Yung Y.L., DeMore W.B. Photochemistry of Planetary Atmospheres
Подождите немного. Документ загружается.

226
Photochemistry
of
Planetary
Atmospheres
The
most likely fate
of CN is to
react
with
CH
4
,
restoring HCN:
The net
result
of
reactions
(6.50)
and
(6.5
la) is
CH
4
-»•
CH
3
+H.
Thus,
the
photolysis
of HCN
results
in the
photosensitized dissociation
of
CH
4
.
This accounts
for the
remarkable
stability
of HCN in a
reducing atmosphere.
There
may be a
small branch
for
the
reaction between
CN and
CH
4
to
proceed
by the
insertion
of CN
into
CH
4
:
The
ratio
of the
rate
coefficient
for
(6.5Ib)
to
that
of
(6.5la)
is
less
than
0.005,
but
the
reaction
may be a
significant
source
of
methyl cyanide.
An
alternative source
of
CH
3
CN
is the
recombination reaction:
A
number
of
more complex
nitrile
compounds
can be
produced
from
reactions between
HCN,
CN, and the
hydrocarbons:
The
rate coefficient
for
(6.53)
is
slower than expected
for
this type
of
reaction.
To
account
for the
observed abundance
of
HC
3
N,
another scheme
has
been
proposed:
where
the
excited atom
N(
2
D)
is
derived from energetic
processes
in the
thermosphere.
Most
of the
above speculative chemistry
has not
been quantitatively studied
in the
laboratory.
The
boundary conditions
for the
model
are
summarized
in
table 6.8.
In
addition
to
the
source
of odd
nitrogen from
the
thermosphere, absorption
of
cosmic
rays
by
N
2
in
the
lower stratosphere
is
also
a
source
of odd
nitrogen.
The
model includes both types
of
nitrogen sources. Figure
6.17a
summarizes
the
altitude profiles
of the
important
nitrile
compounds HCN,
HC
3
N,
and
C
2
N
2
.
The
principal source
of
nitrile compounds
is
the
upper atmosphere.
The
primary sink
is
condensation
at the
tropopause (see
ta-
ble
6.9). Since
the
abundances
of the
nitrile
compounds
are
quite high
at
high altitudes
and
these compounds
all
have very
low
saturation vapor pressure,
it is
possible that
they
could
be the
source
of the
photochemical
aerosol
layer observed
by the
Voyager
UVS.
However,
no
quantitative modeling
has
been carried
out to
verify
this possi-
bility.
The
concentrations
of the odd
nitrogen radicals
N, NH, and CN are
shown
in
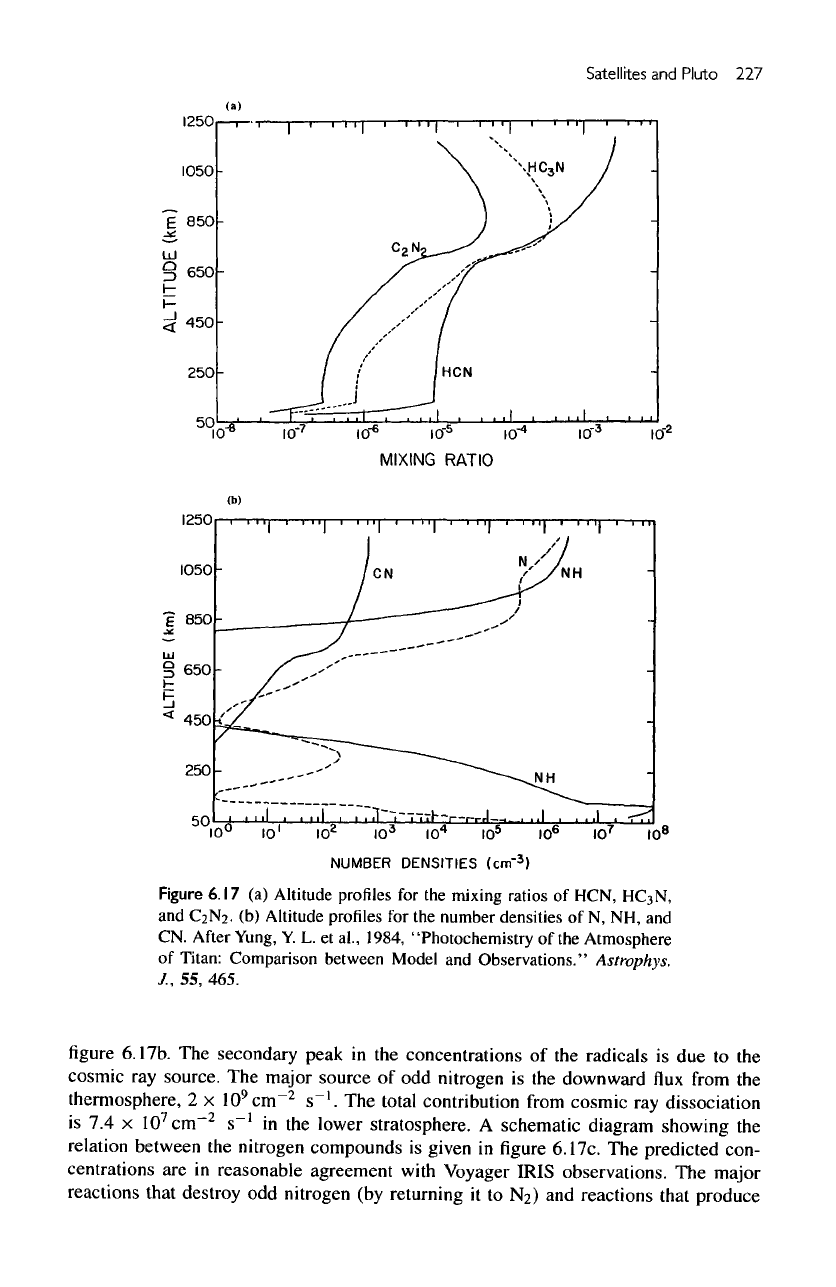
Satellites
and
Pluto
227
Figure
6.17
(a)
Altitude
profiles
for the
mixing
ratios
of
HCN,
HC
3
N,
and
C2N2.
