
x
1(T
7
7.4
x
10-"
5.5 x
10~
9
5.1
x
10~
8
2.0 x
10^
8
2.5 x
10~
7
1.8
x
10~
7
1.0
x
10~
4
1.3
x
10~
6
Reference
Kloster-Jensen
et
al.
(1974)
estimated
Kloster-Jensen
et al.
(1974)
estimated
West
(1975);
Lee
(1980)
Connors
et al.
(1974)
Connors
et al.
(1974)
Nuth
and
Clicker
(1982)
Shemansky
(1972)
Demore
and
Patapoff
(1972)
Allen
et al.
(1981)
Pinto
et al.
(1980)
Pinto
et al.
(1980)
Okabe
(1978)
"Excited
atom
or
molecule:
N(
2
D),
O('D),
'CH
2
=
CH
2
(a
]
A
l
);
CR =
cosmic rays.
b
The
values
for the
diurnally
averaged dissociation
coefficient
refer
to the top of the
atmosphere
in
units
of
s~
l
(a)
C\ and
C
2
Species,
and
H
and H2
The
primary driving force
of the
photochemistry
of
hydrocarbons
in
Titan's atmo-
sphere
is
photolysis. Figure 6.12 presents
the
diurnally averaged dissociation coef-
ficients
for
the
important hydrocarbons
in the
model.
The
major absorber
at
short
wavelengths (below 1600
A) is CTLi.
Absorption
by
C2H2,
€2^4,
and HCN
becomes
important
at
longer wavelengths. Aerosol opacity provides
significant
attenuation
of
solar radiation below
240 km.
The
mixing
ratios
of
PL^
and the
major hydrocarbon
species
CH4,
C
2
H
2
,
C
2
H4,
and
C2H
0
are
given
in figure
6.13a.
The
number densities
of the
important radicals
H,
CH,
'CH
2
,
CH
2
,
CH
3
,
C
2
H
3
,
C
2
H
5
,
C
2
H,
C
4
H,
and
C
6
H
are
shown
in figure
6.13,
b and c. The
computed mixing ratio
at
1140
km is
8.1%,
in
good
agreement
with
the
value
8 ± 3%
deduced
from
Voyager
UVS
observations.
The
increase
of CH4
at
high altitudes
is due to
diffusive
separation above
the
homopause.
The
maximum
abundances
of all
photochemically produced
species
occur near
the
homopause region
in
the
mesosphere, where
CFU
dissociation
is
greatest.
The
most abundant radical
is
H,
with
peak
concentration
equal
to 5 x
10
8
cm""
3
.
Since
H
atoms
escape
from
Titan,
their
abundance
is an
order
of
magnitude less than that
in the
Jovian atmosphere.
The
consequence
of
this
for
photochemistry
on
Titan
is
discussed later
in
this section.
The
rapid
decrease
in the
concentrations
of the
higher hydrocarbons near
the
tropopause
is
due to
condensation. Table
6.9
gives
the
saturation mixing ratio
of
selected
species
in
the
model.
It is
clear that most
of the
heavier hydrocarbons would condense
and
be
removed from
the gas
phase.
The
radicals
CH
3
,
C2H
3
,
and
C
2
H
5
all
exhibit
a
sec-
ondary
maximum
at
about
200 km.
This
is
driven
by the
photosensitized dissociation
of CH4 by
photons
at
long wavelength (see discussion
in the
following paragraph).
