Yung Y.L., DeMore W.B. Photochemistry of Planetary Atmospheres
Подождите немного. Документ загружается.

Table
6.8
Boundary
conditions
for
selected species
in the
photochemical model
for
Titan
Species"
H
H
2
CH4
N
NH
CH
3
OH
H
2
O
CO
Lower
boundary
1
"
0 = 0
0 = 0
f
= 2.0 x
10-
2
0
= 0
0=0
0 = 0
0 = 0
v
= 2.0 x
10-
4
0=0
Upper
boundary
0
v
= 2.5 x
10
4
v-6.\
x
10
3
0=
1.0 x
I0
9d
0 =
-1.0
x
10
9
0 =
-1.0
x
10
9
0
=
-1.0
x
10
9
0 = 3.3 x
I0
4
0 =
-6.1
x
10
5
0 = 8.8 x
10
4
From Yung,
Y. L. et
al.
(1984).
"For
species
not
given here, their boundary
conditions
are
zero
flux at the
upper boundary
and
maximum deposition velocity
at the
lower boundary.
The
symbols
/,
(/>,
and v
refer
to
mixing ratio,
flux
(cm"
2
s~'),
and
velocity
(cm
s"
1
),
respectively.
The
sign convention
for
<t>
and
u
is
positive
for
upward
flow.
b
At
tropopause
z = 45 km
(n
=
1.1
x
10
19
cm"
3
).
c
At
z =
1160
km
(n
= 1.2 x
10
9
cm"
3
).
For all
species
except
H and
H
2
,
for
which
the
upper
boundary
is at
1425
km (n = 4.9 x
10
7
cm"
3
).
d
All
fluxes
refer
to
the
surface.
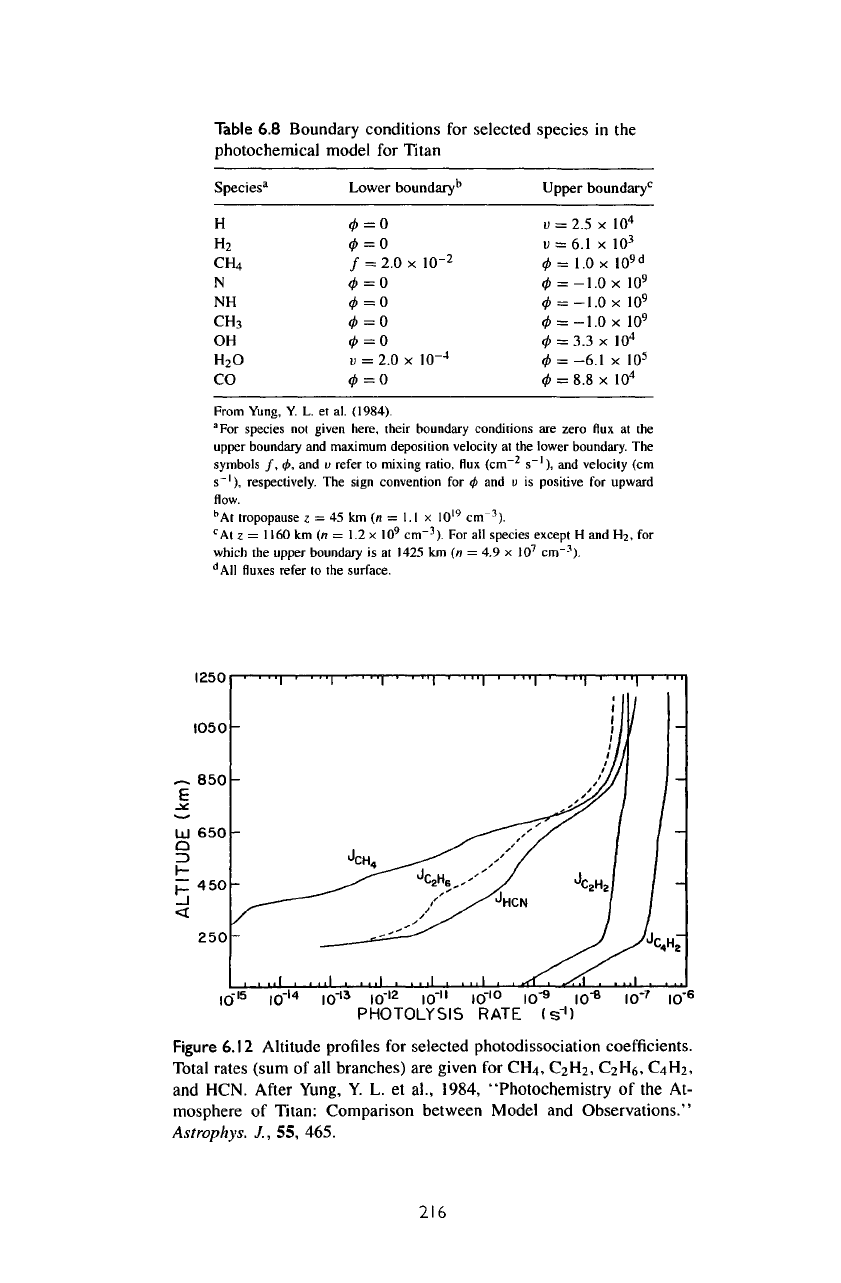
Figure
6.12
Altitude
profiles
for
selected photodissociation
coefficients.
Total
rates (sum
of all
branches)
are
given
for
CH4,
C2H2,
C2H6,
C4H2,
and
HCN.
After
Yung,
Y. L. et
al.,
1984,
"Photochemistry
of the At-
mosphere
of
Titan: Comparison between Model
and
Observations."
Astrophys.
/,
55,
465.