(b)
Altitude
profiles
for the
number
densities
of N, NH, and
CM.
After
Yung,
Y. L. et
al.,
1984,
"Photochemistry
of the
Atmosphere
of
Titan:
Comparison
between
Model
and
Observations."
Astrophys.
J.,
55,
465.
figure
6.17b.
The
secondary peak
in the
concentrations
of the
radicals
is due to the
cosmic
ray
source.
The
major source
of odd
nitrogen
is the
downward
flux
from
the
thermosphere,
2 x
10
9
cm~
2
s~'.
The
total contribution
from
cosmic
ray
dissociation
is
7.4 x
10
7
cm~
2
s~'
in the
lower stratosphere.
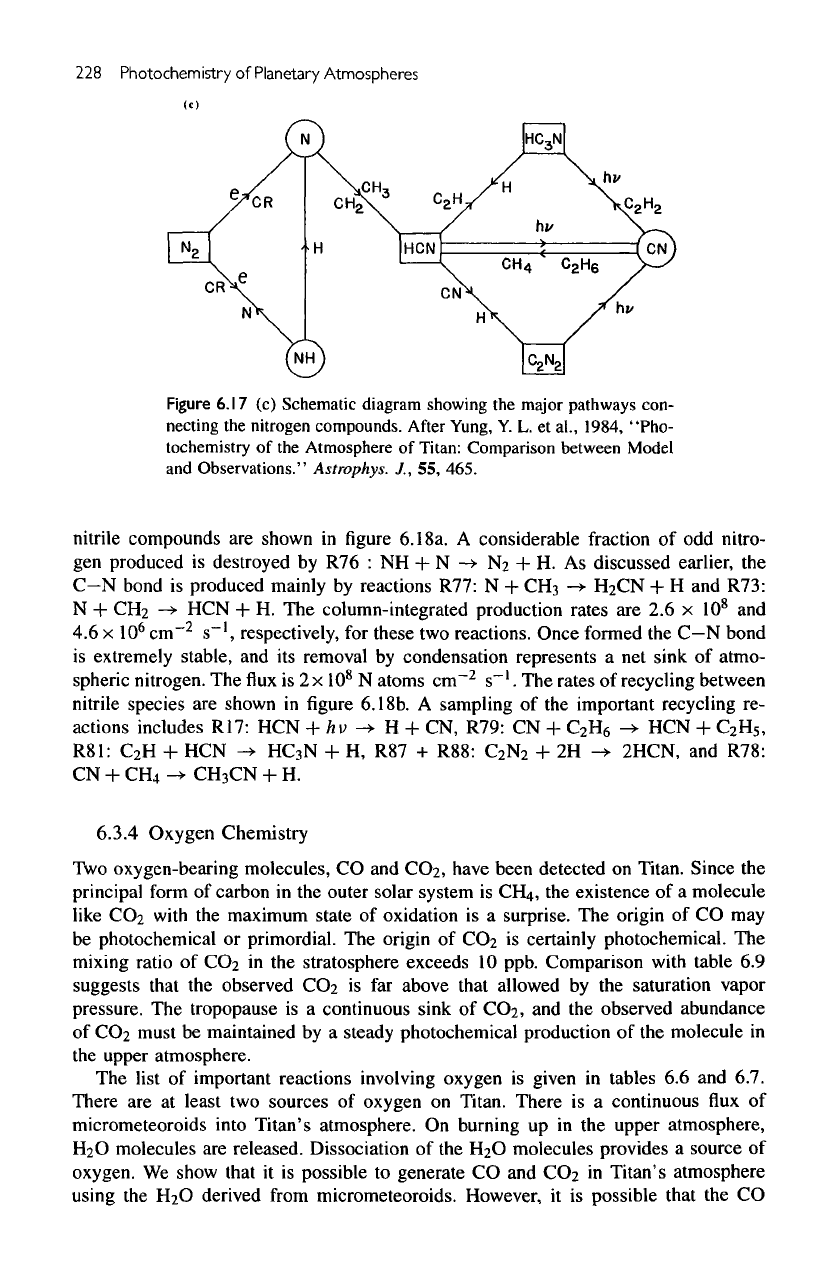
A
schematic diagram showing
the
relation
between
the
nitrogen compounds
is
given
in
figure
6.17c.
The
predicted
con-
centrations
are in
reasonable agreement
with
Voyager IRIS observations.
The
major
reactions that destroy
odd
nitrogen
(by
returning
it to
N
2
)
and
reactions
that
produce
228
Photochemistry
of
Planetary
Atmospheres
Figure
6.17
(c)
Schematic diagram showing
the
major pathways con-
necting
the
nitrogen compounds.
After
Yung,
Y. L. et
al.,
1984, "Pho-
tochemistry
of the
Atmosphere
of
Titan: Comparison between
Model
and
Observations."
Astmphys.
J., 55,
465.
nitrile
compounds
are
shown
in figure
6.18a.
A
considerable fraction
of odd
nitro-
gen
produced
is
destroyed
by R76 : NH + N
-»
N2 + H. As
discussed earlier,
the
C-N
bond
is
produced mainly
by
reactions R77:
N +
CH
3
->
H
2
CN
+ H and
R73:
N
+ CH2
->
HCN + H. The
column-integrated production rates
are 2.6 x
10
8
and
4.6 x
10
6
cm"
2
s~',
respectively,
for
these
two
reactions. Once formed
the
C—N
bond
is
extremely stable,
and its
removal
by
condensation represents
a net
sink
of
atmo-
spheric
nitrogen.
The flux is 2x
10
8
N
atoms
cm~
2
s~'.
The
rates
of
recycling between
nitrile
species
are
shown
in figure
6.18b.