Satellites
and
Pluto
213
Table
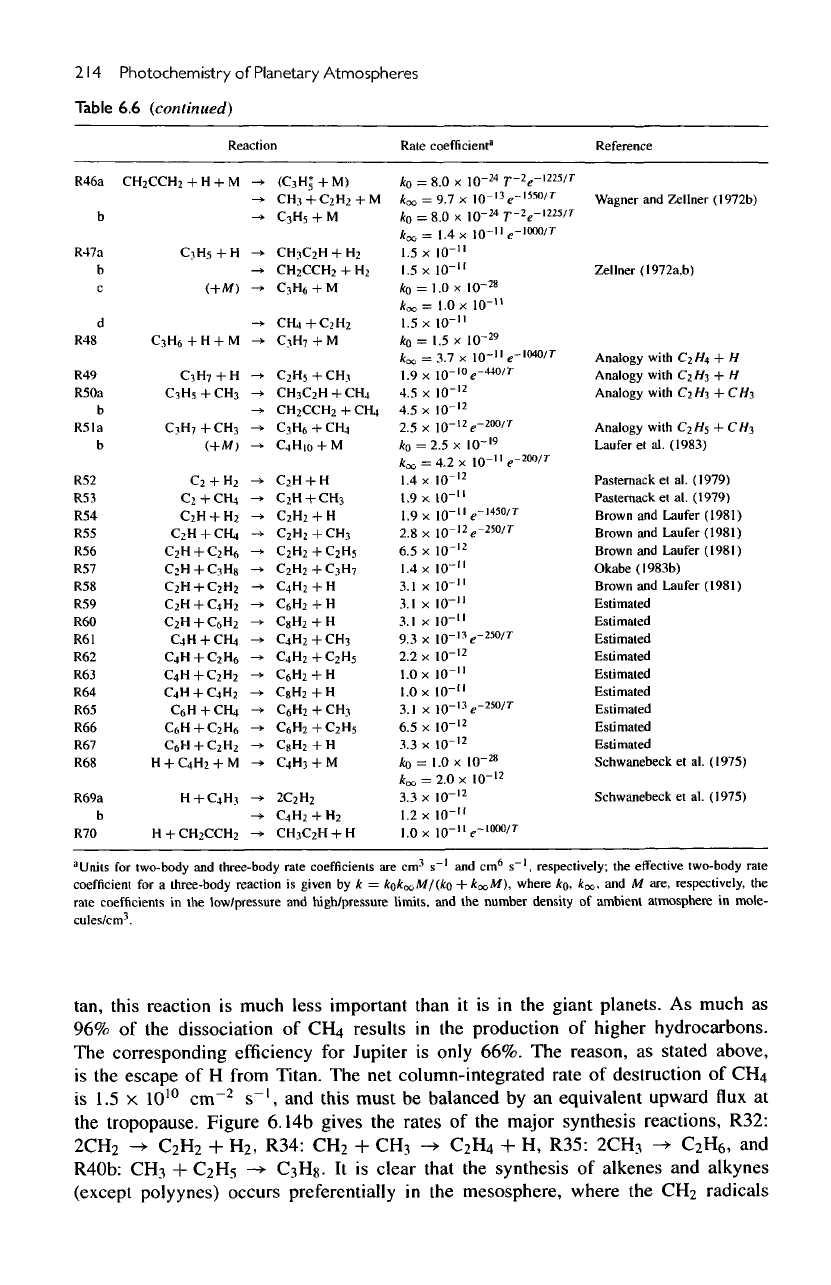
6.6
List
of
essential
hydrocarbon
reactions
for the
atmosphere
of
Titan
Reaction
R25
R26
R27
R28
R29a
b
R30a
b
R31a
b
R32
R33
R34
R35
R36
R37a
b
R38
R39
R40a
b
R4la
b
R42a
b
R43
R44a
b
R45a
b
H+C
2
H
2
+ M
H+C
2
H
4
+M
CH+CHj
'CH
2
+
N
2
'CH
2
+
H
2
1
CH
2
+CH
4
CH
2
+
C
2
H
2
+ M
2CH
2
CH
5
+ H + M
CH
3
+CH
2
2CH
3
+ M
C
2
H
3
+ H
C
2
H
3
+CH
3
(+M)
2C
2
H
3
C
2
Hi
+ H
C
2
H
5
+CH
3
(+ M)
C
2
H
5
+C
2
H
3
2C
2
H
5
(+ M)
C
3
H
2
+ H + M
C
3
H
3
+ H + M
CH
3
C
2
H
+ H +
M
-
_>
_>
—
*
-»•
—
>
-»
->
_>
->.