216
Satellites
and
Pluto
217
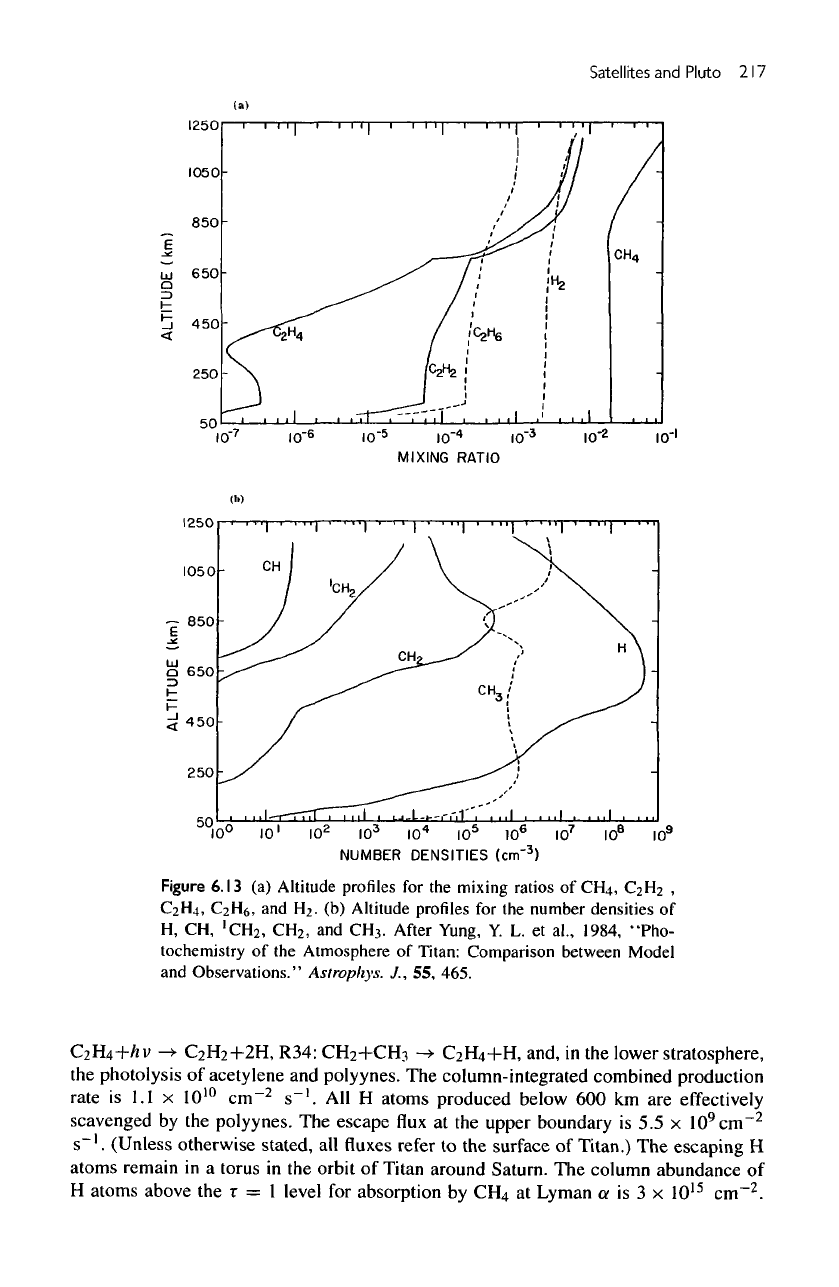
Figure
6.13
(a)
Altitude profiles
for the
mixing
ratios
of
CH
4
,
C
2
H
2
,
C2H4,
C
2
H6,
and
H
2
.
(b)
Altitude profiles
for the
number densities
of
H,
CH,
'CH
2
,
CH
2
,
and
CH
3
.
After Yung,
Y. L. et
al.,
1984, "Pho-
tochemistry
of the
Atmosphere
of
Titan:
Comparison
between
Model
and
Observations."
Asiropliys.
J., 55,
465.
C
2
H
4
+hv
-H>
C
2
H
2
+2H,
R34:
CH
2
+CH
3
->
C
2
H
4
+H,
and,
in the
lower stratosphere,
the
photolysis
of
acetylene
and
polyynes.
The
column-integrated combined production
rate
is 1.1 x
10
10
cm~
2
s~'.
All H
atoms produced below
600 km are
effectively
scavenged
by the
polyynes.
The
escape
flux at the
upper boundary
is 5.5 x
10
9
cm~
2
s~'.
(Unless otherwise
stated,
all
fluxes refer
to the
surface
of
Titan.)
The
escaping
H
atoms remain
in a
torus
in the
orbit
of
Titan around Saturn.
The
column abundance
of
H
atoms above
the r = 1
level
for
absorption
by
CH
4
at
Lyman
a is 3 x
10
15
cm~
2
.
218
Photochemistry
of
Planetary
Atmospheres
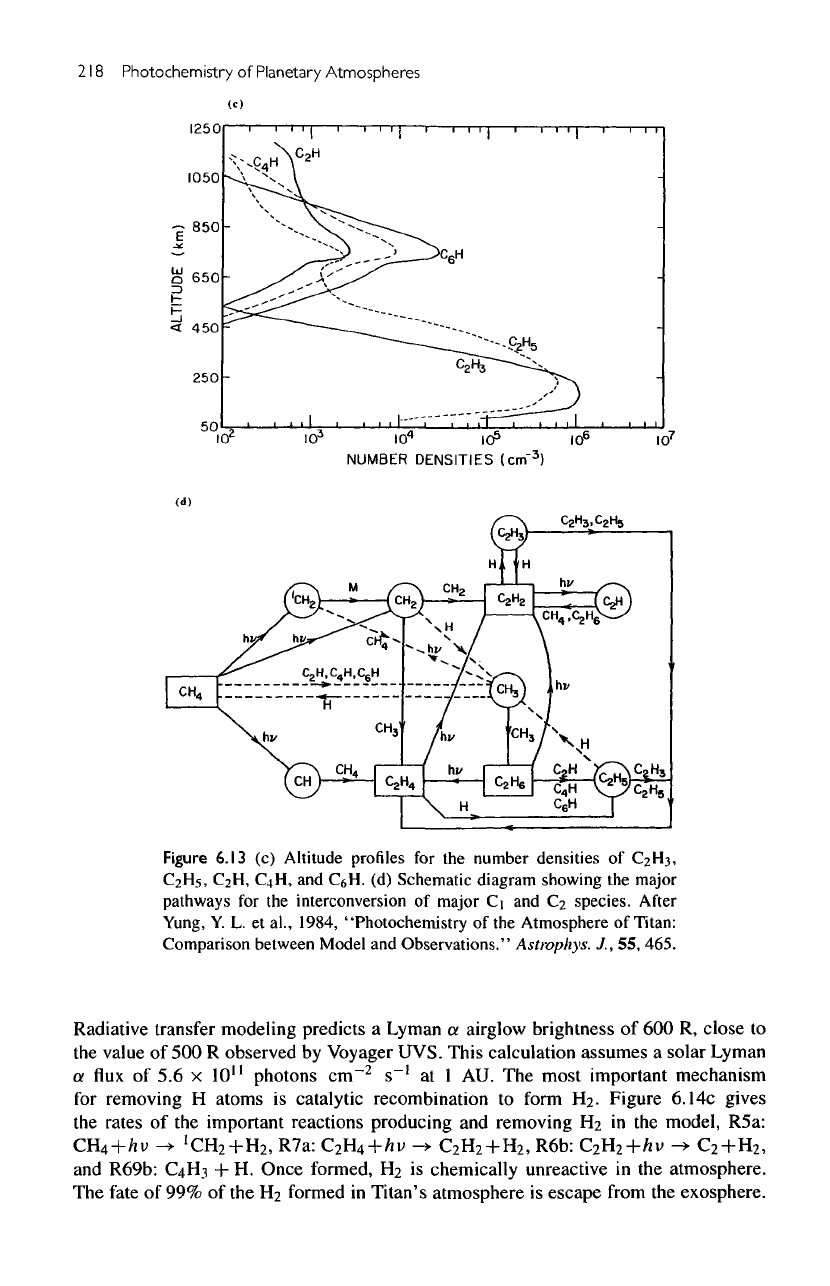
Figure
6.13
(c)
Altitude profiles
for the
number
densities
of
C2H3,
C2Hs,
C2H, C4H,
and
C&H.
(d)
Schematic
diagram
showing
the
major
pathways
for the
interconversion
of
major
Ci
and
C-i
species.
After
Yung,
Y. L. et
al.,
1984,
"Photochemistry
of the
Atmosphere
of
Titan:
Comparison
between
Model
and
Observations."
Astmphys.
J., 55,
465.
Radiative
transfer
modeling predicts
a
Lyman
a
airglow brightness
of 600 R,
close
to
the
value
of 500 R
observed
by
Voyager UVS. This calculation
assumes
a
solar Lyman
a flux of 5.6 x 10"
photons
cm~
2
s~
!
at 1
AU.
The
most
important mechanism
for
removing
H
atoms
is
catalytic recombination
to
form
H
2
.
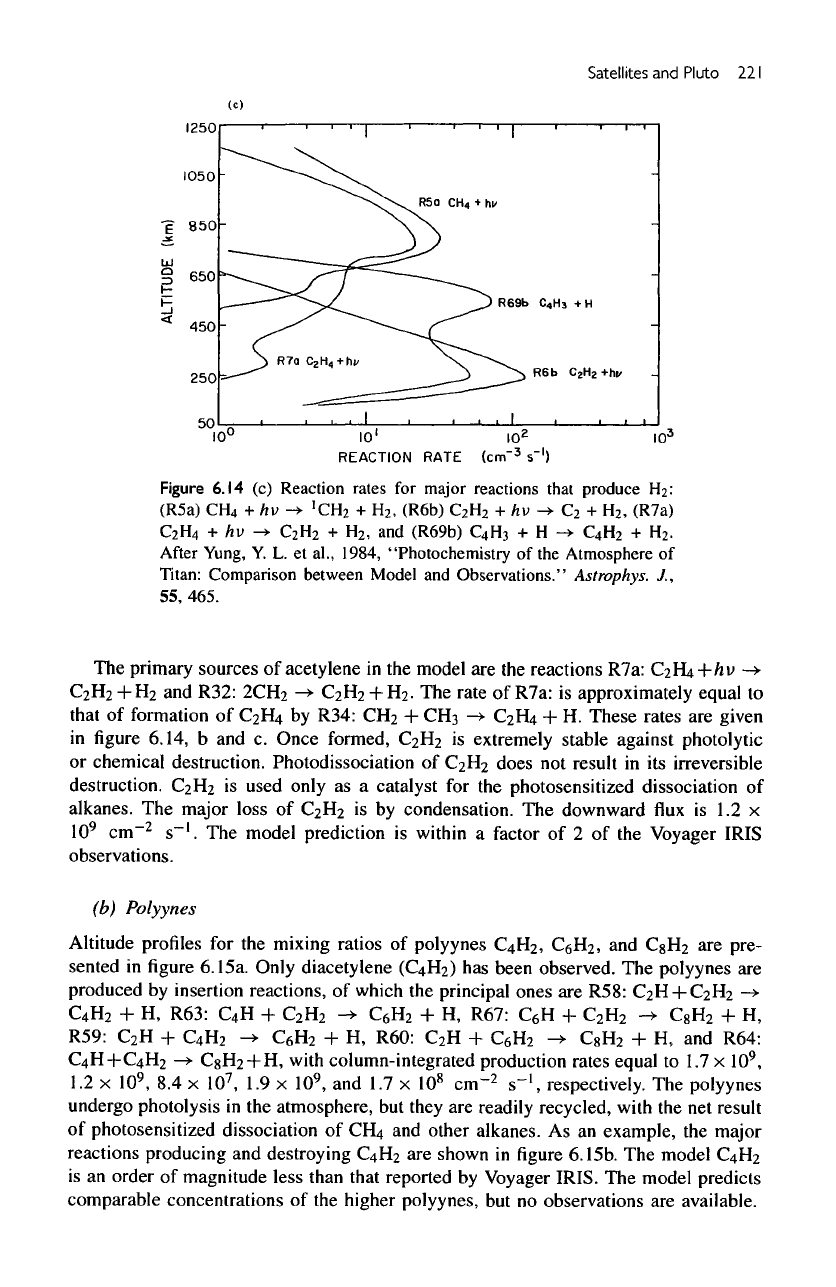
Figure
6.14c
gives
the
rates
of the
important reactions producing
and
removing
H
2
in the
model, R5a:
CH
4
+/iu
->
'CH
2
+H
2
,
R7a:
C
2
H
4
+
/Jv
->
C
2
H
2
+
H2,
R6b:
C
2
H
2
+fcv
->
C
2
+H
2
,
and
R69b:
C4H.i
+ H.
Once
formed,
H
2
is
chemically unreactive
in the
atmosphere.
The
fate
of 99% of the
H
2
formed
in
Titan's
atmosphere
is
escape
from
the
exosphere.