A
sampling
of the
important recycling
re-
actions includes R17:
HCN + h v
-»
H + CN,
R79:
CN +
C
2
H
6
->•
HCN +
C
2
H
5
,
R81:
C
2
H
+ HCN
->
HC
3
N
+ H, R87 +
R88:
C
2
N
2
+ 2H
->
2HCN,
and
R78:
CN
+
CH
4
-»
CH
3
CN
+ H.
6.3.4 Oxygen Chemistry
Two
oxygen-bearing molecules,
CO and
CO2, have been detected
on
Titan. Since
the
principal
form
of
carbon
in the
outer solar system
is
CH4,
the
existence
of a
molecule
like
CO2
with
the
maximum state
of
oxidation
is a
surprise.
The
origin
of CO may
be
photochemical
or
primordial.
The
origin
of CO2 is
certainly photochemical.
The
mixing
ratio
of
CO
2
in the
stratosphere exceeds
10
ppb. Comparison
with
table
6.9
suggests
that
the
observed
CO
2
is far
above that allowed
by the
saturation vapor
pressure.
The
tropopause
is a
continuous sink
of
CO2,
and the
observed abundance
of
CO
2
must
be
maintained
by a
steady photochemical production
of the
molecule
in
the
upper atmosphere.
The
list
of
important reactions involving oxygen
is
given
in
tables
6.6 and
6.7.
There
are at
least
two
sources
of
oxygen
on
Titan.
There
is a
continuous
flux of
micrometeoroids into Titan's atmosphere.
On
burning
up in the
upper atmosphere,
H2O
molecules
are
released. Dissociation
of the H2O
molecules provides
a
source
of
oxygen.
We
show
that
it is
possible
to
generate
CO and CO2 in
Titan's atmosphere
using
the H2O
derived from
micrometeoroids.
However,
it is
possible that
the CO
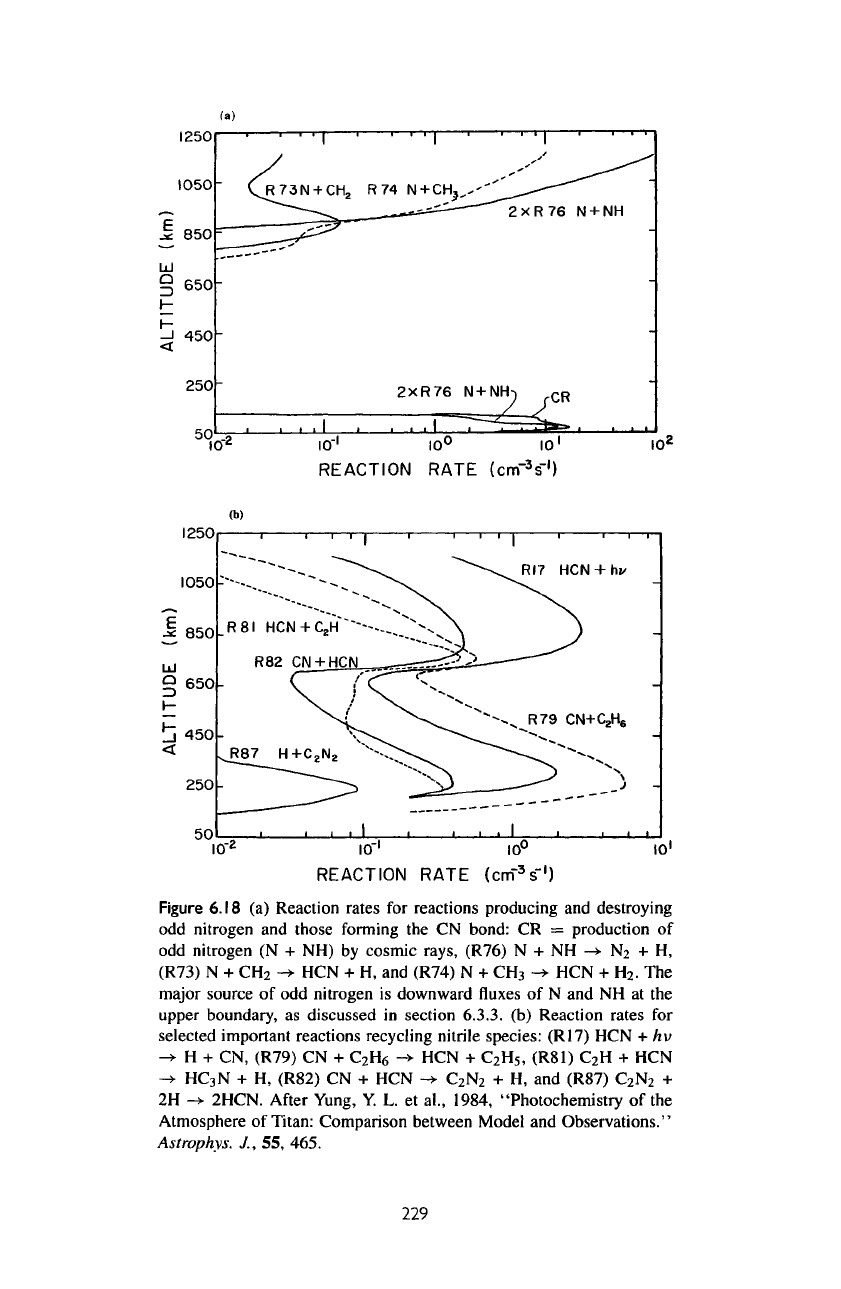
Figure
6.18
(a)
Reaction rates
for
reactions producing
and
destroying
odd
nitrogen
and
those
forming
the CN
bond:
CR =
production
of
odd
nitrogen
(N + NH) by
cosmic
rays, (R76)
N + NH
->
N
2
+ H,
(R73)
N +
CH
2
->•
HCN + H, and
(R74)
N +
CH
3
-»
HCN +
H
2
.
The
major
source
of odd
nitrogen
is
downward
fluxes of N and NH at the
upper
boundary,
as
discussed
in
section
6.3.3.
(b)
Reaction rates
for
selected important reactions recycling
nitrile
species:
(R17)
HCN +
hv
-»
H + CN,
(R79)
CN +
C
2
H
6
->•
HCN +
C
2
H
5
,
(R81)
C
2
H
+ HCN
-»•
HC
3
N
+ H,
(R82)
CN + HCN
-»•
C
2
N
2
+ H, and
(R87)
C
2
N
2
+
2H
->
2HCN.
After
Yung,
Y. L. et
al.,
1984, "Photochemistry
of the
Atmosphere
of
Titan: Comparison between Model
and
Observations."
Astrophys.
J.,
55,
465.
229
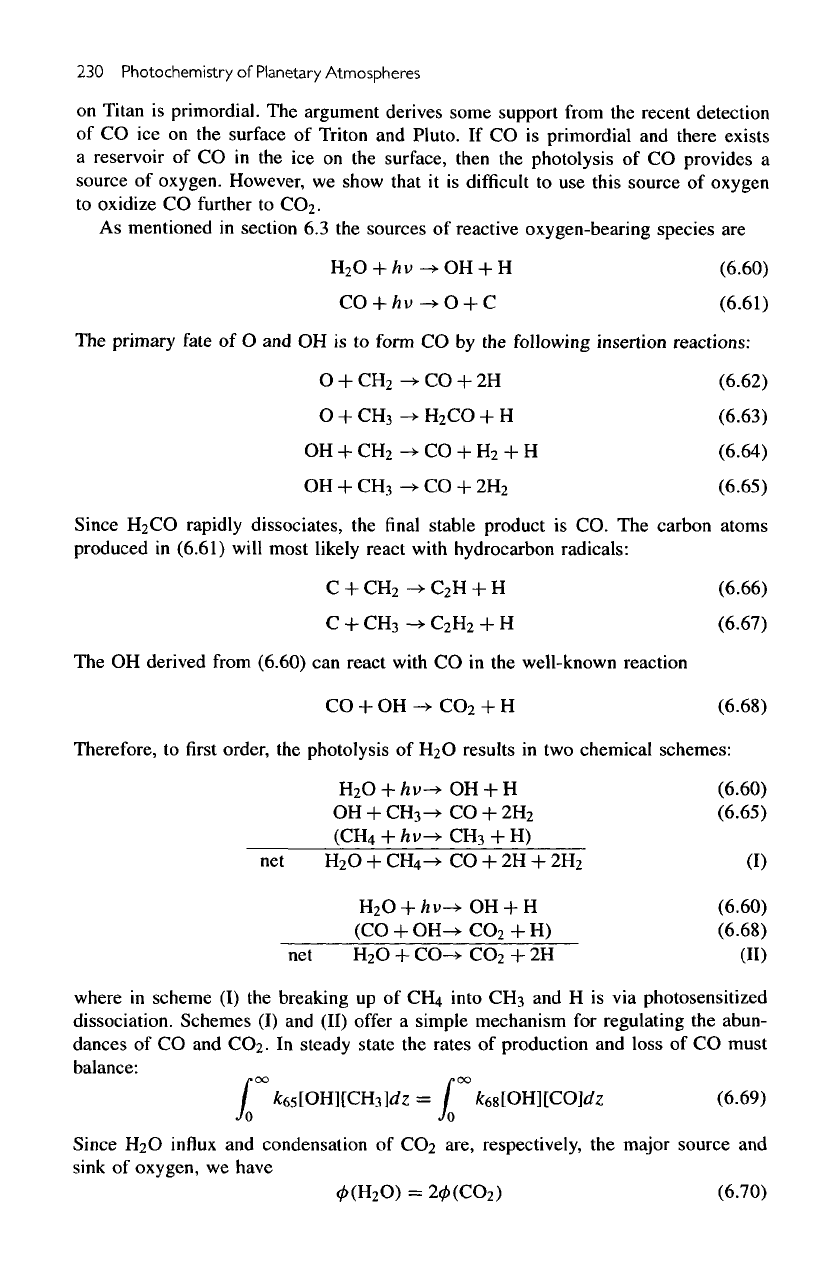
230
Photochemistry
of
Planetary
Atmospheres
on
Titan
is
primordial.
The
argument derives some support from
the
recent detection
of
CO ice on the
surface
of
Triton
and
Pluto.
If CO is
primordial
and
there exists
a
reservoir
of CO in the ice on the
surface,
then
the
photolysis
of CO
provides
a
source
of
oxygen. However,
we
show
that
it is
difficult
to use
this source
of
oxygen
to
oxidize
CO
further
to
CO2.
As
mentioned
in
section
6.3 the
sources
of
reactive oxygen-bearing
species
are
The
primary fate
of O and OH is to
form
CO by the
following insertion
reactions:
Since H2CO rapidly dissociates,
the final
stable product
is CO. The
carbon atoms
produced
in
(6.61)
will most
likely
react with hydrocarbon radicals:
The OH
derived
from
(6.60)
can
react
with
CO in the
well-known reaction
Therefore,
to first
order,
the
photolysis
of
H
2
O
results
in two
chemical
schemes:
where
in
scheme
(I) the
breaking
up of
CFLt
into
CH
3
and H is via
photosensitized
dissociation. Schemes
(I) and
(II)
offer
a
simple mechanism
for
regulating
the
abun-
dances
of CO and
CO
2
.
In
steady state
the
rates
of
production
and
loss
of CO
must
balance:
Since
H
2
O
influx
and
condensation
of
CO
2
are, respectively,
the
major source
and
sink
of
oxygen,
we
have
Satellites
and
Pluto
231
Figure
6.19
(a)
Altitude profiles
for the
mixing ratios
of CO,
CO2,
H
2
O,
H
2
CO,
and
CH
2
CO.
(b)
Altitude profiles
for the
number
den-
sities
of O,
O('D),
OH, and
HCO.
After Yung,
Y. L. et
al.,
1984,
"Photochemistry
of the
Atmosphere
of
Titan:
Comparison
between
Model
and
Observations."
Astrophys.
J., 55,
465.
where
0(H
2
O)
is the
meteoritic
H
2
O
flux and
0(CO
2
)
is the
downward
flux of
CO
2
into
the
troposphere.
Altitude
profiles
for the
mixing ratios
of CO,
CO
2
,
H
2
O,
H
2
CO,
and
CH
2
CO
and
the
number densities
of
O('D),
O, OH, and HCO are
presented
in figure
6.19,
a and b,
respectively.
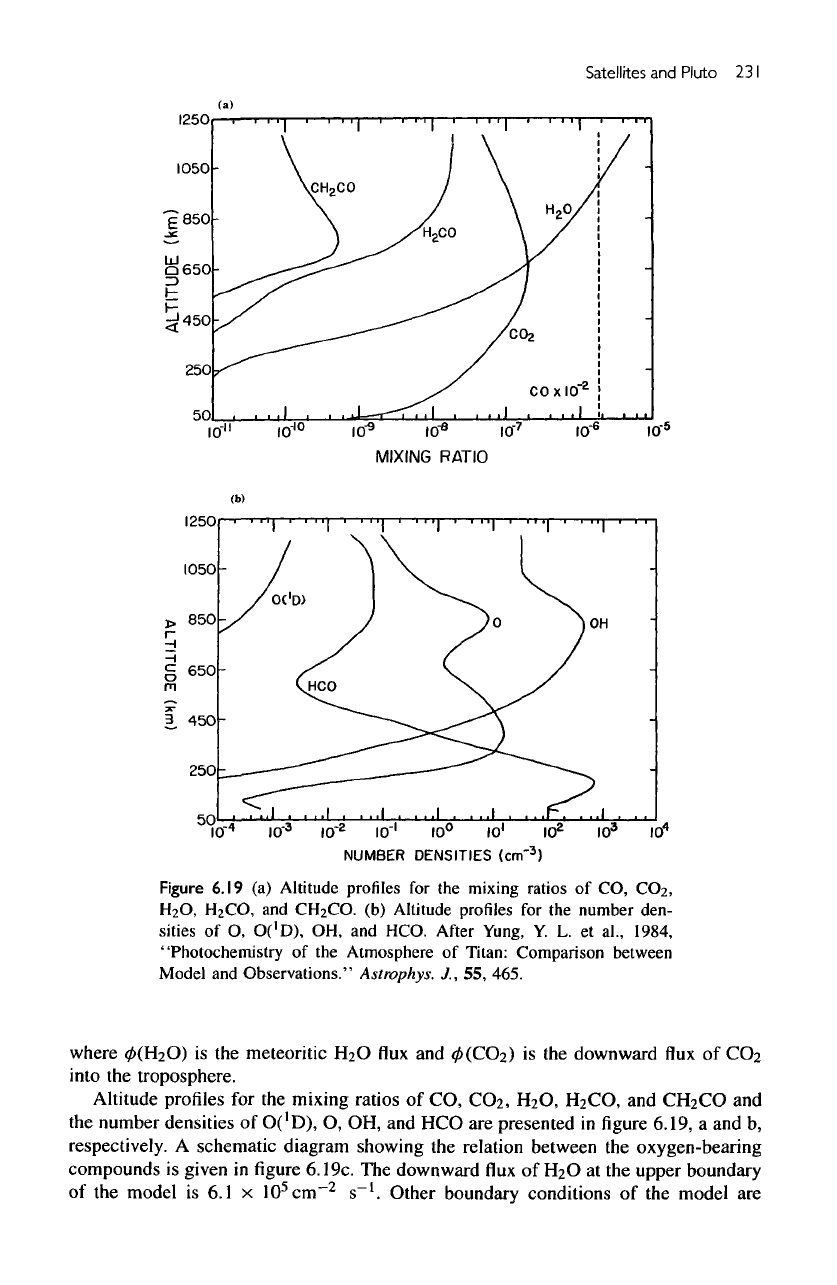
A
schematic diagram showing
the
relation between
the
oxygen-bearing
compounds
is
given
in figure
6.19c.
The
downward
flux of
H
2
O
at the
upper boundary
of
the
model
is 6.1 x
10
5
cm~
2
s"
1
.
Other boundary conditions
of the
model
are
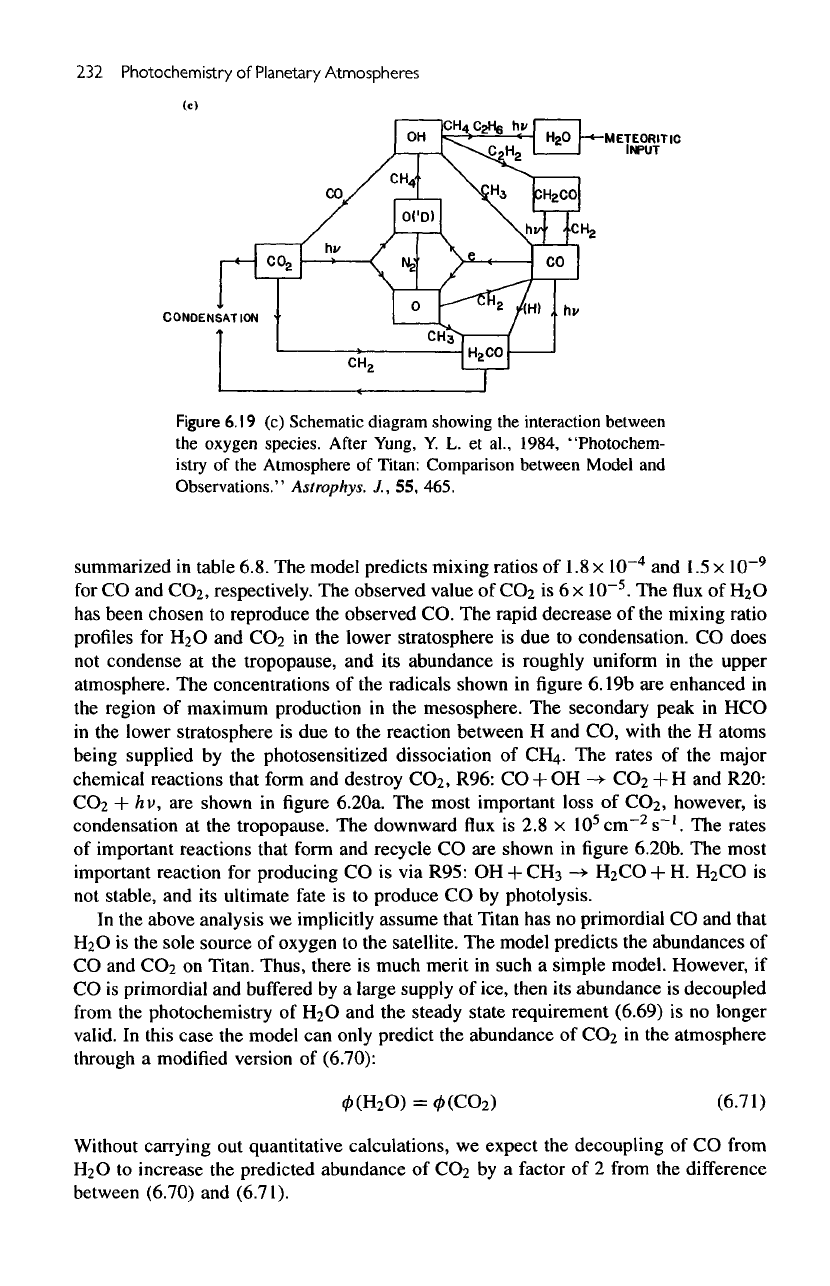
232
Photochemistry
of
Planetary
Atmospheres
Figure
6.19
(c)
Schematic diagram showing
the
interaction between
the
oxygen species.
After
Yung,
Y. L. et
al.,
1984,
"Photochem-
istry
of the
Atmosphere
of
Titan: Comparison between Model
and
Observations."
Astrophys.
J., 55,
465.
summarized
in
table 6.8.
The
model predicts mixing ratios
of
1.8
x 10
4
and
1.5
x 10
9
for
CO and
CO
2
,
respectively.
The
observed value
of
CO
2
is 6 x
10~
5
.
The flux of
H
2
O
has
been chosen
to
reproduce
the
observed
CO. The
rapid
decrease
of the
mixing ratio
profiles
for H2O and
CO
2
in the
lower stratosphere
is due to
condensation.
CO
does
not
condense
at the
tropopause,
and its
abundance
is
roughly uniform
in the
upper
atmosphere.
The
concentrations
of the
radicals shown
in
figure
6.19b
are
enhanced
in
the
region
of
maximum production
in the
mesosphere.
The
secondary peak
in HCO
in
the
lower stratosphere
is due to the
reaction between
H and CO,
with
the H
atoms
being
supplied
by the
photosensitized dissociation
of
CH4.
The
rates
of the
major
chemical reactions that form
and
destroy
CO
2
,
R96:
CO + OH
-*
CO
2
+ H and
R20:
CO
2
+ hv, are
shown
in
figure
6.20a.
The
most important loss
of
CO
2
,
however,
is
condensation
at the
tropopause.
The
downward
flux is 2.8 x
10
5
cm~
2
s~'.
The
rates
of
important reactions that form
and
recycle
CO are
shown
in figure
6.20b.
The
most
important
reaction
for
producing
CO is via
R95:
OH +
CH
3
->
H
2
CO
+ H.
H
2
CO
is
not
stable,
and its
ultimate
fate
is to
produce
CO by
photolysis.
In
the
above analysis
we
implicitly assume that Titan
has no
primordial
CO and
that
H
2
O
is the
sole source
of
oxygen
to the
satellite.
The
model predicts
the
abundances
of
CO and
CO
2
on
Titan. Thus, there
is
much merit
in
such
a
simple model. However,
if
CO is
primordial
and
buffered
by a
large supply
of
ice, then
its
abundance
is
decoupled
from
the
photochemistry
of
H
2
O
and the
steady state requirement
(6.69)
is no
longer
valid.
In
this
case
the
model
can
only predict
the
abundance
of
CO
2
in the
atmosphere
through
a
modified
version
of
(6.70):
Without
carrying
out
quantitative calculations,
we
expect
the
decoupling
of CO
from
H
2
O
to
increase
the
predicted abundance
of
CO
2
by a
factor
of 2
from
the
difference
between
(6.70)
and
(6.71).
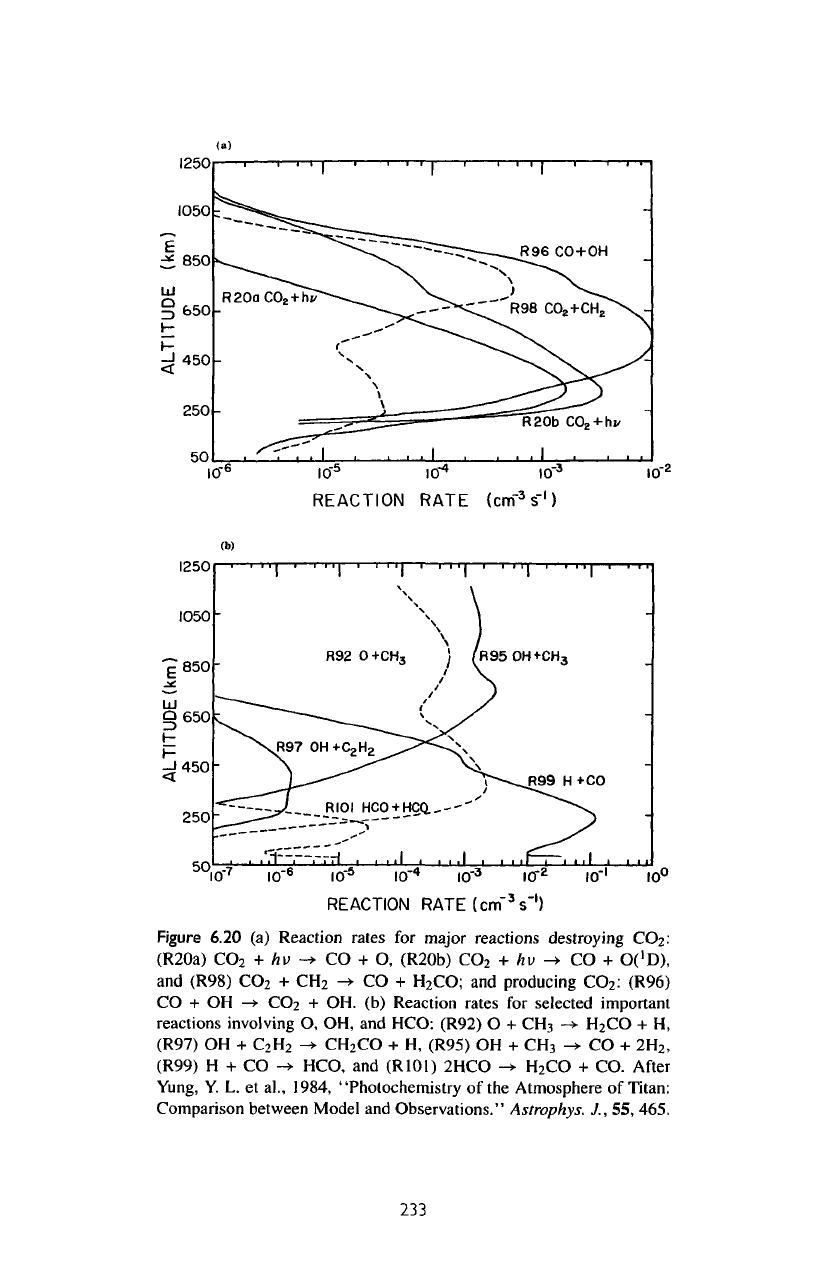
Figure
6.20
(a)
Reaction
rates
for
major
reactions
destroying
(R20a)
CO
2
+
hv
-*
CO
H-
O,
(R20b)
CO
2
+
hv
->•
CO +
O('D),
and
(R98)
CO
2
+
CH
2
->•
CO +
H
2
CO;
and
producing
CO
2
:
(R96)
CO + OH
->•
CO
2
+ OH. (b)
Reaction rates
for
selected
important
reactions involving
O, OH, and
HCO:
(R92)
O +
CH
3
->•
H
2
CO
+
H,
(R97)
OH +
C
2
H
2
-»•
CH
2
CO
H-
H,
(R95)
OH +
CH
3
->•
CO +
2H
2>
(R99)
H + CO
->
HCO,
and
(R101)
2HCO
-»•
H
2
CO
+ CO.
After
Yung,
Y. L. et
al.,
1984,
"Photochemistry
of the
Atmosphere
of
Titan:
Comparison between Model
and
Observations." Astrophys.
J.,
55,
465.
233

234
Photochemistry
of
Planetary
Atmospheres
6.3.5 Comparison with Giant Planets
A
comparison
of the
abundances
of the
principal hydrocarbon species,
CHU,
and
C2H2,
in the
giant
planets
and
Titan
is
summarized
in
chapter
5
(table
5.14).
There
is
more CH4,
C2H
6
,
and
C
2
H
2
in
Titan.
The
abundance
of
CFU
in the
atmosphere
determines
the
optical depth
of
unity
at
Lyman
a.
Thus,
to first
order,
the
primary
photolysis
of CH4
does
not
depend
on the
mixing ratio
of
CFU.
The
efficiency
of
the
synthesis
of the
higher
hydrocarbons depends
on a
large number
of
factors such
as
solar
flux,
temperature, eddy
mixing,
removal
of H
atoms,
and
condensation.
A
systematic
sensitivity
study
of
these factors
has
been carried
out
only
for
Jupiter.
We
qualitatively
discuss
the
causes
of the
difference
in
C2H6
and
C2H2
in the
atmospheres
of
the
outer solar system.
As
discussed
in
section
6.3.2,
the
quantum yield
for
organic
synthesis
is
higher
on
Titan than
in the
giant
planets because
H and H2 can
escape
from
Titan.
The
extremely
low
concentrations
of
CiH-s
and
CjHb
on
Uranus
are due
to the
extreme quiescent nature
of its
upper atmosphere.
The
higher hydrocarbons
synthesized
at
high
altitude
are
locally destroyed before they
can be
transported
to the
lower
stratosphere.
A
more extensive comparison between
the
composition
of
Titan
and
Jupiter
is
given
in figure
6.6.
The
results support
the
general picture that
the
higher
hydrocarbons
are
more abundant
on
Titan.
6.4
Triton
The
Voyager
flyby of
Triton
in
1989 revealed
an
extremely cold satellite covered
by
nitrogen ice.
The
surface temperature
was
measured
at 38 K. The
predominantly
N
2
atmosphere
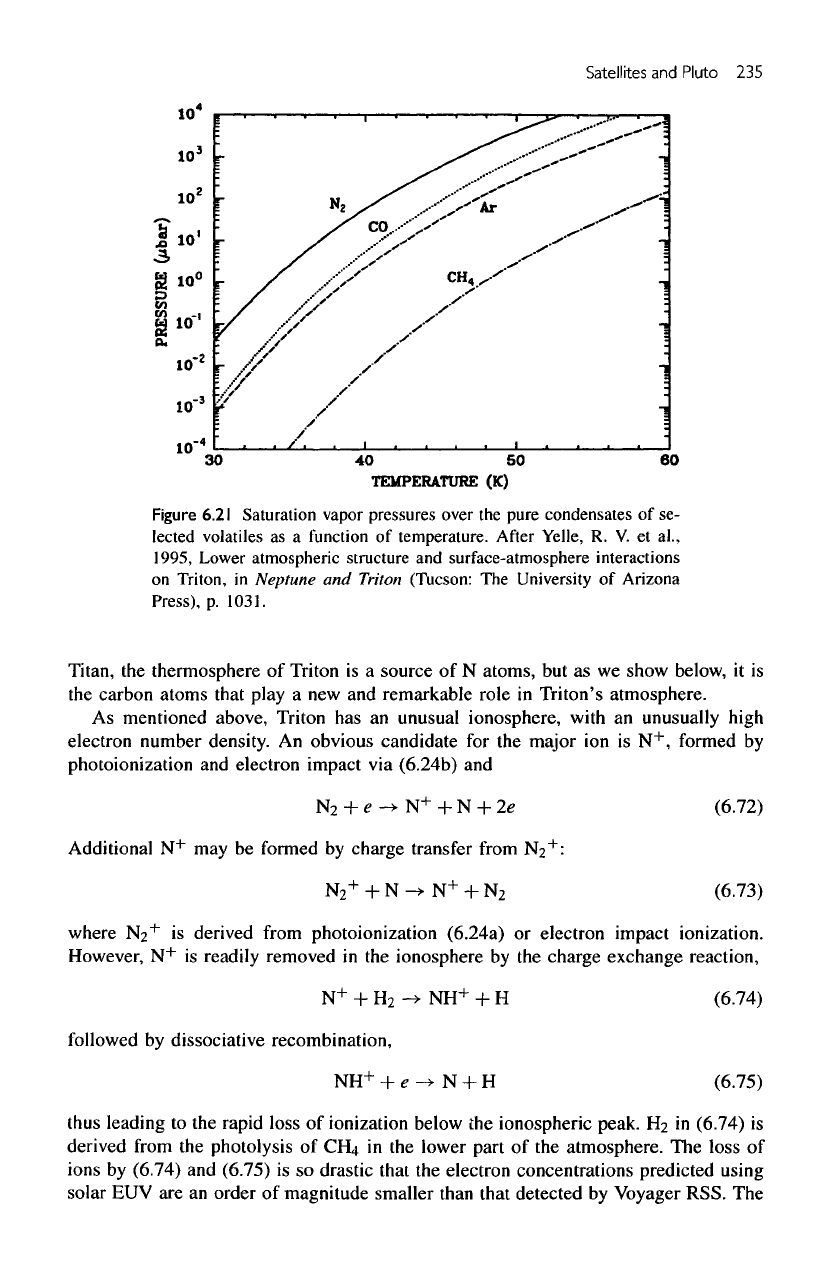
of
17.5
//.bar
is in
equilibrium
with
N2 ice
(see
figure
6.21).
A
trace
amount
of CH4 in the
atmosphere
was
detected
by the
Voyager
UVS.
Post-Voyager
observations revealed
the
presence
of
CH4,
CO, and
CC>2
on the
surface
of
Triton.
The
variation
of
vapor pressure
of N2,
CFLt,
CO and Ar
over
the
temperature range
appropriate
for the
outer solar system
is
shown
in figure
6.21. Thus,
the
presence
of
small amounts
of CO,
CH4,
and Ar at 38 K
would
be
consistent with their respective
vapor
pressures.
The
temperature
of the
upper atmosphere increases
with
altitude
and
reaches
95 K
above
400 km. A
tropopause
may
exist
at 8 km
above
the
surface.
A
haze layer near
the
surface
has
been detected. Perhaps
the
most surprising discovery
by the
Voyager
encounter
is the
ionosphere
with
peak electron densities
in
excess
of
10
4
cm~
3
,
a
value
much
greater
than
the
upper
limit
of
electron densities
in the
atmosphere
of
Titan.
The
atmosphere
of
Triton
is
sufficiently
thin
that there
is
considerable influence
on the
atmospheric
composition from both
the
thermosphere
above
and the
surface below.
6.4.1 Chemical Kinetics
The
photochemistry
of
hydrocarbons, nitrogen,
and
oxygen
species
on
Triton
is
similar
to
that
of
Titan,
and we do not
repeat
the
discussion here. There
is
more uncertainty
in
our
knowledge
of
chemical kinetics
at the
lower temperatures
in the
atmosphere
of
Triton.
In
addition, because
of the low
temperature,
the
higher hydrocarbons
and the
nitrile
species readily condense near
the
surface
to
form
aerosols.
As in the
case
of
Satellites
and
Pluto
235
60
Figure
6.21
Saturation vapor pressures over
the
pure condensates
of se-
lected volatiles
as a
function
of
temperature.
After
Yelle,
R. V. et
al.,
1995, Lower atmospheric structure
and
surface-atmosphere interactions
on
Triton,
in
Neptune
and
Triton (Tucson:
The
University
of
Arizona
Press),
p.
1031.
Titan,
the
thermosphere
of
Triton
is a
source
of N
atoms,
but as we
show below,
it is
the
carbon atoms
that
play
a new and
remarkable role
in
Triton's atmosphere.
As
mentioned above, Triton
has an
unusual ionosphere, with
an
unusually high
electron number density.
An
obvious candidate
for the
major
ion is
N
+
,
formed
by
photoionization
and
electron impact
via
(6.24b)
and
Additional
N
+
may be
formed
by
charge transfer from
where
N2
+
is
derived from photoionization
(6.24a)
or
electron impact ionization.
However,
N
+
is
readily removed
in the
ionosphere
by the
charge exchange reaction,
followed
by
dissociative recombination,
thus
leading
to the
rapid loss
of
ionization below
the
ionospheric peak.
H
2
in
(6.74)
is
derived
from
the
photolysis
of CH4 in the
lower part
of the
atmosphere.
The
loss
of
ions
by
(6.74)
and
(6.75)
is so
drastic that
the
electron concentrations predicted using
solar
EUV are an
order
of
magnitude smaller than that detected
by
Voyager RSS.
The