^
_>
->
_>
—
»
—
»
->
->
-»
->
->
_>
->
-»
_>
->
_>
->
—
>
->
->
C
2
H
3
+ M
C
2
H
5
+
M
C
2
H
4
+ H
CH
2
+
N
2
CH
2
+
H
2
CH
3
+ H
CH
2
+CH
4
2CH
3
CHjC
2
H
+
M
CH
2
CCH
2
+ M
C
2
H
2
+
H
2
CH
4
+ M
C
2
H
4
+ H
C
2
H
6
+ M
C
2
H
2
+
H
2
C
2
H
2
+
CH
4
C
3
H
6
+ M
C
2
H
4
+C
2
H
2
2CH
3
C
2
H
4
+CH
4
C
3
H
8
+M
C
2
H
6
+C
2
H
2
2C
2
Hj
C
2
H
6
+C
2
H
2
C
4
H
IO
+ M
C
3
H
3
+ M
CH
3
C
2
H
+
M
CH
2
CCH
2
+ M
(C
3
H^
+M)
CH,+C
2
H
2
+ M
C
3
H
5
+ M
Rate
coefficient
3
k
= 9 2 x
io-
|2
c~'
200
/
r
/t-x,
= 3.7 x
IO""
e
~
lata/T
l.Ox
l<r'
0
7.9 x
I0~'
2
l.Ox
IO-'
2
7.0 x
IQ-'
2
l.6x
I0~
l2
1
.9
x
I0-'
2
*o
= 3.8 x
IO-
25
/too
= 2.2 x
IO-'
2
/t
0
= 3.8 x
I0~
25
k.
x
= 3.7 x
IO-'
2
5.3
x
IO-"
/t
0
=
1.
7
x
I0~
27
*oo
=
l.5x
10-'°
7.0 x
IO-"
/to
=
1.
3
x
10~
23
^
=
5.5
x
10""
1.5
x
10-"
9.1
x
10~
12
/t
0
= 1.3 x
10~
22
/too
=9.1
x
10'
l2
5.3 x
I0~
12
1.9
x
IO-'
0
,?-
4
*"
7
'
1.7
x
I0-
|2
e-
200/r
to = 5.6 x
10~
22
k
— 4 2 x
10""
e~
200/rr
6.0 x
10~
12
3.0 x
10~
12
1.7
x
10-'
2
*-'
0
"'
/to
= 2.9 x
I0~
21
*«,
=
1.3
x
lO-"?-
95
'
7
/t
0
= 1.7 x
10"
26
/too
=
1-5
x
1<T
10
*o=
1.7 x
IO-
26
/too
= 1.5 x
10-'°
same
as
branch'
/to
=
8.0x
io-2
4
7-
2
f-
|225/:
A'oc,
= 9.7 x
io-
12
e
-
1550
'
7
'
same
as
branch
3
Reference
r
Payne
and
Stief
(
1976)
r
Michael
el al.
(1973);
Leeet
al.
(1978)
Butler
el al.
(1981)
Ashfold
el al.
(1980);
Laufer
(198
la)
Laufer
(198
la)
Laufer
et al.
(1983):
Laufer
(198
la)
Banyardet
al.
(1980)
Laufer
et al.
(1983);
Patrick
et al.
(1980)
Laufer
et
al.
(1981
a)
Laufer
et al.
(1983);
Callear
and
Metcalfe
(1976)
Keiletal.
(1976)
estimated
Laufer
et al.
(1983)
MacFadden
and
Currie
(1973)
Teng
and
Jones
(1972)
Teng
and
Jones
(1972)
Laufer
el al.
(1983)
Laufer
et al.
(1983)
Teng
and
Jones
(1972)
Laufer
et al.
(1983)
Laufer
el al.
(1983)
r
Whylock
et al.
(1976)
Wagner
and
Zellner
(1972a)
(continued)
A
schematic diagram showing
the
pathways
for the
interconversion
of
major
Ci
and
C
2
species
is
given
in
figure
6.13d.
The
destruction
of
CFU
is the
ultimate source
of all
higher hydrocarbons.
Fig-
ure
6.14a
presents
the
rates
of the
major reactions destroying
CFLi,
R5:
CH
4
+
hv,
R55:
CH4
+
C
2
H,
R61:
CH
4
+
C
4
H,
and
R65:
CH
4
+
C
6
H
(reaction
numbers refer
to
tables
6.5-6.7).