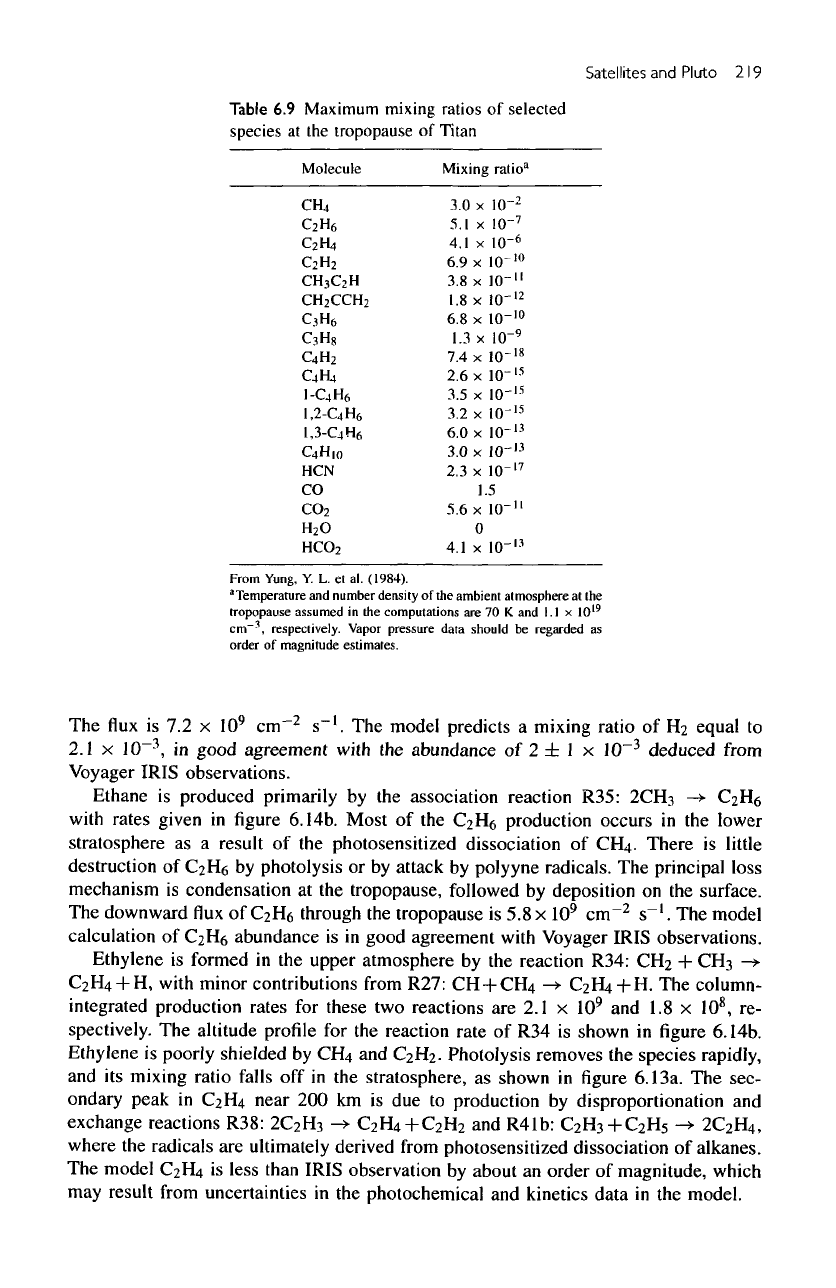
Table
6.9
Maximum mixing
ratios
of
selected
species
at the
tropopause
of
Titan
Satellites
and
Plirto
219
Molecule
CR,
C
2
H
6
C
2
H4
C
2
H
2
CH
3
C
2
H
CH
2
CCH
2
C
3
H
6
C
3
H
8
C
4
H
2
C
4
H4
1-C
4
H
6
1,2-C
4
H
6
1,3-C
4
H
6
C
4
H
IO
HCN
CO
C0
2
H
2
O
HCO
2
Mixing
ratio"
3.0 x
10~
2
5.1
x
1(T
7
4.1
x
1CT
6
6.9
x
10'
10
3.8
x
10-"
1.8
x
10"
12
6.8
x
10-'°
1.3
x
10~
9
7.4
x
lO'
18
2.6
x
10"
15
3.5
x
10'
15
3.2
x
10-'
5
6.0
x
10-'
3
3.0
x
10-'
3
2.3
x 10-
"
1.5
5.6
x
10-"
0
4.1
x
10-'
3
From
Yung,
Y. L. et
al.
(1984).
"Temperature
and
number density
of the
ambient
atmosphere
at the
tropopause
assumed
in the
computations
are 70
K
and
I.I
x
10
19
cm-',
respectively.
Vapor
pressure
data should
be
regarded
as
order
of
magnitude
estimates.
The flux is 7.2 x
10
9
cm
2
s
'.
The
model predicts
a
mixing
ratio
of H2
equal
to
2.1
x
10~
3
,
in
good
agreement
with
the
abundance
of 2 ± 1 x
1(T
3
deduced
from
Voyager IRIS observations.
Ethane
is
produced primarily
by the
association reaction R35:
2CH
3
->
C2He
with
rates given
in figure
6.14b.
Most
of the
C2H
6
production occurs
in the
lower
stratosphere
as a
result
of the
photosensitized dissociation
of
CH
4
.
There
is
little
destruction
of
C2Hg
by
photolysis
or by
attack
by
polyyne radicals.
The
principal loss
mechanism
is
condensation
at the
tropopause, followed
by
deposition
on the
surface.
The
downward
flux of
C2Hg
through
the
tropopause
is
5.8x
10
9
cm~
2
s~'.
The
model
calculation
of
C2H
6
abundance
is in
good agreement
with
Voyager IRIS observations.
Ethylene
is
formed
in the
upper atmosphere
by the
reaction R34:
CH
2
+
CH
3
-»•
C
2
H
4
+ H,
with
minor contributions
from
R27:
CH +
CH
4
->
C
2
H4
+ H. The
column-
integrated
production rates
for
these
two
reactions
are
2.1
x
10
9
and 1.8 x
10
8
,
re-
spectively.
The
altitude
profile
for the
reaction rate
of R34 is
shown
in figure
6.14b.
Ethylene
is
poorly
shielded
by
CH
4
and
C2H2-
Photolysis removes
the
species
rapidly,
and
its
mixing ratio
falls
off in the
stratosphere,
as
shown
in figure
6.13a.
The
sec-
ondary
peak
in
C2H
4
near
200 km is due to
production
by
disproportionation
and
exchange reactions R38:
2C
2
H
3
->
C
2
H
4
+C
2
H
2
and
R41b:
C
2
H
3
-|-C
2
H
5
->
2C
2
H
4
,
where
the
radicals
are
ultimately
derived
from
photosensitized dissociation
of
alkanes.
The
model
C2H
4
is
less
than
IRIS observation
by
about
an
order
of
magnitude, which
may
result
from
uncertainties
in the
photochemical
and
kinetics data
in the
model.

Figure
6.14
(a)
Reaction rates
for
major reactions destroying
and
pro-
ducing
CH
4
:
(R5)
CH
4
+
hv
-»•
products, (R55)
CH
4
+
C
2
H
-»
CH
3
+
C
2
H
2
,
(R61)
CH
4
+
C
4
H
->
CH
3
+
C
4
H
2
,
(R65)
CH4
+
C
6
H
->
CH
3
+
C
6
H
2
,
and
(R33)
CH
3
+ H
->
CH
4
.
(b)
Reaction rates
for
important
synthesis reactions: (R31)
CH
2
+
C
2
H
2
->•
CH
3
C
2
H,
and
CH
2
CCH
2
(the
sum is
shown here), (R32)
2CH
2
->
C
2
H
2
+
H
2
,
(R34)
CH
2
+
CH
3
->•
C
2
H
4
+ H,
(R35)
2CH
3
->•
C
2
H
6
,
and
(R40b)
CH
3
+
C
2
H
5
->•
C
3
H
8
.
After
Yung,
Y. L. et
al.,
1984,
"Photochemistry
of the
Atmosphere
of
Titan: Comparison between Model
and
Observations."
Aslmphys.
J., 55,
465.
220
Satellites
and
Pluto
221
Figure
6.14
(c)
Reaction rates
for
major reactions
that
produce
H2:
(R5a)
CH
4
+
hv
-*
'CH
2
+
H
2
,
(R6b)
C
2
H
2
+ hv
->•
C
2
+
H
2
,
(R7a)
C
2
H
4
+
Ai>
-»•
C
2
H
2
+
H
2
,
and
(R69b)
C
4
H
3
+ H
->
C
4
H
2
+
H
2
.
After
Yung,
Y. L. et
al.,
1984, "Photochemistry
of the
Atmosphere
of
Titan:
Comparison between Model
and
Observations."
Astrophys.
J.,
55,
465.
The
primary sources
of
acetylene
in the
model
are the
reactions R7a:
C
2
H4+/zi>
—>
C
2
H
2
+
H
2
and
R32:
2CH
2
->
C
2
H
2
+H
2
.
The
rate
of
R7a:
is
approximately equal
to
that
of
formation
of
C
2
H
4
by
R34:
CH
2
+
CH
3
->•
C
2
H
4
+ H.
These
rates
are
given
in
figure
6.14,
b and c.
Once
formed,
C
2
H
2
is
extremely stable against photolytic
or
chemical destruction. Photodissociation
of
C
2
H
2
does
not
result
in its
irreversible
destruction.
C
2
H
2
is
used only
as a
catalyst
for the
photosensitized dissociation
of
alkanes.
The
major
loss
of
C
2
H
2
is by
condensation.
The
downward
flux is 1.2 x
10
9
cm~
2
s~'.
The
model prediction
is
within
a
factor
of 2 of the
Voyager IRIS
observations.
(b)
Polyynes
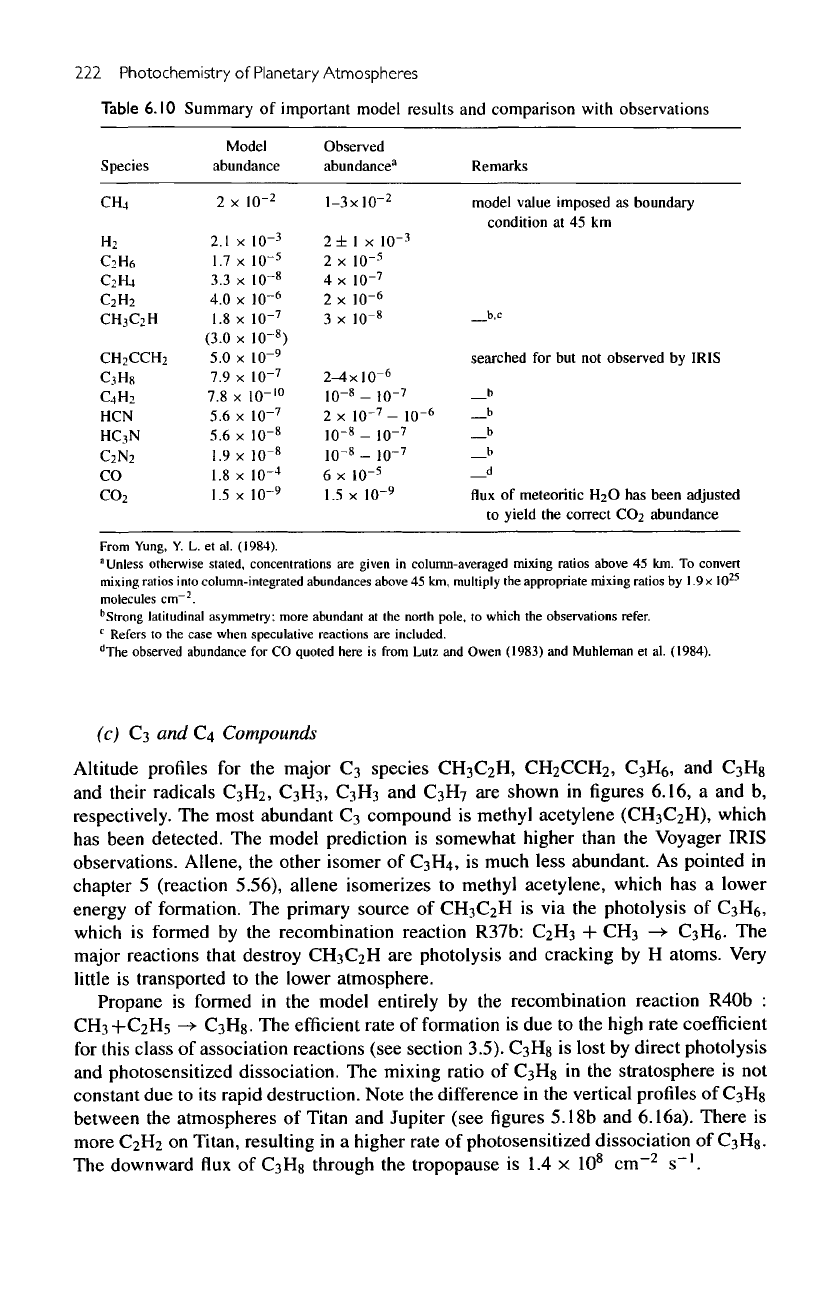
Altitude
profiles
for the
mixing ratios
of
polyynes
C
4
H
2
,
CeH
2
,
and
CgH
2
are
pre-
sented
in figure
6.15a.
Only diacetylene
(C
4
H
2
)
has
been
observed.
The
polyynes
are
produced
by
insertion reactions,
of
which
the
principal
ones
are
R58:
C
2
H
+
C
2
H
2
->
C
4
H
2
+ H,
R63:
C
4
H
+
C
2
H
2
-»•
C
6
H
2
+ H,
R67:
C
6
H
+
C
2
H
2
-*
C
8
H
2
+ H,
R59:
C
2
H
+
C
4
H
2
->
C
6
H
2
+ H,
R60:
C
2
H
+
C
6
H
2
->•
C
8
H
2
+ H, and
R64:
C
4
H+C
4
H
2
->
CgH
2
+ H,
with
column-integrated production rates equal
to 1.7 x
10
9
,
1.2
x
10
9
,
8.4 x
10
7
,
1.9 x
10
9
,
and 1.7 x
10
8
cm"
2
s"
1
,
respectively.
The
polyynes
undergo photolysis
in the
atmosphere,
but
they
are
readily recycled,
with
the net
result
of
photosensitized dissociation
of
CH
4
and
other alkanes.
As an
example,
the
major
reactions producing
and
destroying
C
4
H
2
are
shown
in figure
6.15b.
The
model
C
4
H
2
is
an
order
of
magnitude
less
than
that
reported
by
Voyager IRIS.
The
model predicts
comparable concentrations
of the
higher polyynes,
but no
observations
are
available.
222
Photochemistry
of
Planetary
Atmospheres
Table
6.10
Summary
of
important model results
and
comparison
with
observations
Species
CH
4
H
2
C
2
H
6
C
2
Hj
C
2
H
2
CH
3
C
2
H
CH
2
CCH
2
C
3
H
8
QH
2
HCN
HC
3
N
C
2
N
2
CO
C0
2
Model
abundance
2
x
IO-
2
2.1
x
IO-
3
1.7
x
10"
5
3.3 x
IO"
8
4.0 x
10~
6
1.8
x
10-
7
(3.0
x
10~
8
)
5.0
x
10-
9
7.9 x
10~
7
7.8
x
10-'°
5.6 x
10~
7
5.6 x
10~
8
1.9
x
10-
8
1.8
x
10~
4
1.5
x
10~
9
Observed
abundance
3
l-3xlO~
2
2± 1 x
10~
3
2
x
10-
5
4 x
10~
7
2
x
10~
6
3 x
10~
8
Remarks
model
value
imposed
as
boundary
condition
at 45 km
b.c
searched
for but not
observed
by
IRIS
2^xlO~
6
10-
8
-
Itr
7
2
x
10'
7
-
10-
6
10~
8
-
10~
7
10-S-
)0~
7
6 x
10-
5
1.5
x
10~
9
b
b
b
b
d
flux
of
meteoritic
H
2
O
has
to
yield
the
correct
CO
2
been
adjusted
abundance
From
Yung,
Y. L. et
al.
(1984).
"Unless
otherwise stated,
concentrations
are
given
in
column-averaged
mixing
ratios
above
45 km. To
convert
mixing
ratios into column-integrated abundances
above
45 km,
multiply
the
appropriate mixing ratios
by
1.9x
10
25
molecules
cm~
2
.
b
Strong
latitudinal
asymmetry;
more
abundant
at the
north
pole,
to
which
the
observations refer.
c
Refers
to the
case
when speculative reactions
are
included.
d
The
observed
abundance
for CO
quoted
here
is
from
Lulz
and
Owen
(1983)
and
Muhleman
et al.
(1984).
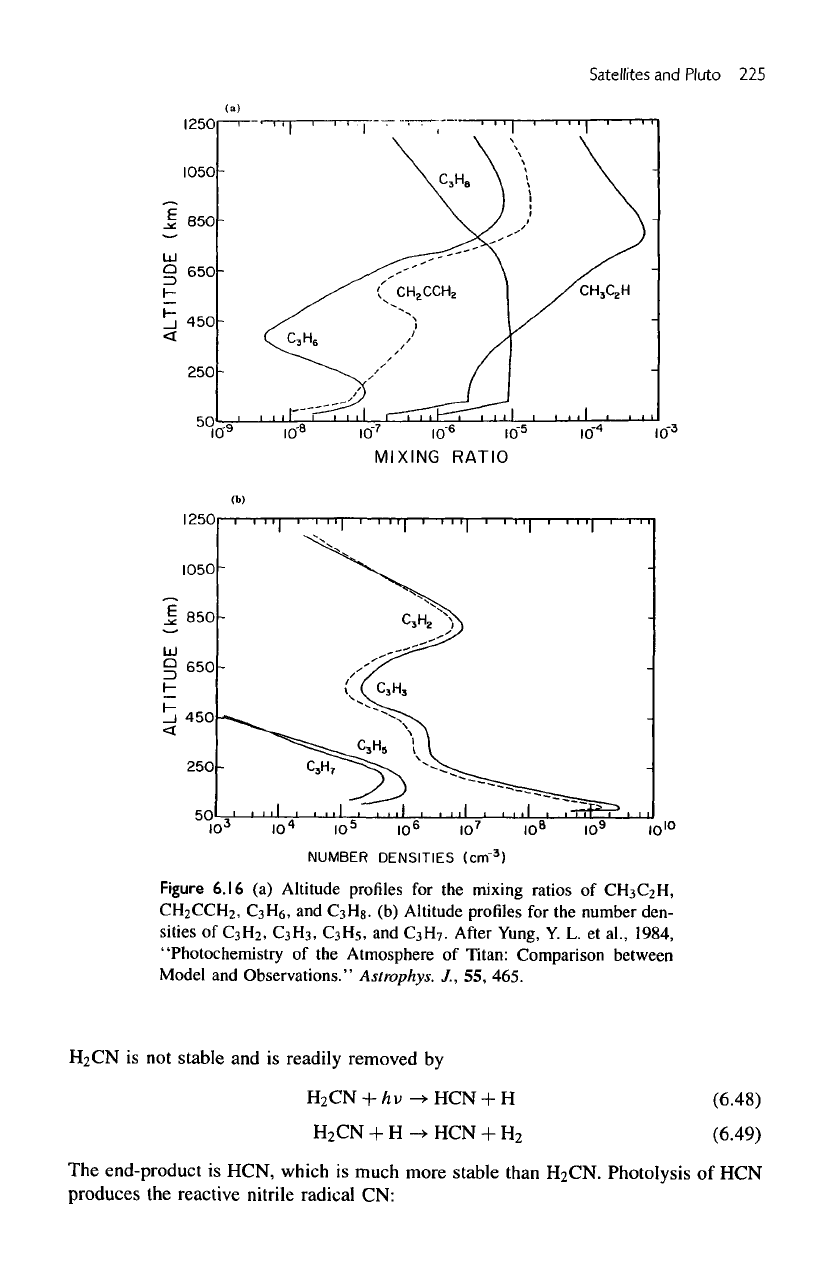
(c)
C
3
and
Cn
Compounds
Altitude
profiles
for the
major
C
3
species
CH
3
C
2
H,
CH
2
CCH
2
,
C
3
H
6
,
and
C
3
H
g
and
their radicals
C
3
H2,
C
3
H
3)
C
3
H
3
and
C
3
H
7
are
shown
in figures
6.16,
a and b,
respectively.
The
most abundant
C
3
compound
is
methyl acetylene
(CH
3
C
2
H),
which
has
been detected.
The
model prediction
is
somewhat higher than
the
Voyager IRIS
observations.
Allene,
the
other isomer
of
C
3
H4,
is
much less abundant.
As
pointed
in
chapter
5
(reaction
5.56),
allene isomerizes
to
methyl acetylene, which
has a
lower
energy
of
formation.
The
primary source
of
CH
3
C
2
H
is via the
photolysis
of
C
3
He,
which
is
formed
by the
recombination reaction R37b:
C
2
H
3
+
CH
3
—>
C
3
H6.
The
major
reactions
that
destroy
CH3C
2
H
are
photolysis
and
cracking
by H
atoms. Very
little
is
transported
to the
lower atmosphere.
Propane
is
formed
in the
model entirely
by the
recombination reaction R40b
:
CH
3
+C
2
Hs
->
C
3
Hg.
The
efficient
rate
of
formation
is due to the
high
rate
coefficient
for
this
class
of
association reactions (see section 3.5).
CjHg
is
lost
by
direct photolysis
and
photosensitized dissociation.
The
mixing ratio
of
C
3
Hg
in the
stratosphere
is not
constant
due to its
rapid destruction.
Note
the
difference
in the
vertical profiles
of
C
3
Hg
between
the
atmospheres
of
Titan
and
Jupiter (see
figures
5.18b
and
6.16a).
There
is
more
C
2
H
2
on
Titan, resulting
in a
higher rate
of
photosensitized dissociation
of
C
3
Hg.
The
downward
flux of
C
3
Hg
through
the
tropopause
is 1.4 x
10
8
cm~
2
s~'.
Satellites
and
Pluto
223
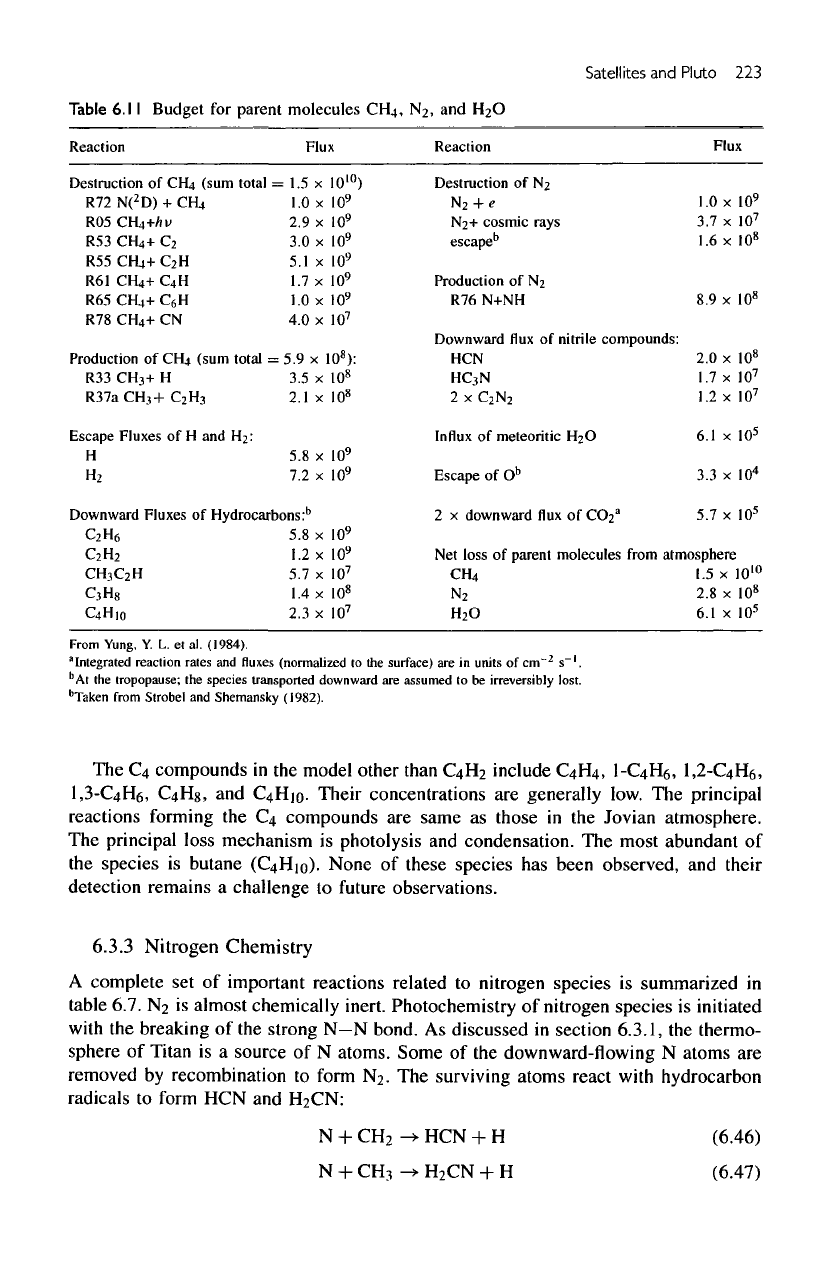
Table
6.11
Budget
for
parent
molecules
CH4,
N
2
,
and H2O
Reaction
Destruction
of
CH
4
(sum
R72
N(
2
D)
+
CH4
R05
CH4+hv
R53
CRj+
C
2
R55
CH4+
C
2
H
R61
CH
4
+
C
4
H
R65
CR,+
C
6
H
R78
CH
4
+
CN
Flux
total
=
1.5
x
10'°)
1.0
x
10
9
2.9 x
10
9
3.0 x
10
9
5.1
x
10
9
1.7
x
10
9
1.0
x
10
9
4.0 x
10
7
Reaction
Destruction
of N2
N
2
+ e
N
2
+
cosmic rays
escape
1
"
Production
of
N
2
R76
N+NH
Flux
l.Ox
10
9
3.7
x
10
7
1.6
x
10
8
8.9 x
10
8
Production
of
CH
4
(sum total
= 5.9 x
10
s
):
R33
CH
3
+
H 3.5 x
10
8
R37aCH
3
+
C
2
H
3
2.1
x
10
8
Escape Fluxes
of H and H2:
H
5.8 x
10
9
H
2
7.2 x
10
9
Downward Fluxes
of
Hydrocarbons:
b
C
2
H
6
5.8 x
10
9
C
2
H
2
I.2xl0
9
CH
3
C
2
H
5.7 x
10
7
CjH
8
1.4x
10
s
C
4
H,o
2.3 x
10
7
Downward
flux of
nitrile
compounds:
HCN
2.0 x
10
8
HC
3
N
1.7 x
10
7
2
xC
2
N
2
1.2 x
10
7
Influx
of
meteoritic
H
2
O
6.1 x
10
s
Escape
of
O
b
3.3 x
10
4
2
x
downward
flux of
CO
2
a
5.7 x
10
5
Net
loss
of
parent molecules
from
atmosphere
CH
4
1.5
x
10'°
N
2
2.8 x
10
8
H
2
O
6.1 x
10
5
From
Yung,
Y. L. et
al.
(1984).
a
Integrated reaction
rales
and fluxes
(normalized
to the
surface)
are in
units
of
cm~
2
s~'.
b
At
the
Iropopause;
the
species
transported
downward
are
assumed
to be
irreversibly lost.
b
Taken
from
Strobel
and
Shemansky
(1982).
The
€4
compounds
in the
model other
than
C4H
2
include
C4H4,
1-C4H6,
1,
1,3-GtHe,
C4Hg,
and
C4Hio-
Their concentrations
are
generally low.
The
principal
reactions
forming
the
€4
compounds
are
same
as
those
in the
Jovian atmosphere.
The
principal loss mechanism
is
photolysis
and
condensation.
The
most abundant
of
the
species
is
butane
(C4Hio).
None
of
these species
has
been observed,
and
their
detection remains
a
challenge
to
future
observations.
6.3.3 Nitrogen Chemistry
A
complete
set of
important reactions related
to
nitrogen
species
is
summarized
in
table 6.7.
N2 is
almost chemically
inert.
Photochemistry
of
nitrogen
species
is
initiated
with
the
breaking
of the
strong
N—N
bond.
As
discussed
in
section
6.3.1,
the
thermo-
sphere
of
Titan
is a
source
of N
atoms. Some
of the
downward-flowing
N
atoms
are
removed
by
recombination
to
form
N
2
.
The
surviving
atoms react
with
hydrocarbon
radicals
to
form
HCN and
H
2
CN:
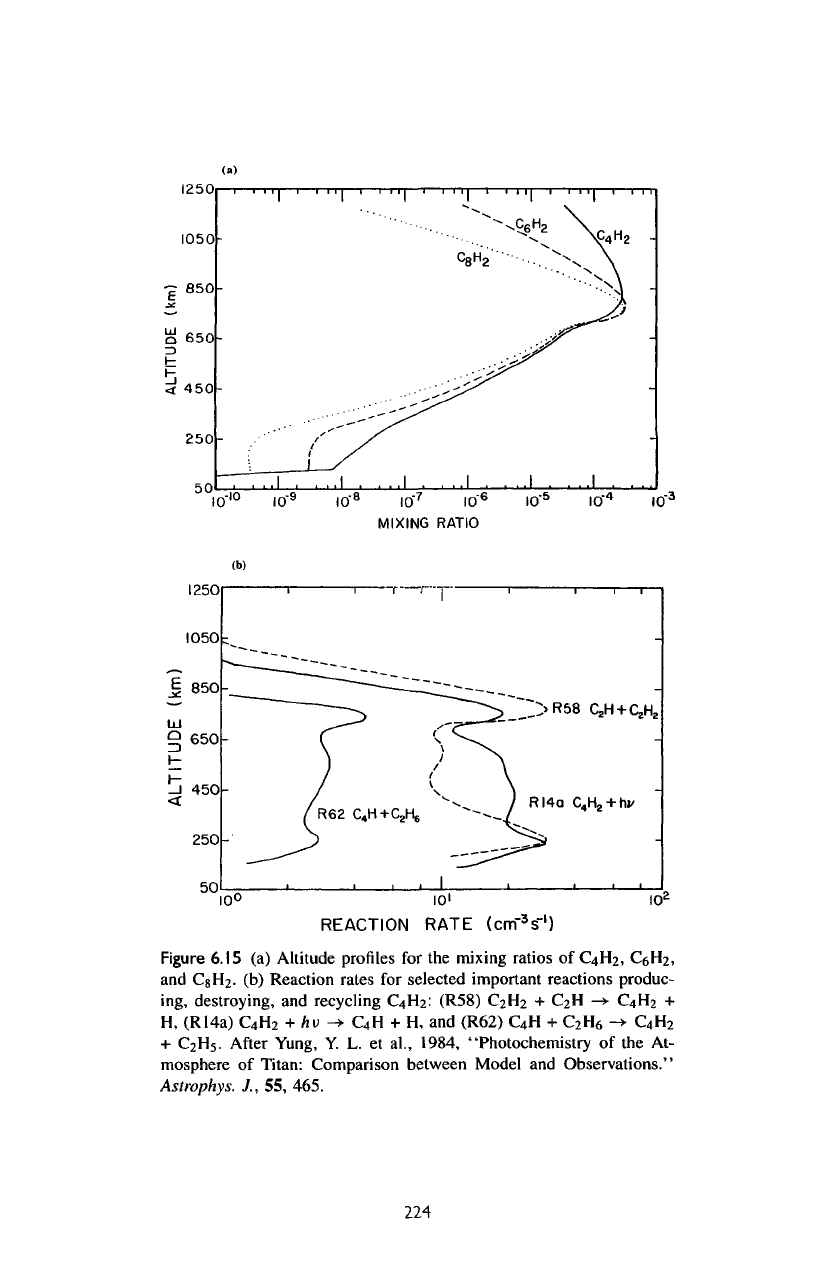
Figure
6.15
(a)
Altitude profiles
for the
mixing ratios
of
C
4
H
2
,
CeH
2
,
and
CgH
2
.
(b)
Reaction rates
for
selected important reactions produc-
ing,
destroying,
and
recycling
C
4
H
2
:
(R58)
C
2
H
2
+
C
2
H
-»
C
4
H
2
+
H,
(R14a)
C
4
H
2
+
hv
->•
C
4
H
+ H, and
(R62)
C
4
H
+
C
2
H
6
->
C
4
H
2
+
C
2
H
5
.
After
Yung,
Y. L. et
al,
1984,
"Photochemistry
of the At-
mosphere
of
Titan: Comparison between Model
and
Observations."
Astrophys.
J.,
55,
465.
224
Satellites
and
Pluto
225
Figure
6.16
(a)
Altitude profiles
for the
mixing
ratios
of
CH
3
C
2
H,
CH
2
CCH
2
,
C
3
H
6
,
and
C
3
H
8
.
(b)
Altitude profiles
for the
number den-
sities
of
C
3
H
2
,
C
3
H
3
,
C
3
H
5
,
and
C
3
H
7
.
After Yung,
Y. L. et
al.,
1984,
"Photochemistry
of the
Atmosphere
of
Titan:
Comparison
between
Model
and
Observations."
Astrophys.
J., 55,
465.
H
2
CN
is not
stable
and is
readily removed
by
The
end-product
is
HCN, which
is
much
more
stable than
H
2
CN.
Photolysis
of HCN
produces
the
reactive
nitrile
radical
CN:
