Yung Y.L., DeMore W.B. Photochemistry of Planetary Atmospheres
Подождите немного. Документ загружается.

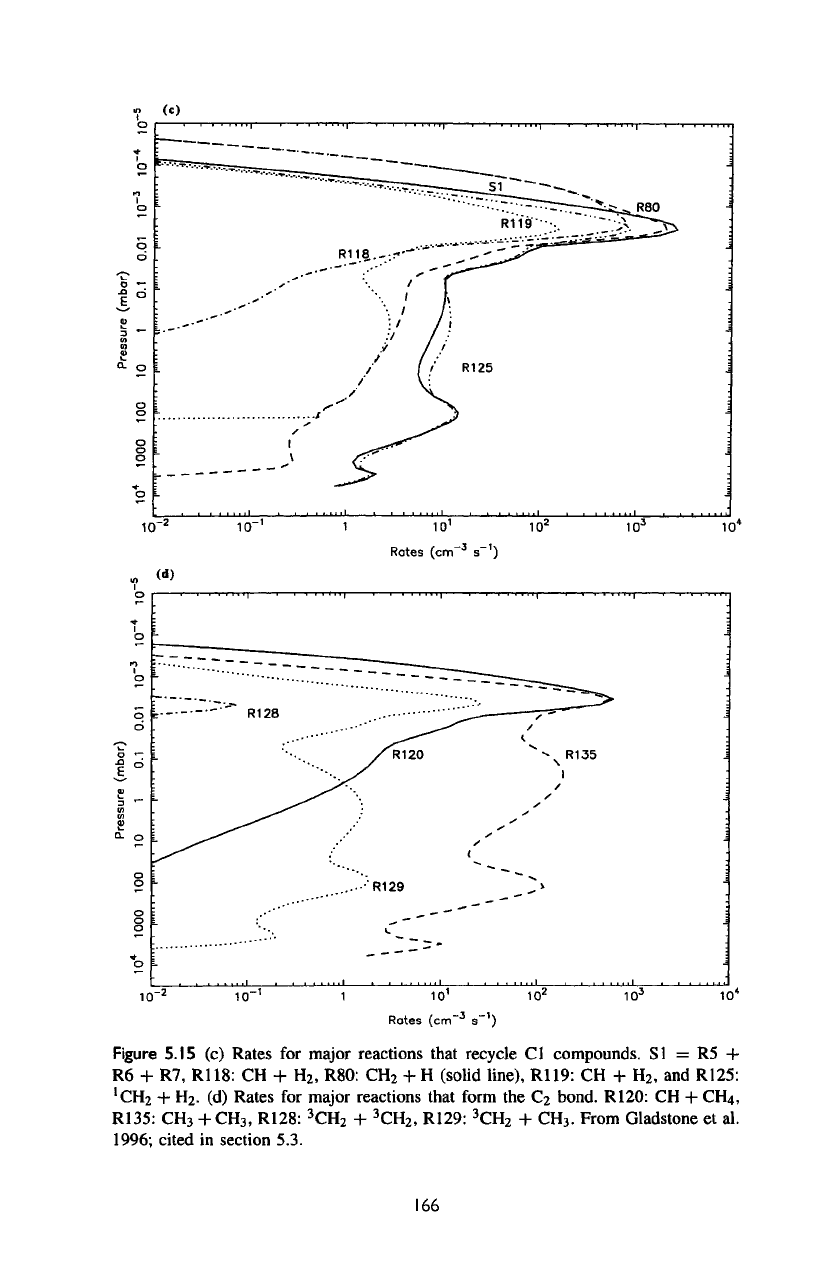
Figure
5.15
(c)
Rates
for
major reactions that recycle
Cl
compounds.
SI
= R5 +
R6
+ R7,
R118:
CH +
H
2)
R80:
CH
2
+ H
(solid line),
R119:
CH +
H
2
,
and
R125:
'CH
2
+
H
2
.
(d)
Rates
for
major reactions that form
the
C
2
bond. R120:
CH +
CH
4
,
R135:
CH
3
+CH
3
,
R128:
3
CH
2
+
3
CH
2
,
R129:
3
CH
2
+
CH
3
.
From Gladstone
et
al.
1996; cited
in
section 5.3.
166
Jovian
Planets
167
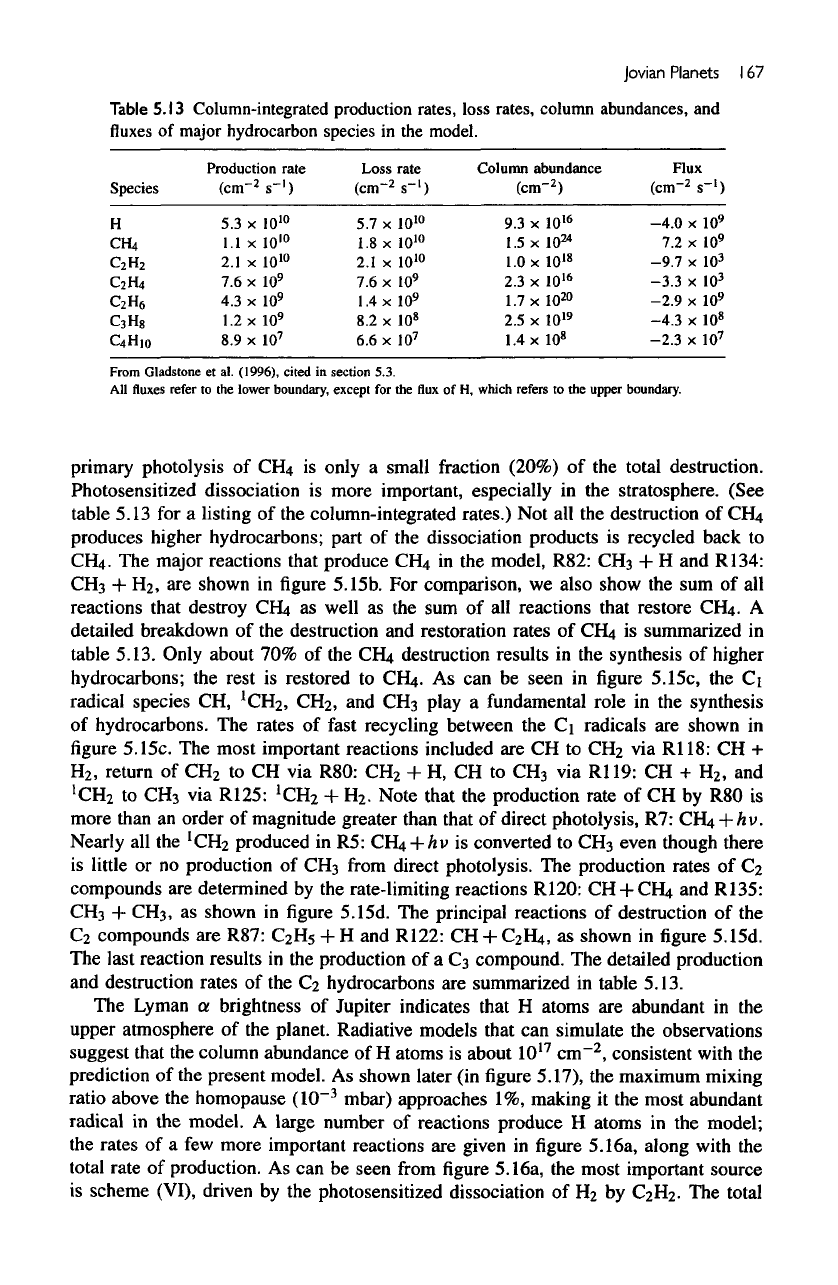
Table
5.13
Column-integrated production rates, loss rates, column abundances,
and
fluxes of
major hydrocarbon species
in the
model.
Species
H
ca,
C
2
H
2
C
2
rt,
C
2
H
6
C
3
H
8
C
4
H,
0
Production
rate
(cm~
2
s~')
5.3 x
10
10
1.1
x
10
10
2.1
x
10'°
7.6 x
10
9
4.3 x
10
9
1.2x
10
9
8.9 x
10
7
Loss
rate
(cm~
2
s~')
5.7
x
10
10
1.8
x
10
10
2.1
x
10'°
7.6 x
10
9
1.4x
10
9
8.2 x
10
8
6.6 x
10
7
Column
abundance
(cm-
2
)
9.3
x
10
16
1.5x
10
24
1.0
x
10
18
2.3
x
10
16
1.7x
10
20
2.5
x
10
19
1.4x
10
8
Flux
(cm-
2
s-')
-4.0
x
10
9
7.2
x
10
9
-9.7
x
10
3
-3.3
x
10
3
-2.9
x
10
9
-4.3
x
10
8
-2.3
x
10
7
From Gladstone
et
al.
(1996), cited
in
section 5.3.
All
fluxes
refer
to the
lower boundary, except
for the flux of H,
which refers
to the
upper boundary.
primary
photolysis
of CH4 is
only
a
small
fraction
(20%)
of the
total destruction.
Photosensitized dissociation
is
more important, especially
in the
stratosphere. (See
table
5.13
for a
listing
of the
column-integrated rates.)
Not all the
destruction
of
CH4
produces higher hydrocarbons; part
of the
dissociation products
is
recycled back
to
CH
4
.
The
major reactions that produce
CH4
in the
model, R82:
CH
3
+ H and
R134:
CH3
+ H2, are
shown
in
figure
5.15b.
For
comparison,
we
also show
the sum of all
reactions that destroy
CH\
as
well
as the sum of all
reactions that restore
CFU.
A
detailed breakdown
of the
destruction
and
restoration rates
of
CELi
is
summarized
in
table 5.13. Only about
70% of the
CH4
destruction results
in the
synthesis
of
higher
hydrocarbons;
the
rest
is
restored
to
CHU.
As can be
seen
in figure
5.15c,
the
Q
radical
species
CH,
J
CH2,
CH2,
and
CH
3
play
a
fundamental
role
in the
synthesis
of
hydrocarbons.
The
rates
of
fast recycling between
the
Ci
radicals
are
shown
in
figure
5.15c.
The
most important reactions included
are CH to CH2 via
R118:
CH +
H
2
,
return
of
CH
2
to CH via
R80:
CH
2
+
H,
CH to
CH
3
via
R119:
CH +
H
2
,
and
'CH
2
to
CH
3
via
R125:
'CH2
+
H
2
.
Note
that
the
production
rate
of CH by R80 is
more
than
an
order
of
magnitude greater than that
of
direct photolysis,
R7:
CH
4
+ hv.
Nearly
all the
'CH2
produced
in R5:
CHt
+ hv is
converted
to
CH
3
even though there
is
little
or no
production
of
CH
3
from
direct photolysis.
The
production
rates
of
C
2
compounds
are
determined
by the
rate-limiting reactions R120:
CH +
CH*
and
R135:
CH
3
+
CH
3
,
as
shown
in figure
5.15d.
The
principal reactions
of
destruction
of the
C
2
compounds
are
R87:
C
2
H
5
+ H and
R122:
CH +
C
2
H4,
as
shown
in figure
5.15d.
The
last reaction results
in the
production
of a
C
3
compound.
The
detailed production
and
destruction rates
of the C2
hydrocarbons
are
summarized
in
table
5.13.
The
Lyman
a
brightness
of
Jupiter indicates that
H
atoms
are
abundant
in the
upper atmosphere
of the
planet. Radiative models that
can
simulate
the
observations
suggest that
the
column abundance
of H
atoms
is
about
10
17
cm~
2
,
consistent
with
the
prediction
of the
present model.
As
shown later
(in figure
5.17),
the
maximum mixing
ratio above
the
homopause
(10~
3
mbar)
approaches
1%,
making
it the
most abundant
radical
in the
model.
A
large number
of
reactions
produce
H
atoms
in the
model;
the
rates
of a few
more important reactions
are
given
in figure
5.16a,
along
with
the
total
rate
of
production.
As can be
seen
from figure
5.16a,
the
most important source
is
scheme (VI), driven
by the
photosensitized dissociation
of H2 by
C2H
2
.
The
total
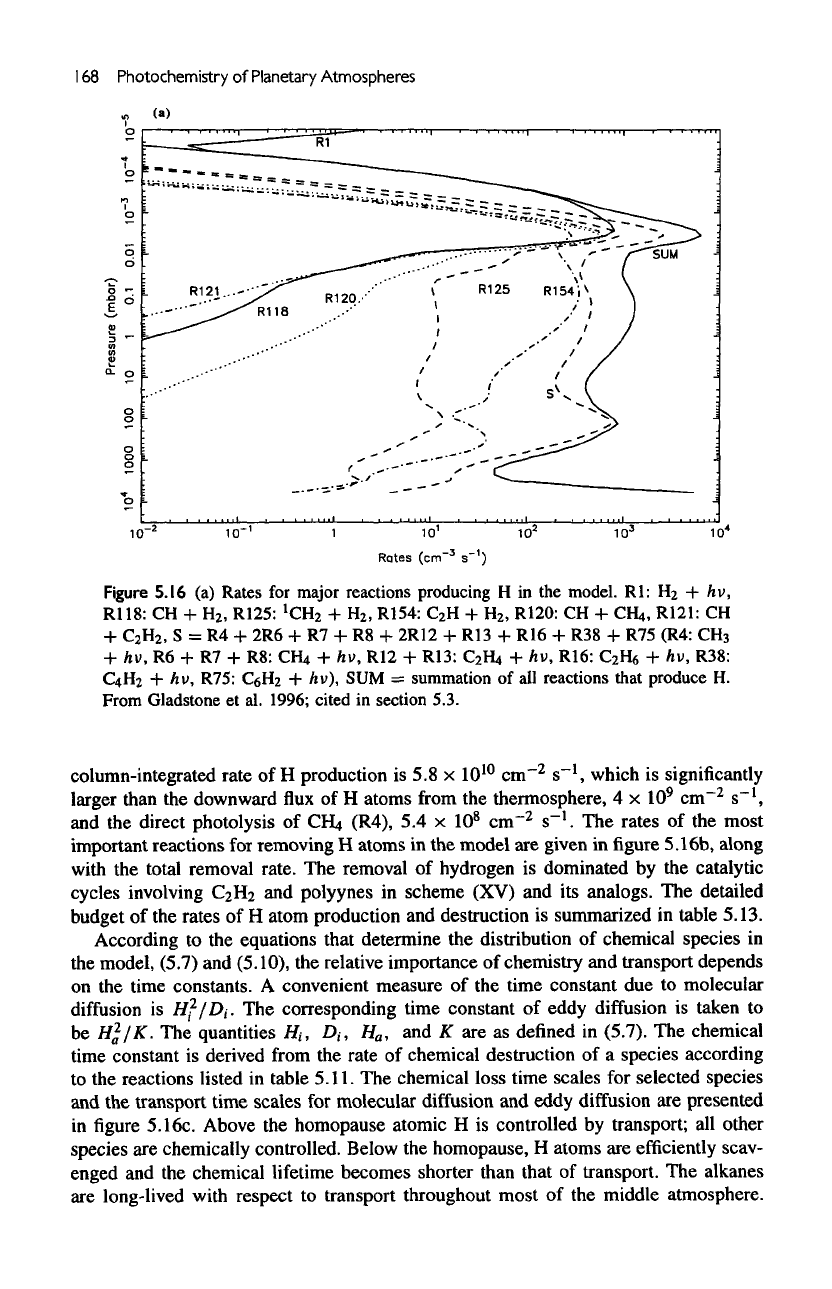
168
Photochemistry
of
Planetary Atmospheres
Figure
5.16
(a)
Rates
for
major
reactions
producing
H in the
model.
Rl:
H2 +
hv,
R118:
CH +
H
2
,
R125:
'CH
2
+
H
2
,
R154:
C
2
H
+
H
2)
R120:
CH +
CU,,
R121:
CH
+
C
2
H
2
,
S = R4 + 2R6 + R7 + R8 +
2R12
+ R13 + R16 + R38 + R75
(R4:
CH
3
+ hv, R6 + R7 + R8:
CUt
+ hv, R12 +
R13:
C
2
Ht
+ hv,
R16:
C
2
H
6
+ hv,
R38:
C4H
2
+ hv,
R75:
CeH
2
+
hv),
SUM
=
summation
of all
reactions
that
produce
H.
From
Gladstone
et
al.
1996;
cited
in
section
5.3.
column-integrated rate
of H
production
is 5.8 x
10
10
cm
2
s
',
which
is
significantly
larger than
the
downward
flux of H
atoms
from
the
thermosphere,
4 x
10
9
cm~
2
s"
1
,
and
the
direct photolysis
of
CEL»
(R4),
5.4 x
10
8
cm~
2
s"
1
.
The
rates
of the
most
important
reactions
for
removing
H
atoms
in the
model
are
given
in
figure
5.16b,
along
with
the
total removal rate.
The
removal
of
hydrogen
is
dominated
by the
catalytic
cycles involving
€2^2
and
polyynes
in
scheme (XV)
and its
analogs.
The
detailed
budget
of the
rates
of H
atom production
and
destruction
is
summarized
in
table
5.13.
According
to the
equations that determine
the
distribution
of
chemical species
in
the
model,
(5.7)
and
(5.10),
the
relative importance
of
chemistry
and
transport depends
on
the
time constants.
A
convenient measure
of the
time constant
due to
molecular
diffusion
is
//?/D,.
The
corresponding time constant
of
eddy
diffusion
is
taken
to
be
H%/K.
The
quantities
H
t
,
D,,
H
a
,
and K are as
defined
in
(5.7).
The
chemical
time
constant
is
derived
from
the
rate
of
chemical destruction
of a
species according
to the
reactions listed
in
table
5.11.
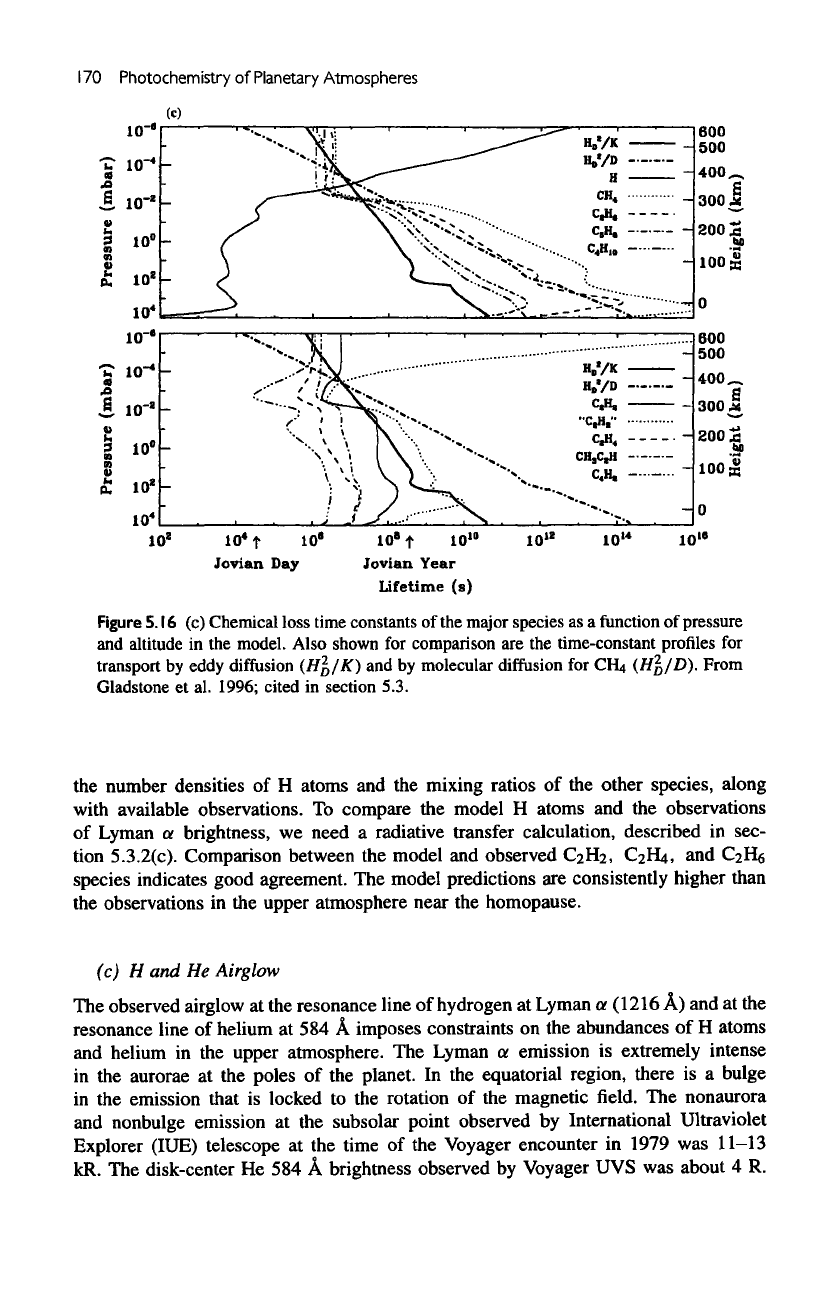
The
chemical loss time scales
for
selected species
and the
transport time
scales
for
molecular
diffusion
and
eddy
diffusion
are
presented
in
figure
5.16c.
Above
the
homopause atomic
H is
controlled
by
transport;
all
other
species
are
chemically controlled. Below
the
homopause,
H
atoms
are
efficiently
scav-
enged
and the
chemical
lifetime
becomes shorter than that
of
transport.
The
alkanes
are
long-lived with respect
to
transport throughout most
of the
middle atmosphere.
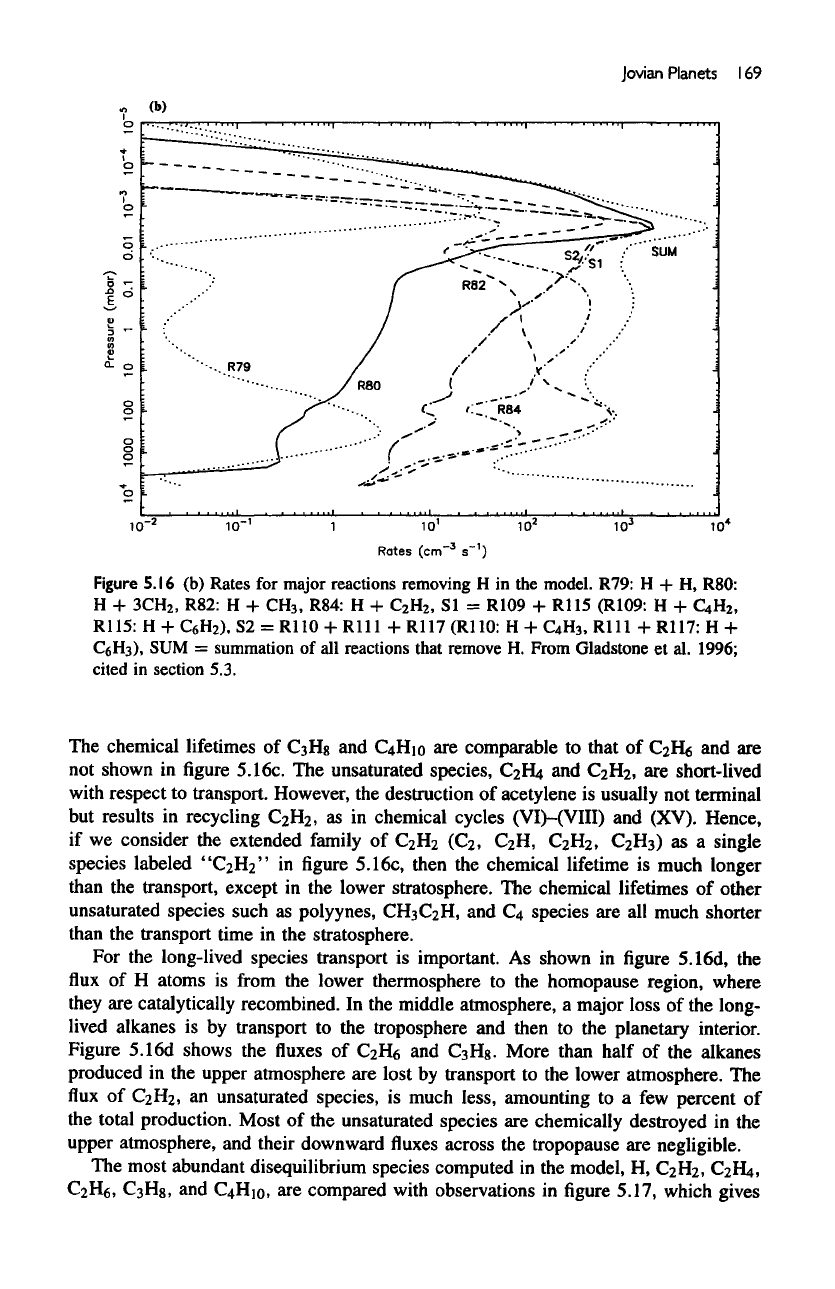
Jovian
Planets
169
Figure
5.16
(b)
Rates
for
major
reactions
removing
H in the
model.
R79:
H + H,
R80:
H
+
3CH
2
,
R82:
H +
CH
3
,
R84:
H +
C
2
H
2
,
SI
=
R109
+
R115
(R109:
H +
C,H
2
,
R115:
H +
C
6
H
2
),
S2 =
R110
+
Rill
+
R117
(R110:
H +
C
4
H
3
,
Rill
+
R117:
H +
QjHs),
SUM =
summation
of all
reactions
that
remove
H.
From
Gladstone
et
al.
1996;
cited
in
section
5.3.
The
chemical lifetimes
of
CsHg
and
C4Hio
are
comparable
to
that
of
C
2
H«
and are
not
shown
in
figure
5.16c.
The
unsaturated
species,
C
2
ftt
and
C
2
H2,
are
short-lived
with
respect
to
transport. However,
the
destruction
of
acetylene
is
usually
not
terminal
but
results
in
recycling
C
2
H
2
,
as in
chemical cycles
(VI)-(VIII)
and
(XV). Hence,
if
we
consider
the
extended
family
of
C
2
H
2
(C
2
,
C
2
H,
C
2
H
2
,
C
2
Hs)
as a
single
species
labeled
"C
2
H2"
in figure
5.16c,
then
the
chemical lifetime
is
much longer
than
the
transport, except
in the
lower stratosphere.
The
chemical lifetimes
of
other
unsaturated
species such
as
polyynes,
CH3C
2
H,
and
€4
species
are all
much shorter
than
the
transport time
in the
stratosphere.
For the
long-lived species transport
is
important.
As
shown
in figure
5.16d,
the
flux
of
H
atoms
is
from
the
lower
thermosphere
to the
homopause
region, where
they
are
catalytically
recombined.
In the
middle atmosphere,
a
major
loss
of the
long-
lived
alkanes
is by
transport
to the
troposphere
and
then
to the
planetary interior.
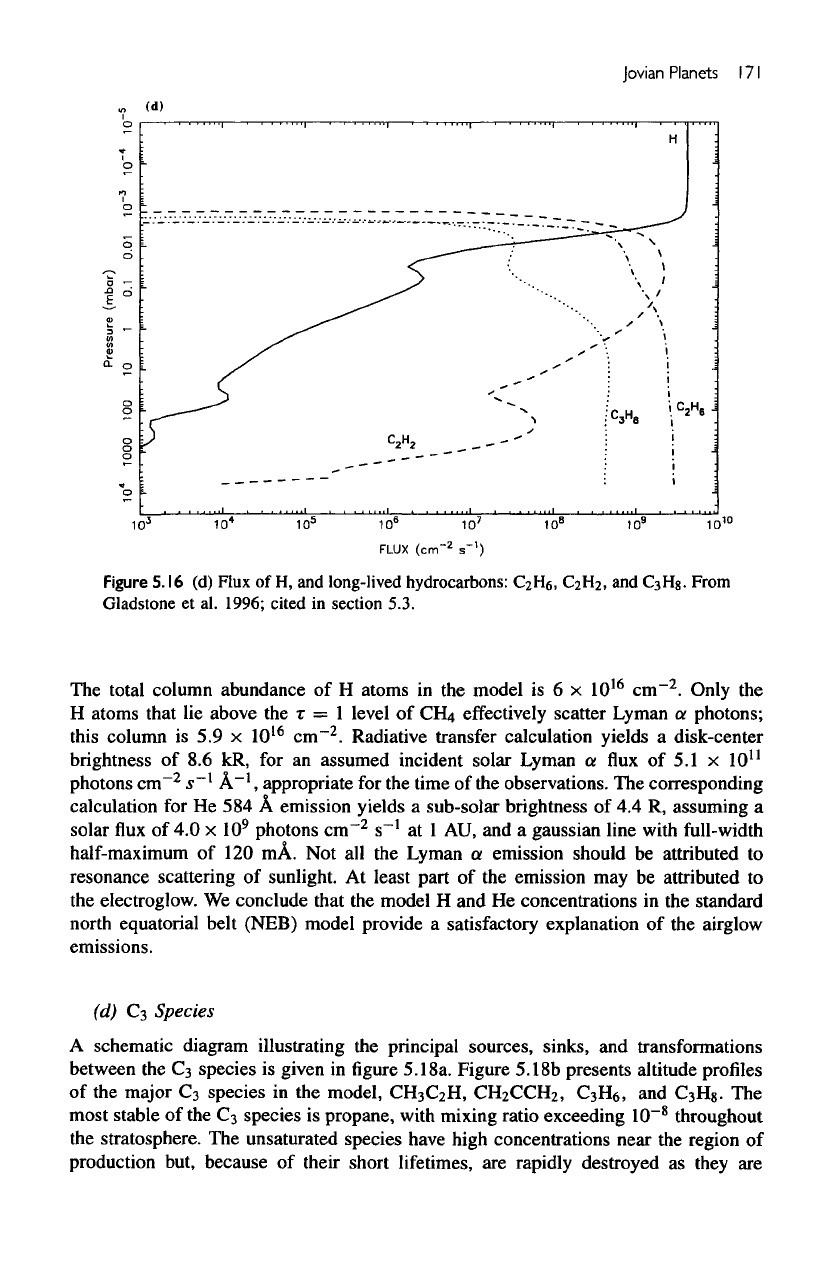
Figure
5.16d
shows
the fluxes of
C
2
He
and
C
3
Hg.
More than half
of the
alkanes
produced
in the
upper atmosphere
are
lost
by
transport
to the
lower atmosphere.
The
flux of
C
2
H
2
,
an
unsaturated species,
is
much less, amounting
to a few
percent
of
the
total production. Most
of the
unsaturated species
are
chemically destroyed
in the
upper
atmosphere,
and
their
downward
fluxes
across
the
tropopause
are
negligible.
The
most abundant disequilibrium species computed
in the
model,
H,
C
2
H
2
,
C
2
H4,
C
2
He,
CsHg,
and
C4Hio,
are
compared
with
observations
in figure
5.17, which gives
170
Photochemistry
of
Planetary Atmospheres
Figure
5.16
(c)
Chemical
loss
time
constants
of the
major
species
as a
function
of
pressure
and
altitude
in the
model.
Also
shown
for
comparison
are the
time-constant
profiles
for
transport
by
eddy
diffusion
(H%/K)
and by
molecular
diffusion
for
CtLt
(H^/D).
From
Gladstone
et
al.
1996;
cited
in
section
5.3.
the
number densities
of H
atoms
and the
mixing ratios
of the
other
species,
along
with
available observations.
To
compare
the
model
H
atoms
and the
observations
of
Lyman
a
brightness,
we
need
a
radiative transfer calculation, described
in
sec-
tion
5.3.2(c).
Comparison between
the
model
and
observed
Calfe,
€2^4,
and
C2Hg
species
indicates good agreement.
The
model predictions
are
consistently higher than
the
observations
in the
upper atmosphere near
the
homopause.
(c)
H and He
Airglow
The
observed airglow
at the
resonance line
of
hydrogen
at
Lyman
or
(1216
A) and at the
resonance line
of
helium
at 584 A
imposes constraints
on the
abundances
of H
atoms
and
helium
in the
upper atmosphere.
The
Lyman
a
emission
is
extremely intense
in
the
aurorae
at the
poles
of the
planet.
In the
equatorial region, there
is a
bulge
in
the
emission that
is
locked
to the
rotation
of the
magnetic
field. The
nonaurora
and
nonbulge emission
at the
subsolar
point observed
by
International Ultraviolet
Explorer (IUE)
telescope
at the
time
of the
Voyager encounter
in
1979
was
11-13
kR.
The
disk-center
He 584 A
brightness observed
by
Voyager
UVS was
about
4 R.
Jovian Planets
171
Figure
5.
1
6
(d)
Flux
of H, and
long-lived hydrocarbons:
Gladstone
et
al.
1996; cited
in
section 5.3.
,
C2H2,
and
C^Hg.
From
The
total column abundance
of H
atoms
in the
model
is 6 x
10
16
cm
2
.
Only
the
H
atoms that
lie
above
the r = 1
level
of CH4
effectively
scatter
Lyman
a
photons;
this
column
is 5.9 x
10
16
cm""
2
.
Radiative transfer calculation yields
a
disk-center
brightness
of 8.6 kR, for an
assumed incident solar Lyman
a flux of 5.1 x
10
11
photons
cm~
2
s~
l
A"
1
,
appropriate
for the
time
of the
observations.
The
corresponding
calculation
for He 584 A
emission yields
a
sub-solar brightness
of 4.4 R,
assuming
a
solar
flux of 4.0 x
10
9
photons
cm"
2
s"
1
at 1 AU, and a
gaussian line with
full-width
half-maximum
of 120
mA.
Not all the
Lyman
a
emission should
be
attributed
to
resonance scattering
of
sunlight.
At
least part
of the
emission
may be
attributed
to
the
electroglow.
We
conclude that
the
model
H and He
concentrations
in the
standard
north
equatorial belt (NEB) model provide
a
satisfactory explanation
of the
airglow
emissions.
(d)
€3
Species
A
schematic diagram illustrating
the
principal sources, sinks,
and
transformations
between
the
€3
species
is
given
in figure
5.18a.
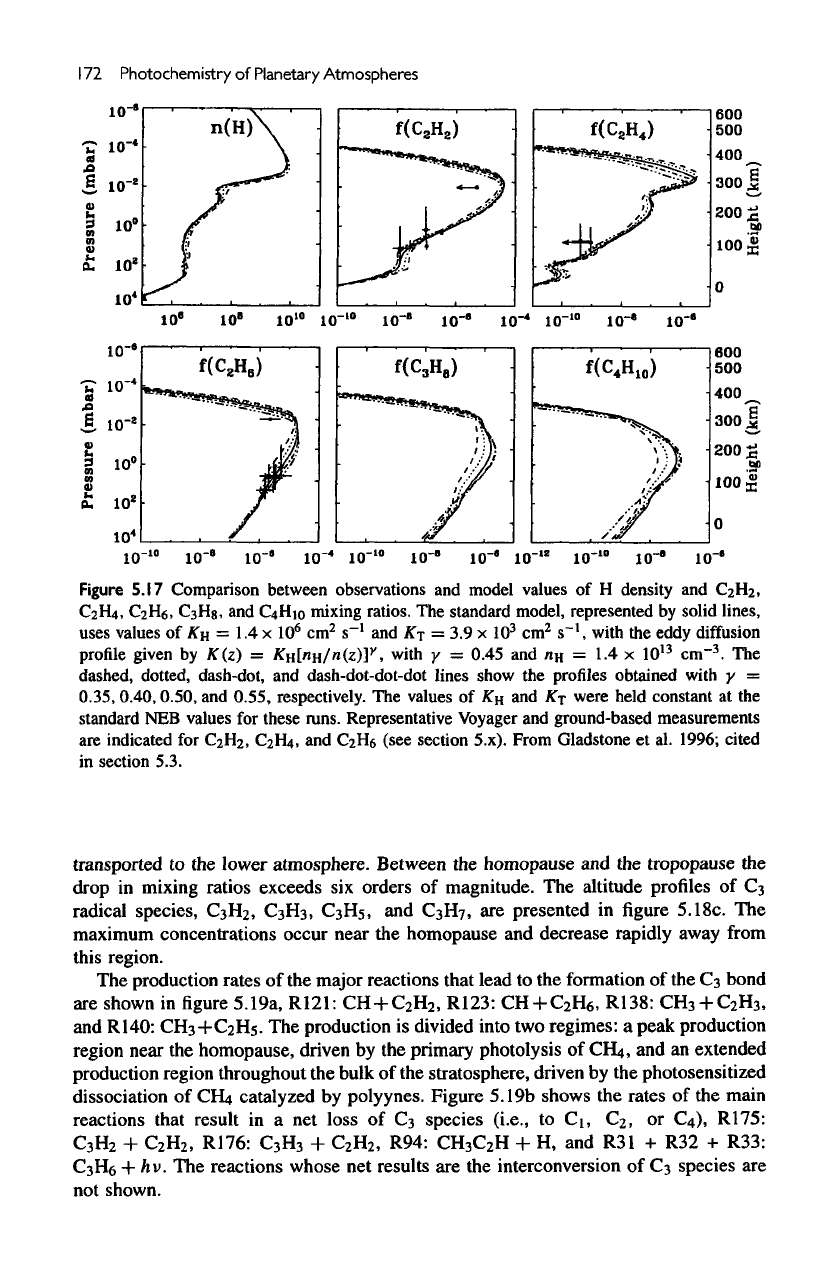
Figure
5.18b
presents altitude profiles
of
the
major
C
3
species
in the
model,
CH
3
C
2
H,
CH
2
CCH
2
,
C
3
H
6
,
and
C
3
H
8
.
The
most
stable
of the
C
3
species
is
propane,
with mixing
ratio
exceeding
10~
8
throughout
the
stratosphere.
The
unsaturated
species
have high concentrations near
the
region
of
production
but, because
of
their short lifetimes,
are
rapidly destroyed
as
they
are
172
Photochemistry
of
Planetary
Atmospheres
Figure
5.17
Comparison between observations
and
model values
of H
density
and
/t,
C2Hfi,
CsHg,
and
C4Hio
mixing ratios.
The
standard model, represented
by
solid lines,
uses values
of
KH
=
1.4
x
10
6
cm
2
s"
1
and
KI
= 3.9 x
10
3
cm
2
s"
1
,
with
the
eddy
diffusion
profile
given
by
K(z)
=
Kn[n
H
/n(z)V,
with
y
=
0.45
and
n»
= 1.4 x
10
13
cm~
3
.
The
dashed,
dotted,
dash-dot,
and
dash-dot-dot-dot
lines
show
the
profiles obtained with
y =
0.35, 0.40, 0.50,
and
0.55, respectively.
The
values
of
AT
H
and
AT
T
were held constant
at the
standard
NEB
values
for
these runs. Representative Voyager
and
ground-based measurements
are
indicated
for
CiFh,
C2H4,
and
CiHe
(see section 5.x). From Gladstone
et
al.
1996; cited
in
section 5.3.
transported
to the
lower
atmosphere.
Between
the
homopause
and the
tropopause
the
drop
in
mixing ratios exceeds
six
orders
of
magnitude.
The
altitude profiles
of
C
3
radical species,
C
3
H
2
,
C
3
H
3>
C
3
Hs,
and
C
3
H7,
are
presented
in
figure
5.18c.
The
maximum
concentrations occur near
the
homopause
and
decrease rapidly away
from
this
region.
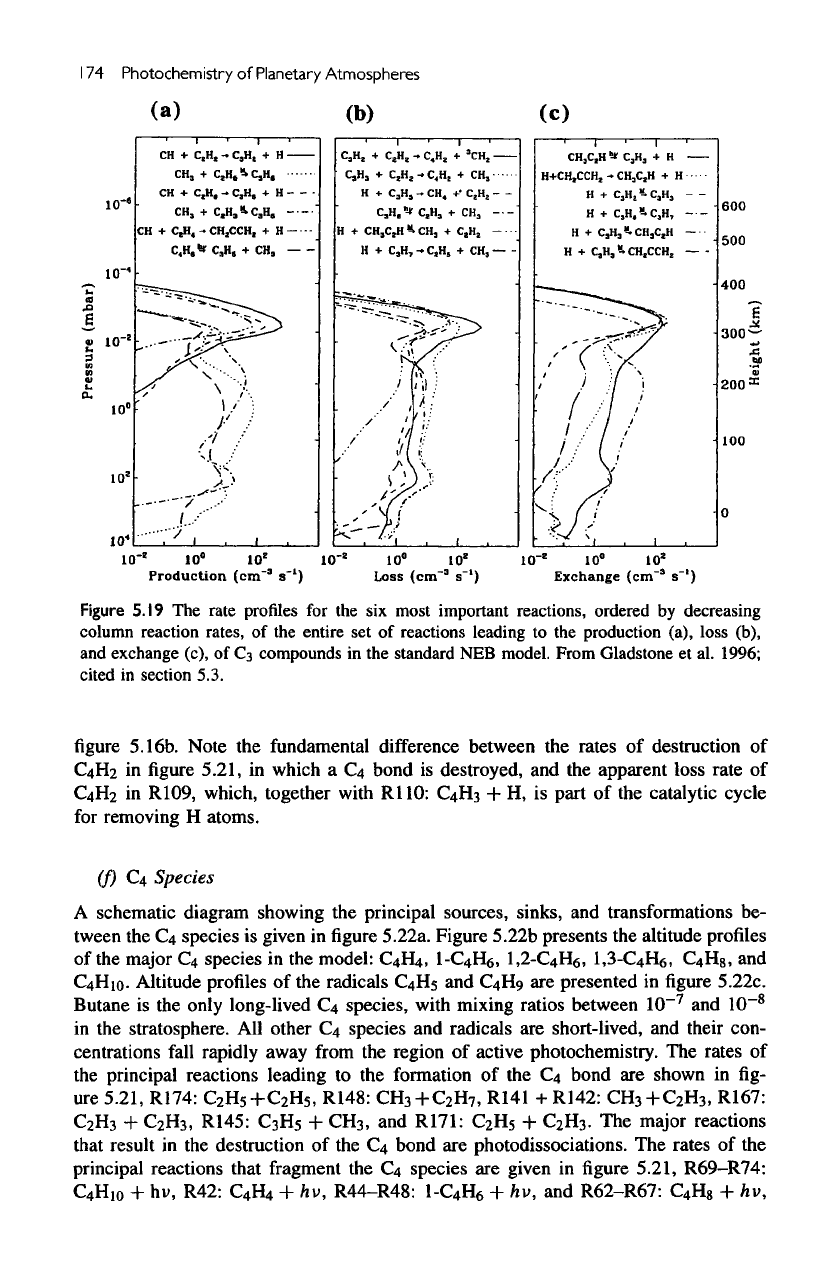
The
production rates
of the
major
reactions that lead
to the
formation
of the
C
3
bond
are
shown
in
figure
5.19a,
R121:
CH +
C
2
H
2)
R123:
CH +
C
2
H
6
,
R138:
CH
3
+
C
2
H
3
,
and
R140:
CH
3
+C
2
H5.
The
production
is
divided into
two
regimes:
a
peak production
region
near
the
homopause, driven
by the
primary photolysis
of
CHj,
and an
extended
production
region throughout
the
bulk
of the
stratosphere, driven
by the
photosensitized
dissociation
of
CHj
catalyzed
by
polyynes. Figure
5.19b
shows
the
rates
of the
main
reactions
that
result
in a net
loss
of
C
3
species (i.e.,
to
Ci,
C
2
,
or
C4>,
R175:
C
3
H
2
+
C
2
H
2
,
R176:
C
3
H
3
+
C
2
H
2
,
R94:
CH
3
C
2
H
+ H, and R31 + R32 +
R33:
C
3
He
+
hv.
The
reactions whose
net
results
are the
interconversion
of
C
3
species
are
not
shown.
Jovian
Planets
173
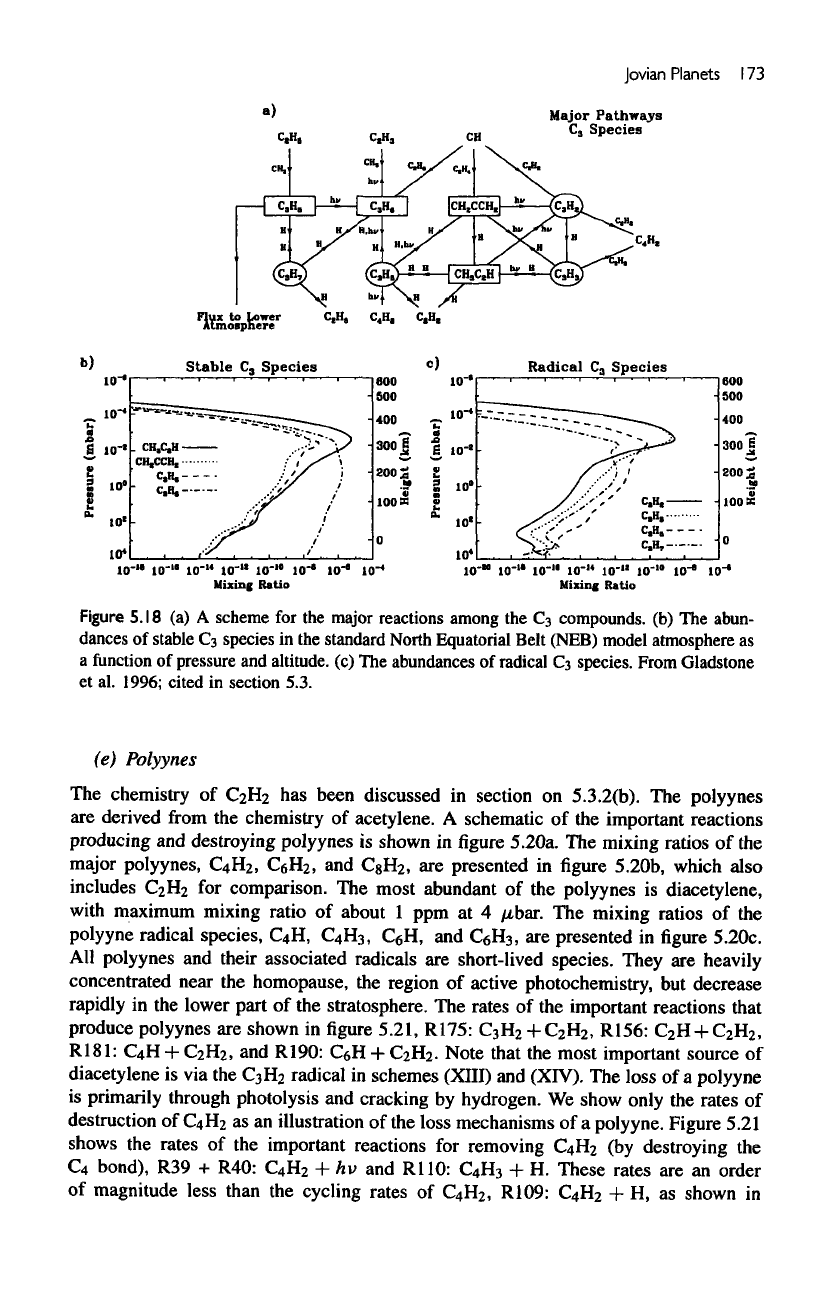
Figure
5.18
(a) A
scheme
for the
major reactions among
the
C
3
compounds,
(b) The
abun-
dances
of
stable
C
3
species
in the
standard North Equatorial Belt (NEB) model atmosphere
as
a
function
of
pressure
and
altitude,
(c) The
abundances
of
radical
C
3
species.
From Gladstone
et
al.
1996; cited
in
section 5.3.
(e)
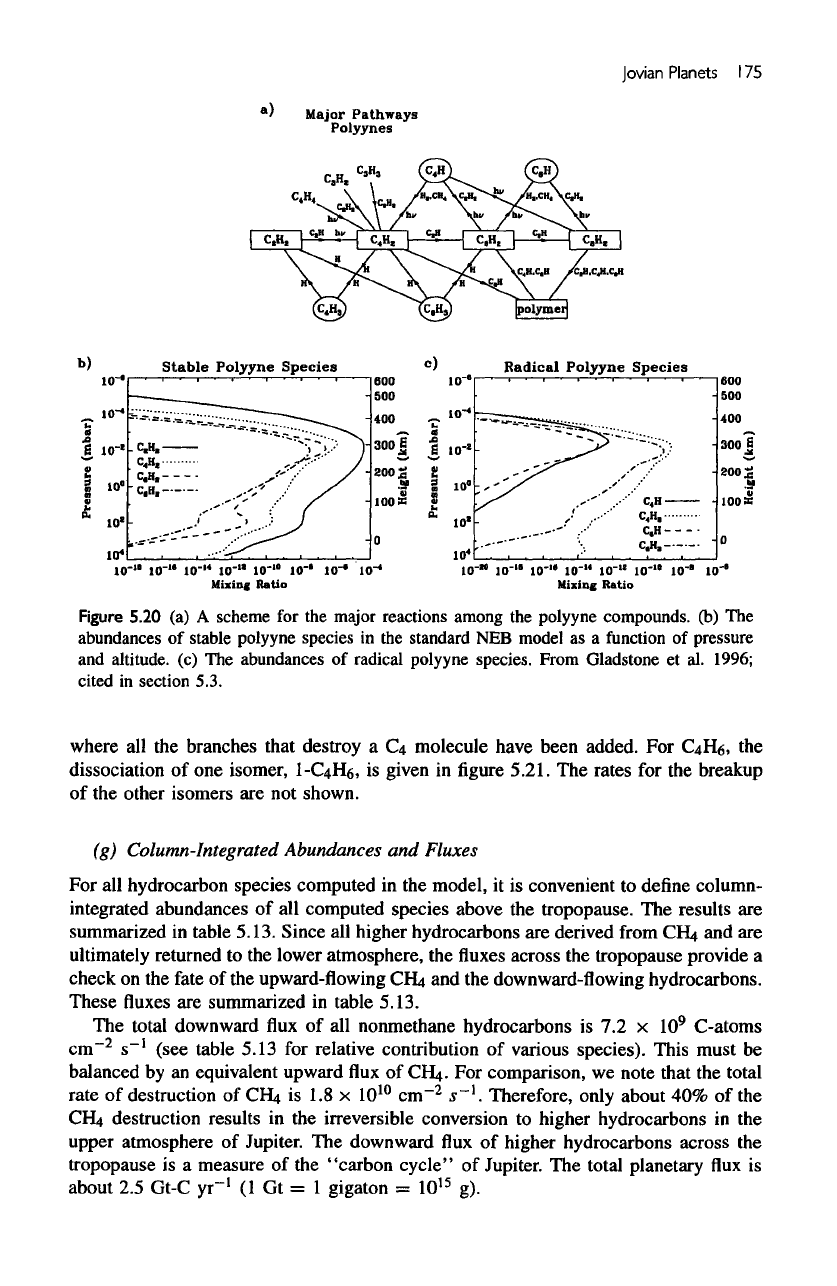
Polyynes
The
chemistry
of
C
2
H
2
has
been discussed
in
section
on
5.3.2(b).
The
polyynes
are
derived
from
the
chemistry
of
acetylene.
A
schematic
of the
important reactions
producing
and
destroying
polyynes
is
shown
in figure
5.20a.
The
mixing
ratios
of the
major
polyynes,
C
4
H
2
,
CeHa,
and
CgH
2
,
are
presented
in figure
5.20b,
which
also
includes
C
2
H
2
for
comparison.
The
most abundant
of the
polyynes
is
diacetylene,
with
maximum mixing ratio
of
about
1
ppm
at 4
/ibar.
The
mixing ratios
of the
polyyne
radical species,
C
4
H,
C
4
H
3
,
C
6
H,
and
C6H
3
,
are
presented
in figure
5.20c.
All
polyynes
and
their associated radicals
are
short-lived
species.
They
are
heavily
concentrated near
the
homopause,
the
region
of
active photochemistry,
but
decrease
rapidly
in the
lower part
of the
stratosphere.
The
rates
of the
important reactions that
produce polyynes
are
shown
in figure
5.21, R175:
C
3
H
2
+
C2H
2
,
R156:
C
2
H
+
C
2
H
2
,
R181:
C
4
H
+
C
2
H
2
,
and
R190:
C
6
H
+
C
2
H
2
.
Note that
the
most important source
of
diacetylene
is via the
C
3
H
2
radical
in
schemes
(XIII)
and
(XIV).
The
loss
of a
polyyne
is
primarily through photolysis
and
cracking
by
hydrogen.
We
show
only
the
rates
of
destruction
of
C
4
H
2
as an
illustration
of the
loss mechanisms
of a
polyyne. Figure 5.21
shows
the
rates
of the
important reactions
for
removing
C
4
H
2
(by
destroying
the
C
4
bond),
R39 +
R40:
C
4
H
2
+ hv and
R110:
C
4
H
3
+ H.
These rates
are an
order
of
magnitude less than
the
cycling rates
of
C
4
H
2
,
R109:
C
4
H
2
+ H, as
shown
in
174
Photochemistry
of
Planetary Atmospheres
(a) (b)
(C)
Figure
5.19
The
rate
profiles
for the six
most
important
reactions,
ordered
by
decreasing
column
reaction
rates,
of the
entire
set of
reactions
leading
to the
production
(a),
loss
(b),
and
exchange
(c),
of
Ci
compounds
in the
standard
NEB
model.
From
Gladstone
et
al.
1996;
cited
in
section
5.3.
figure
5.16b.
Note
the
fundamental
difference between
the
rates
of
destruction
of
in
figure
5.21,
in
which
a
€4
bond
is
destroyed,
and the
apparent loss rate
of
in
R109, which, together
with
RUO:
C4H
3
+ H, is
part
of the
catalytic cycle
for
removing
H
atoms.
(f)
C
4
Species
A
schematic diagram showing
the
principal sources, sinks,
and
transformations
be-
tween
the
€4
species
is
given
in figure
5.22a.
Figure 5.22b presents
the
altitude profiles
of
the
major
€4
species
in the
model:
C4H4,
1-C
4
H6,
1,2-C4H
6
,
1,3-C4H
6
,
C
4
H
8
,
and
C4Hio-
Altitude profiles
of the
radicals
€4*15
and
C4H9
are
presented
in figure
5.22c.
Butane
is the
only
long-lived
C4
species,
with mixing ratios between
10~
7
and
10~
8
in
the
stratosphere.
All
other
C$
species
and
radicals
are
short-lived,
and
their con-
centrations
fall
rapidly away
from
the
region
of
active photochemistry.
The
rates
of
the
principal reactions leading
to the
formation
of the
C*
bond
are
shown
in fig-
ure
5.21, R174:
C
2
H5+C
2
H5,
R148:
CH
3
+C
2
H
7
,
R141
+
R142:
CH
3
+C
2
H
3
,
R167:
C
2
H
3
+
C
2
H
3
,
RMS:
C
3
H
5
+
CH
3
,
and
R171:
C
2
H
5
+
C
2
H
3
.
The
major
reactions
that
result
in the
destruction
of the
€4
bond
are
photodissociations.
The
rates
of the
principal
reactions that
fragment
the C4
species
are
given
in figure
5.21,
R69-R74:
C
4
H
10
+
hi>,
R42:
C
4
H
4
+
hv,
R44-R48:
1-C
4
H
6
+ hv, and
R62-R67:
C
4
H
g
+
hv,
lovian
Planets
175
Figure
5.20
(a) A
scheme
for the
major
reactions
among
the
polyyne
compounds,
(b)
The
abundances
of
stable
polyyne
species
in the
standard
NEB
model
as a
function
of
pressure
and
altitude,
(c) The
abundances
of
radical
polyyne
species.
From
Gladstone
et
al.
1996;
cited
in
section
5.3.
where
all the
branches that destroy
a
€4
molecule have been
added.
For
C^g,
the
dissociation
of one
isomer,
l-C4He,
is
given
in
figure
5.21.
The
rates
for the
breakup
of
the
other isomers
are not
shown.
(g)
Column-Integrated Abundances
and
Fluxes
For all
hydrocarbon
species
computed
in the
model,
it is
convenient
to
define column-
integrated abundances
of all
computed
species
above
the
tropopause.
The
results
are
summarized
in
table
5.13.
Since
all
higher hydrocarbons
are
derived from
CUt
and are
ultimately
returned
to the
lower atmosphere,
the
fluxes
across
the
tropopause provide
a
check
on the
fate
of the
upward-flowing
CHt
and the
downward-flowing hydrocarbons.
These
fluxes are
summarized
in
table 5.13.
The
total downward
flux of all
nonmethane hydrocarbons
is 7.2 x
10
9
C-atoms
cm~
2
s"
1
(see table 5.13
for
relative contribution
of
various
species).
This must
be
balanced
by an
equivalent upward
flux of
CHj.
For
comparison,
we
note that
the
total
rate
of
destruction
of
CUt
is 1.8 x
10
10
cm~
2
j"
1
.
Therefore, only about
40% of the
CHj
destruction results
in the
irreversible conversion
to
higher hydrocarbons
in the
upper atmosphere
of
Jupiter.
The
downward
flux of
higher hydrocarbons across
the
tropopause
is a
measure
of the
"carbon
cycle"
of
Jupiter.
The
total planetary
flux is
about
2.5
Gt-C
yr~'
(1 Gt = 1
gigaton
=
10
15
g).