Direct photolysis
of
CH
4
is
important
in the
mesosphere; photo-
sensitized dissociation driven
by
C
2
Hb
and
polyynes
is
more important
in the
lower
stratosphere.
The
most important reaction
for
producing
CH
4
,
R33:
CH
3
+ H, is
also
included
in figure
6.14a.
Since there
are
fewer
H
atoms
in the
atmosphere
of Ti-
214
Photochemistry
of
Planetary Atmospheres
Table
6.6
(continued)
Reaction
R46a
CH
2
CCH
2
+ H + M
->
b
->
R47a
b
c
d
R48
R49
R50a
b
RSI
a
b
R52
R53
R54
R55
R56
R57
R58
R59
R60
R61
R62
R63
R64
R65
R66
R67
R68
R69a
b
R70
C
3
H
5
+ H
(+M)
C
3
H
6
+ H + M
C
3
H
7
+ H
C
3
H
5
+CH
3
C
3
H
7
+CH
3
(+M>
C
2
+
H
2
C
2
+
CH
4
C
2
H
+
H
2
C
2
H
+
CH4
C
2
H
+
C
2
H(,
C
2
H
+
C
3
Hg
C
2
H
+
C
2
H
2
C
2
H
+
C
4
H
2
C
2
H
+
C
6
H
2
C
4
H
+
CHi
C
4
H
+
C
2
H
6
C
4
H
+
C
2
H
2
C
4
H
+
C
4
H
2
CsH+CH,
C,,H
+
C
2
H
6
C
6
H
+
C
2
H
2
H
+
C
4
H
2
+
M
H
+
C
4
H,
H
+
CH
2
CCH
2
->
->
->
->
-»
->
->
->
-V
-V
->
->
->
->.
-»
_>
—
*
->
->
_>
-t
->
->
->
->
->
-»
—
*
_>
-»
(C
3
H;
+ M)
CH
3
+C
2
H
2
+ M
C
3
H
5
+
M
CH
3
C
2
H
+
H
2
CH
2
CCH
2
+
H
2
C
3
H
6
+ M
CU,+C
2
H
2
C
3
H
7
+ M
C
2
H
5
+CH
3
CH
3
C
2
H
+
CH
4
CH
2
CCH
2
+Crl4
C.,H
6
+
CH
4
C
4
H,
0
+ M
C
2
H
+ H
C
2
H
+
CH
3
C
2
H
2
+
H
C
2
H
2
+CH
3
C
2
H
2
+
C
2
H
5
C
2
H
2
+C
3
H
7
C
4
H
2
+ H
C
6
H
2
+ H
C
8
H
2
+ H
C
4
H
2
+
CH3
C
4
H
2
+C
2
H
5
C
6
H
2
+ H
C
8
H
2
+ H
C
6
H
2
+CH
3
Cf,H2+C
2
H
5
CgH
2
+H
C
4
H
3
+ M
2C
2
H
2
C
4
H
2
+
H
2
CH
3
C
2
H
+ H
Rate
coefficient"
*o
=
too
1.5
1.5
kQ
-
If
"CO
1.5
t
0
=
too
1.9
4.5
4.5
2.5
to =
too
1.4
1.9
1.9
2.8
6.5
1.4
3.1
3.1
3.1
9.3
2.2
1.0
1.0
3.1
6.5
3.3
to
=
£(Xi
3.3
1.2
1.0
-
=
x
x
=
x
=
X
X
X
X
=
x
X
X
X
X
X
X
X
X
X
X
X
X
x
x
X
—
X
X
X
8.0
x
10-"
7-
2
r-
|225
'
r
8 0 x
I0~
24
y-
2
e
-'
2
^
5
/^
1
4 x
10~"
e"
1000
/
7
"
10-"
10-"
1.0
x
10~
28
1.0
x
10-"
10-"
1.5
x
10-
29
3.7
x
10-"
e
-io«o/r
10-io
e
-4
4
o/r
io-'
2
lO^
12
,
0
-12
,.-200/7
2.5
x
IO-
19
4.2
x
\o-"
e
-xx»T
io-
12
10-"
,
0
-ll
e
-l450/7-
,0-12^-250/7
io-
12
10-"
10-"
10-"
10-"
,
0
-13,
-250/7
io-
12
io-"
10-"
lO-
13
^-
250
/
7
"
io-
12
io-
12
1.0 x
IO-
28
2.0 x
IQ-'
2
io-'
2
10-"
,
0
-ii
f
-iooo/r
Reference
Wagner
and
Zellner (I972b)
Zellner(l972a,b)
Analogy
with
C
2
Ha,
+ H
Analogy
with
C
2
W
3
+ H
Analogy
with
C
2
H
3
+
CW
3
Analogy
with
C
2
H$
+
CW
3
Laufer
et al.
(1983)
Pasternack
el al.
(1979)
Pasternack
et al.
(1979)
Brown
and
Laufer
(1981)
Brown
and
Laufer
(1981)
Brown
and
Laufer
(1981)
Okabe(l983b)
Brown
and
Laufer
(1981)
Estimated
Estimated
Estimated
Estimated
Estimated
Estimated
Estimated
Estimated
Estimated
Schwanebeck
et al.
(1975)
Schwanebeck
et al.
(1975)
"Units
for
two-body
and
three-body rate
coefficients
are
cm
3
s"'
and
cm
6
s
',
respectively;
the
effective two-body rate
coefficient
for a
three-body
reaction is
given
by t =
k
e
k
x
M/(ko
+
k
x
M),
where
t
0
,
too.
and M
are,
respectively,
the
rate
coefficients
in the
low/pressure
and
high/pressure
limits,
and the
number
density
of
ambient
atmosphere
in
mole-
cules/cm
5
.
tan,
this
reaction
is
much less important
than
it is in the
giant
planets.
As
much
as
96% of the
dissociation
of CH4
results
in the
production
of
higher
hydrocarbons.
The
corresponding
efficiency
for
Jupiter
is
only
66%.
The
reason,
as
stated above,
is
the
escape
of H
from
Titan.
The net
column-integrated rate
of
destruction
of
CH
4
is
1.5 x
10
10
cm~
2
s~',
and
this must
be
balanced
by an
equivalent upward
flux at
the
tropopause. Figure
6.14b
gives
the
rates
of the
major
synthesis reactions, R32:
2CH
2
->
C
2
H
2
+
H
2
,
R34:
CH
2
+
CH
3
->
C
2
H
4
+ H,
R35:
2CH
3
->
C
2
H
6
,
and
R40b:
CHs
+
C
2
H_«;
->•
CsHg.
It is
clear
that
the
synthesis
of
alkenes
and
alkynes
(except
polyynes)
occurs
preferentially
in the
mesosphere, where
the
CH
2
radicals
Satellites
and
Pluto
215
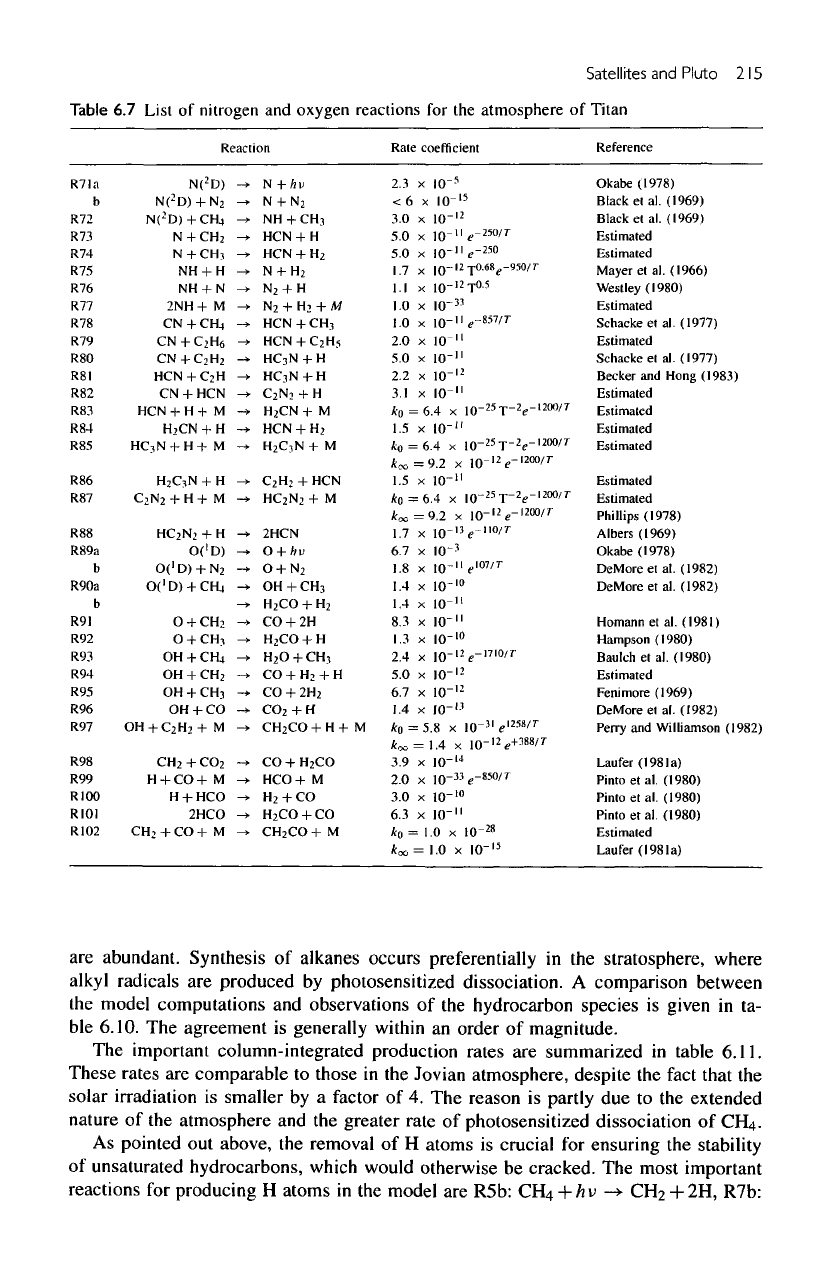
Table
6.7
List
of
nitrogen
and
oxygen
reactions
for the
atmosphere
of
Titan
Reaction
R71a
b
R72
R73
R74
R75
R76
R77
R78
R79
R80
R8I
R82
R83
R84
R85
R86
R87
R88
R89a
b
R90a
b
R9I
R92
R93
R94
R95
R96
R97
R98
R99
RIOO
RI01
R102
N(
2
D)
N(
2
D)
+
N
2
N(
2
D)+CH
4
N+CH
2
N+CHi
NH
+ H
NH
+ N
2NH+
M
CN
+
CK,
CN
+
C
2
H
6
CN
+
C
2
H
2
HCN+C
2
H
CN
+ HCN
HCN
+ H + M
H,CN
+ H
HC
3
N
+ H+ M
H
2
C
3
N
+ H
C
2
N
2
+ H + M
HC
2
N
2
+ H
0('D)
0('D)
+
N
2
0('D)
+
CH
4
O
+
CH
2
0 +
CHi
OH
+
CH
4
OH
+
CH
2
OH
+
CHi
OH
+ CO
OH
+
C
2
H
2
+ M
CH
2
+C0
2
H
+ CO + M
H
+ HCO
2HCO
CH
2
+ CO + M
_^
->
_>
_»
->
->
_>
_>
->
_>.
_*
->
->
->
_».
->
->
_»
->
_>
_>
—
*
->
_>
->
—
>
->
-*
-*
—
>
->
->
-»
—
>
->
N
+AV
N
+N
2
NH
+
CH
3
HCN
+ H
HCN
+
H
2
N
+
H
2
N
2
+ H
N
2
+
H
2
+ M
HCN
+
CH
3
HCN
+
C
2
H
5
HC
3
N
+ H
HC
3
N
+ H
C
2
N
2
+ H
H
2
CN
+ M
HCN
+
H
2
H
2
C
3
N+
M
C
2
H
2
+
HCN
HC
2
N
2
+ M
2HCN
O
+
hv
0 +
N
2
OH
+
CH
3
H
2
CO
+
H
2
CO
+ 2H
H
2
CO
+ H
H
2
0
+
CHj
CO
+
H
2
+ H
CO
+
2H
2
C0
2
+ H
CH
2
CO
+
H+
M
CO
+
H
2
CO
HCO+
M
H
2
+ CO
H
2
CO
+ CO
CH
2
CO+
M
Rale
coefficient
2.3 x
10~
5
< 6 x
I0~
15
3.0 x
10-'
2
5.0 x
lO-"*'-
250
'
7
5.0 x
10""
i?"
2
-''
0
1.7
x
|o-
|2
T°-
68
<?-
950
'
r
1.1
x
10~
12
T
05
1.0
x
ID"
33
1.0
x
10-"*-
857
/
7
'
2.0
x
10-"
5.0
x
10-"
2.2 x
1Q-'
2
3.1
x
10-"
to
= 6.4 x
lo-^T-
2
?-'™/
7
1.5
x
10-
"
t
0
= 6.4 x
10-
25
T-
2
?-
1200
/
7
tno
= 9.2 x
io-
|2
f-
|200
/
r
1.5
x
10-"
to
=6.4
x
K
r
25
T-
2
<r
1200
'
7
'
toe
=9.2
x
io-'
2
e
-
|2
<W
r
1.7
x
\o~"e'"°'
T
6.7
x
10"'
1.8
x
10-"
e
lol/T
1.4
x
10-
I0
1.4
x
10""
8.3
x
10-
"
1.3
x
10-
10
2.4
x
10-'
2
e
-"
lo/r
5.0 x
10-'
2
6.7 x
10-'
2
1.4
x
10-'-
1
to = 5.8 x
IO--
11
e
l258/r
*«,
=
!.
4
x
io-<2
e
+m/T
3.9
x
10-'
4
2.0 x
io-33
e
-«so/r
3.0 x
10-'°
6.3
x
10-"
t
0
= 1.0 x
10~
28
too
= 1.0 X
IO-'
5
Reference
Okabe
(1978)
Black
el al.
(1969)
Black
el al.
(1969)
Estimated
Estimated
Mayer
el al.
(1966)
Weslley(l980)
Estimated
Schackeetal.
(1977)
Estimated
Schackeel
al.
(1977)
Becker
and
Hong (1983)
Estimated
Estimated
Estimated
Estimated
Estimated
Estimated
Phillips
(1978)
Albers
(1969)
Okabe
(1978)
DeMore
et al.
(1982)
DeMore
et al.
(1982)
Homann
et al.
(1981)
Hampson
(1980)
Baulch
elal.
(1980)
Estimated
Fenimore
(1969)
DeMore
el al.
(1982)
Perry
and
Williamson (1982)
Laufer
(198
la)
Pinto
et al.
(1980)
Pinto
et al.
(1980)
Pinto
et
al.
(1980)
Estimated
Laufer
(198
la)
are
abundant. Synthesis
of
alkanes occurs preferentially
in the
stratosphere, where
alkyl
radicals
are
produced
by
photosensitized dissociation.
A
comparison between
the
model computations
and
observations
of the
hydrocarbon
species
is
given
in ta-
ble
6.10.
The
agreement
is
generally
within
an
order
of
magnitude.
The
important column-integrated production rates
are
summarized
in
table
6.11.
These
rates
are
comparable
to
those
in the
Jovian atmosphere, despite
the
fact
that
the
solar irradiation
is
smaller
by a
factor
of 4. The
reason
is
partly
due to the
extended
nature
of the
atmosphere
and the
greater rate
of
photosensitized dissociation
of
CH
4
.
As
pointed
out
above,
the
removal
of H
atoms
is
crucial
for
ensuring
the
stability
of
unsaturated hydrocarbons,
which
would otherwise
be
cracked.
The
most important
reactions
for
producing
H
atoms
in the
model
are
R5b:
CH
4
+ hv
->•
CH
2
+2H,
R7b:
