Yung Y.L., DeMore W.B. Photochemistry of Planetary Atmospheres
Подождите немного. Документ загружается.

156
Photochemistry
of
Planetary Atmospheres
Photosensitized dissociation provides
an
efficient
path
for the
synthesis
of
higher
alkanes. Chemical schemes (VII)
and
(VIII) produce
alkyl
radicals
deep
in the
atmo-
sphere, where ternary reactions
are
efficient.
In
addition
to
methyl radical recombina-
tion
(5.39),
we can now
have alkyl recombinations such
as
We
give
two
examples
of
simple chemical schemes
for
synthesizing propane
(CaHg)
and
butane
(C4Hio)
from
the
simpler alkanes
CFU
and
C
2
He:
Note
the
difference between direct photolysis
and
photosensitized dissociation.
In
photolysis,
the
alkane usually loses
an
even number
of H
atoms
to
become
an
alkene
or
alkyne.
However,
in the
photosensitized dissociation only
one H
atom
is
abstracted.
The
resulting radical
can
then
react
to
form
higher alkanes.
It is
straightforward
to
generalize schemes (IX)
and (X) to
produce even more complex alkanes,
but
there
is
no
motivation
to go
beyond
€4
compounds
due to the
lack
of
observations
of
these
species.
In a
variant
of
schemes (IX)
and
(X),
we may
obtain
the
ethyl radical
from
the
direct recombination
of
C
2
H4
and H
instead
of the
photosensitized dissociation
of
C
2
H
6
.
(c)
C
3
Compounds
Our
knowledge
of
hydrocarbons that
are
more complex than
C
2
is
limited. Many
of
the
rate
coefficients
are not
known,
and we
have
to
estimate their magnitude based
on
analogies with similar reactions. Another problem
is the
large number
of
isomers
associated
with
the
higher hydrocarbons.
The
list compiled
for
table
5.9
does
not
include
all the
isomers.
Few of
these more complex hydrocarbons computed
in the
model have been observed.
The
principal
C
3
compounds considered
in the
model
are the two
isomers
of
C
3
H4,
methyl
acetylene
(CH
3
C
2
H)
and
allene
(CH
2
CCH
2
),
and
propene
(C
3
Hg)
and
propane
(C
3
Hg).
C
3
compounds
can be
formed
by
insertion
of a
Cj
radical into
a
C
2
compound,
as in the
following examples:
Jovian
Planets
157
where
the
methylidyne radical,
CH, is
derived
from
either
CH4
photolysis
or the
reaction
CH2 + H
(5.34).
The
radical
C3H2
can
undergo successive hydrogenation
to
form
methyl acetylene
and
allene:
The
allene formed
in
(5.52)
and
(5.55b)
isomerizes
rapidly through
the
exchange
reaction
This
is
known
as a
telescopic reaction:
The H
atom
is
attached
to one end of
allene,
followed
by the
ejection
of the H
atom
at the
other
end of the
molecule.
The net
result
is the
conversion
of a
pair
of
double bonds into
a
triple
and a
single bond.
The
reaction
is
exothermic
to the
right
by
about
one
kcal
mole"
1
.
The
simplest paths
to
form methyl acetylene
and
allene would
be the
insertion
reactions
Early
laboratory experiments showed that
the
reactions could
be
fast. However,
a
more
recent study showed that
the
reactions have high activation energies
and
cannot play
a
significant role
in the
atmospheres
of the
outer solar system.
Recombination
of
radicals provides alternative pathways
of
forming
C
3
compounds.
Examples include
the
recombination
of
alkyl
radicals mentioned earlier
(5.49)
and the
following
reaction forming propene:
where
the
vinyl
radical
(C
2
H
3
)
is
produced
by
reaction
(5.72)
between
H and
C
2
H
2
[see section
5.3.1(e)].
Photolysis
provides
a
major pathway
for the
destruction
of
C
3
compounds:
1
58
Photochemistry
of
Planetary
Atmospheres
where
C^H^
is
either
CHsCaH
or
CH2CCH2,
and we
have listed only
the
most
im-
portant
channels
of
dissociation (for
a
complete
listing,
see
table
5.10).
Of the
above
reactions,
CsHg
dissociation (5.61)
is
partially shielded
by
CHU
and is not as
fast
as
those
of
CsHe
and
CjlLi.
Most
of the
€3
dissociations result
in the
elimination
of
hydrogen. There
is no net
loss
of
€3
compounds. Only reactions such
as
(5.60d),
(5.61b),
and
(5.61c)
lead
to
fragmentation
of
Cj
into species
of
lower carbon number.
In
addition
to
loss
by
photolysis,
we
show
in
section
5.3.1(e)
that
the
unsaturated
compounds such
as
€3114
and
C^H^
may be
destroyed
by
cracking
by
hydrogen.
(d)
Polyynes
The
polyynes
are
long-chain acetylenelike compounds.
The
simplest example
is di-
acetylene
(C
4
H
2
)
formed
by
This
process
can be
repeated with
the
ethynyl radical inserting into
the
C4H
2
and
higher
polyynes:
where,
for
n
= 1,
chemical scheme (XII)
is the
same
as
scheme (XI).
For n = 2,
3, 4, we
have
the
production
of
CeH
2
,
CgH
2
,
and
CioH
2>
respectively.
In the
model
we
do not go
beyond
CgH
2
.
All
polyynes with more than eight carbon atoms
are
labeled
"products."
They
are
expected
to
condense
in the
form
of
polymers
and
will
not
further
participate
in
chemical reactions.
The
polyynes absorb ultraviolet radiation,
resulting
in the
production
of
radicals that further participate
in the
synthesis
of
higher
polyynes.
The
following example illustrates this mechanism:
There
are
alternative paths
to
synthesize
the
most important
of the
polyynes,
di-
acetylene:
where
the
C3H
2
and
CsH3
radicals
are
derived
from
the
insertion reaction
of CH
(5.51)
and
photolysis
of
C3H
4
(5.59).
The
chemical
schemes
are

Jovian
Planets
159
where
C
3
HU
is
either
CH
3
C
2
H
or
CH
2
CCH
2
;
the
recombination
of
CH
3
and H
(5.68)
is
discussed
in
section
5.3.1(e).
By
analogy
with
acetylene, photolysis
of
polyynes
can
also result
in the
photo-
sensitized dissociation
of
H
2
,
CH
4
,
and
higher alkanes.
The
primary reaction
is the
production
of the
C
2n
H
radical, where
«
= 2, 3, 4, in
reaction
(5.64).
The
C
2n
H
radical
readily attacks
H
2
and
alkanes:
The net
results
may be
summarized
by
chemical schemes similar
to
(VI)-(VIII)
with
the
radical
C2«H
taking
the
place
of
C
2
H.
Since
the
polyynes absorb ultraviolet
ra-
diation
at
even longer wavelengths
than
C
2
H
2
,
the
contribution
of
higher polyynes
to
photosensitized dissociation
is
appreciable.
(e)
H
Atoms
Direct photolysis
of
H
2
by the
absorption
of
EUV
radiation
is a
minor source
of H
atoms
in the
upper atmosphere
of
Jupiter.
The
most important
sources
are
photolysis
of
CH
4
and
C
2
H
4
,
photosensitized dissociation
by
schemes (VI),
(Xlla),
and
(Xllb)
and
abstraction reactions such
as
(5.32)
and
(5.33).
In
addition, impact dissociation
by
energetic electrons
in the
aurorae
at the
polar atmosphere
of the
planet provides
a
net
source
of H
atoms.
The
presence
of
large quantities
of H
atoms
has a
serious impact
on the
chemical
composition
of the
atmosphere.
In
section
5.3.
l(d)
we
discussed
the
formation
of
higher
hydrocarbons
from
CH
4
,
a
molecule
with
the
highest
H/C
ratio. Since
the
higher
hydrocarbons
have
a
lower
H/C
ratio,
this
organic synthesis
is
always accompanied
by
the
production
of
hydrogen,
as
either
H
2
or H. If the
by-product
is
H
2
,
there
is
no
further
reaction
due to the
great stability
of
H
2
.
However,
if the
by-product
is
H,
this
can
lead
to
further
reactions. First,
H
atoms
can
scavenge
CH
3
radicals
via
reaction
(5.68).
The net
result
is to
restore
the
dissociation products back
to
CH
4
.
Indeed,
a
substantial fraction
of the
dissociation (direct photolysis
or
photosensitized
dissociation)
of
CH
4
does
not
result
in the
synthesis
of
higher hydrocarbons
but
returns
to
CH
4
via
(5.68).
The
efficiency
at
which
the
higher hydrocarbons
can be
synthesized depends
to a
large
extent
on how
rapidly
H
atoms
can be
removed.
The
direct recombination
of H
atoms
to
form
a
simple molecule like
H
2
is too
slow (see section 3.5). There
is no
effective
sink
for H
atoms above
the
homopause. This explains
the
high abundance
of H in the
thermosphere,
where
its
maximum mixing ratio becomes
as
high
as
1%.
The
primary loss
of H is via
transport
to
below
the
homopause, where catalytic
recombination
can
take place.
The
most important scheme
for
removing
H
atoms
is
160
Photochemistry
of
Planetary
Atmospheres
where
the
rate-limiting step
is the
formation
of the
vinyl
radical
(C
2
H3).
There
are
similar
schemes involving diacetylene
and
higher polyynes,
and
these schemes
are
important
for
scavenging
H
atoms.
Hydrogen
atoms that
are not
catalytically recombined
can
drive
a
number
of
inter-
esting
reactions, including
hydrogenation,
as
shown
by
where
abstraction
of a
hydrogen atom
from
H
2
by the
vinyl radical
is a
slow reaction
with
a
large activation energy.
It is
important
in the
atmosphere
of
Jupiter
but is
expected
to be
less important
in the
colder atmospheres
of the
other giant planets.
In
the
presence
of H,
further
hydrogenation
of
C
2
H4
is
possible:
The
general tendency
is
that unsaturated hydrocarbons
can be
hydrogenated
in the
presence
of H
atoms
to
become more saturated compounds.
At the
same time,
H
atoms
can be
recombined using
the
unsaturated hydrocarbons
as
catalysts.
A
variant
of
scheme (XVII)
may
result
in
hydrogenation
of
C
2
H4
all the way
back
to
CH
4
:
In
general, unsaturated compounds
may be
"cracked"
by H
atoms,
as in the
following
examples:
Note that
the
unsaturated species
can
scavenge
H
atoms
as in
catalytic cycle
(XV)
and its
analogs,
but the
unsaturated compounds
may in
turn
be
cracked
by H
atoms.
There
is
thus
a
complicated interaction between unsaturated species
and H
atoms.
They mutually regulate each others' abundances.
Jovian
Planets
1
6
1
(f)
C
4
Compounds
and
The
important
€4
compounds
are
vinyl
acetylene
(€4114),
1-butyne
(l^Hg),
1,2-
butadiene
(1,2-C
4
H
6
),
1,3-butadiene
(1,3-C
4
H
6
),
l-butene
(C
4
H
8
),
diacetylene
(C
4
H
2
),
and
butane (C4H[o).
The
last
two
molecules
are
discussed
in
sections 5.3. l(b)
and
5.3.1(d)
and are not
further
discussed here. With
the
exception
of
C4H2,
none
of the
above molecules
has
been detected
in the
atmospheres
of the
giant planets.
The
principal reactions that
form
the
€4
bond
are the
following radical-radical
reactions:
where
the
origin
of the
alkyl
radicals explained
in
section
5.3.
l(b);
the
vinyl
radical
(C
2
H
3
)
is
from
the
reaction
H +
C
2
H
2
(5.72),
and the
propargyl
radical
(C
3
H
3
)
is
from
the
photolysis
of
C
3
Ht
(5.59a).
The
C
3
Hs
radical
is
produced
by
where
C
3
HU
includes both
CH
3
C
2
H
and
CH
2
CCH
2
.
Photolysis transforms
one C4
compound into another
€4
compound:
Loss
of
C
4
compounds
occurs
when
the
photolysis
leads
to the
fragmentation
of the
C
4
species,
as in the
following
examples:
where
we
have shown only
the
more important branches
(for
a
complete listing
of
dissociation branches,
see
table
5.10).
The
dissociative
losses
are
sufficiently
fast
that
most
C
4
species
except butane
are
destroyed
in the
stratosphere before they
can be
transported
to the
lower atmosphere.
Besides
the
polyynes,
the
only
species
of
complex hydrocarbon containing more
than
four
carbon atoms considered
in the
model
is
benzene
(CeHg),
formed
by
162
Photochemistry
of
Planetary
Atmospheres
The
primary source
of the
C
4
Hs
radical
is
C
2
H
2
:
The
chemical scheme
for
synthesizing benzene
from
acetylene
is
The
efficiency
for
forming
benzene
is
reduced
by the
reaction with
H
2
:
This reaction competes with (5.91)
for the
C
4
Hs
radical.
It is
conceivable that syn-
thesis reaction (5.91) could
be
accelerated
if
C
2
H
2
were
in an
excited state
or by
ion
reactions
in
auroral regions
of the
atmosphere. Benzene
is a
very stable molecule.
Once formed,
it
cannot
be
chemically destroyed
in the
upper atmosphere.
The
ultimate
sink
is
pyrolysis
in the
interior
of the
planet.
5.3.2 Modeling Results
The
results
of a
model that
includes
the
photochemistry outlined
in
section
5.3.1
are
briefly
described.
The
standard model
is
appropriate
for the
north
equatorial
belt
(at
a
latitude
of 10° N) of
Jupiter. There
are a
total
of 39
species
and 194
reactions
in
the
model,
as
listed
in
tables 5.9, 5.10,
and
5.11,
respectively.
The set of
continuity
(5.10)
and
diffusion
(5.7) equations
are
solved
for all
species.
The
eddy
diffusivity
profile
is
given
in figure
5.11.
The
molecular
diffusion
coefficients
are
evaluated using
standard kinetic theory.
The
lower boundary
is at 6
bar, well below
the
tropopause
region (0.1 bar), where most
of the
long-lived species
and
photochemical aerosols
tend
to
accumulate.
At the
lower boundary
the
mixing ratios
of
H
2
,
He, and
CH
4
are
fixed
at
0.89,
0.11,
and 2.2 x
10~
3
,
respectively.
All
other species
(i) are
assumed
to
be
lost
from
the
lower boundary
at the
maximum
deposition
velocity
u>t
given
by
where
the
number density
(n,-),
eddy
diffusion
coefficient
(AT),
and
mean scale height
(H
a
)
all
refer
to 6
bar.
The
upper boundary
is
placed
at
10~
9
bar, well above
the
homopause region
(10~
6
bar) where most
of the
primary photochemistry occurs.
We
impose
a
downward
flux of
hydrogen atoms
of 4 x
10
9
cm~
2
s"
1
from
the
dissociation
of
H
2
in the
thermosphere.
Zero
flux
condition
is
imposed
for all
other
species.
(a)
Photodissociation
Table
5.10
lists
78
photodissociation reactions, along
with
their rate coefficients
at the
upper boundary
(1
nbar)
and in the
lower stratosphere (8.9
mbar).
The
solar
flux
used
corresponds
to
solar maximum
at a
distance
of 5.3 AU,
conditions that
are
appropriate
for
the
Voyager encounters.
Jovian
Planets
163
Figure
5.13
Photoabsorption
rate
coefficients
for the
major
gases
as a
function
of
pressure
and
altitude
in the
standard
NEB
model
atmosphere,
(a) J
values
for the
alkanes,
CH
4>
C
2
H
6
,
C
3
H
8
,
and
C
4
Hi
0
.
(b)
J
values
for the
species
H
2
,
C
2
H
2
,
C
2
H4,
and
C
4
H
2
.
From
Gladstone
et
al.
1996;
cited
in
section
5.3.
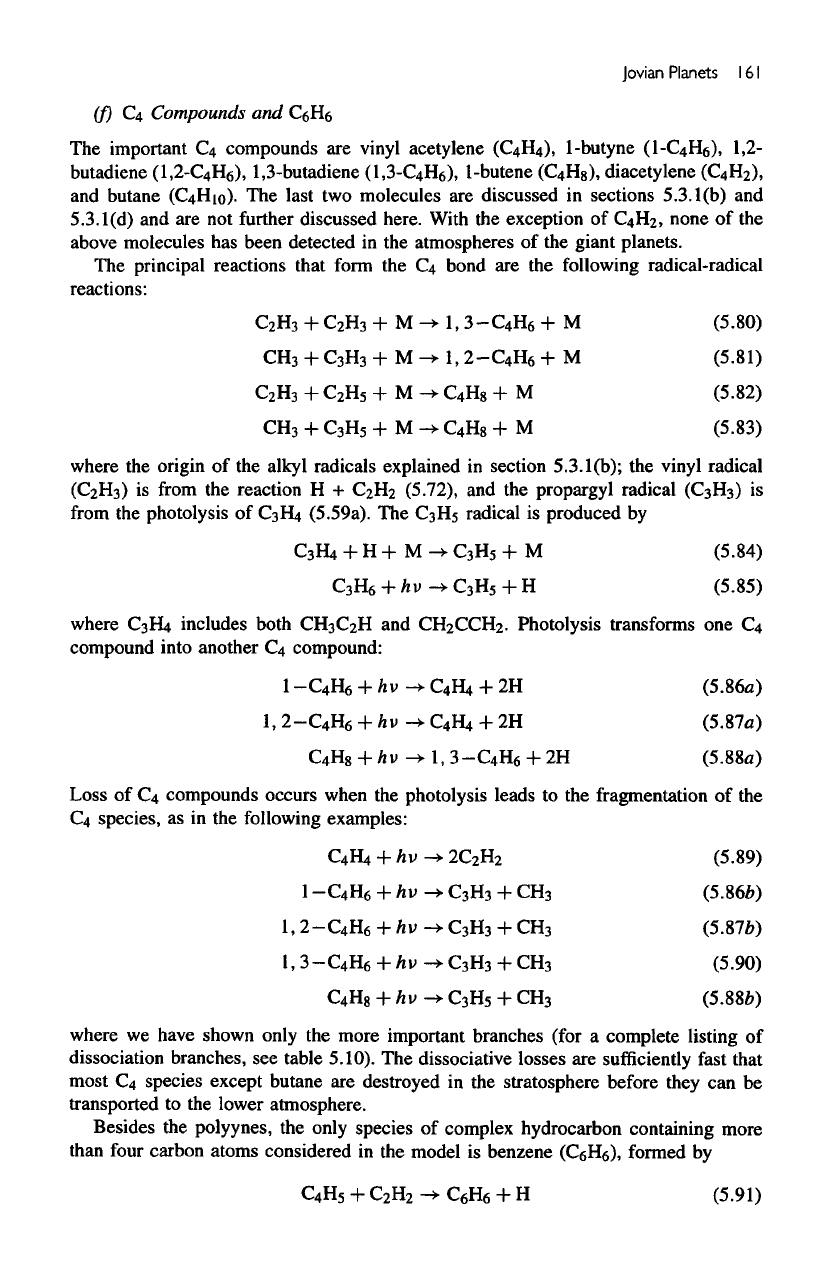
Figure
5.13a
shows
the
photodissociation coefficients
(J) of the
alkanes
in the
model.
The J
values
are of the
order
of
10~
6
s"
1
above
the
homopause
but
decrease
rapidly
below
the
homopause,
due to
self-shielding
in the
case
of
CH4
and due to
partial shielding
by CH4 for the
higher alkanes.
As we
show
in
section 5.3.2(b), when
the
photolytic
process
becomes
inefficient,
other
processes
such
as
transport would
determine
the
distribution
of the
species.
Figure
5.13b
shows
the J
values
for H2,
€2^2,
C2H4,
and
C,»H2.
JH
Z
drops rapidly near
the
upper boundary
of the
model
due to
self
shielding.
Jc
2
H
2
i
s
high
above
the
homopause
but
decreases
by an
order
of
magnitude
in the
stratosphere
due to
shielding
by
CHt.
C4Hb
absorbs
at
longer
wavelengths,
and the
lack
of
shielding makes
the J
value nearly constant throughout
the
middle atmosphere.
€2Hi
is
partially shielded
by
CH4
and
C2H2.
The
vertical profiles
of the J
values
of the
higher hydrocarbons
are not
plotted.
As can be
seen
from
table 5.10, their values
are
generally high,
in the
range
of
10~
6
to
10~
8
,
with
little
attenuation between
the
mesosphere
and the
stratosphere. This
accounts
for the
instability
of
Cj
and
€4
species
in the
model.
(b)
C, and
C
2
Species,
and
H
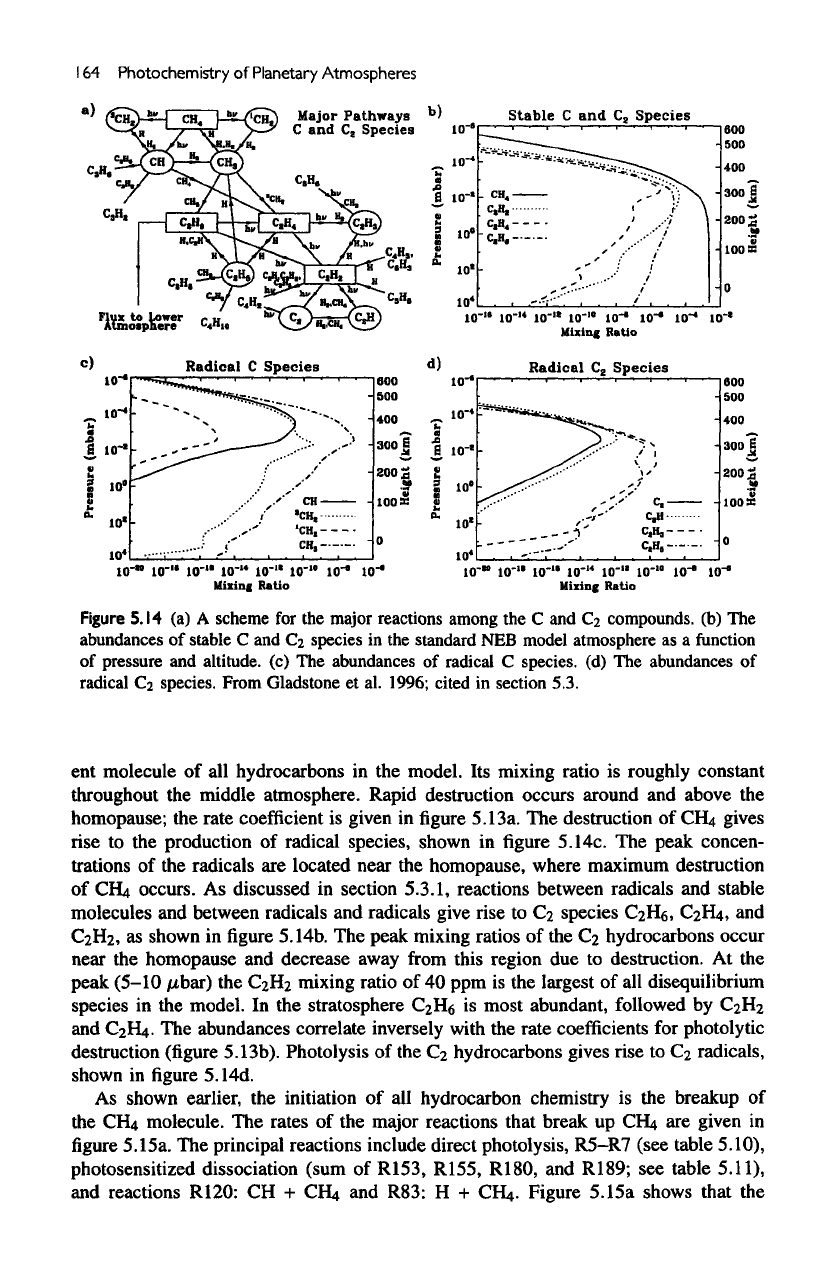
A
schematic diagram showing
the
principal pathways
by
which
the
€2
species
are
formed
from
the
dissociation products
of
CH
4
is
given
in figure
5.14a.
The
mixing
ratios
of the
stable
Q
and
€2
hydrocarbons
are
shown
in figure
5.14b.
The
Ci
and
C2
radical species
are
given
in figures
5.14c
and d,
respectively.
CH
4
is the
par-
164
Photochemistry
of
Planetary Atmospheres
Figure
5.14
(a) A
scheme
for the
major
reactions
among
the C and
€2
compounds,
(b) The
abundances
of
stable
C and
€2
species
in the
standard
NEB
model
atmosphere
as a
function
of
pressure
and
altitude,
(c) The
abundances
of
radical
C
species,
(d) The
abundances
of
radical
Cz
species.
From
Gladstone
et
al.
1996;
cited
in
section
5.3.
ent
molecule
of all
hydrocarbons
in the
model.
Its
mixing ratio
is
roughly constant
throughout
the
middle atmosphere. Rapid destruction occurs around
and
above
the
homopause;
the
rate
coefficient
is
given
in figure
5.13a.
The
destruction
of
CFLt
gives
rise to the
production
of
radical species, shown
in
figure
5.14c.
The
peak concen-
trations
of the
radicals
are
located near
the
homopause, where maximum destruction
of
CH4
occurs.
As
discussed
in
section
5.3.1,
reactions between radicals
and
stable
molecules
and
between radicals
and
radicals give rise
to
Ca
species
CaHg,
CaHi,
and
CaHz,
as
shown
in figure
5.14b.
The
peak mixing ratios
of the
Cz
hydrocarbons occur
near
the
homopause
and
decrease
away
from
this region
due to
destruction.
At the
peak (5-10
nbar)
the
Catb
mixing ratio
of 40 ppm is the
largest
of all
disequilibrium
species
in the
model.
In the
stratosphere
CaHg
is
most abundant, followed
by
CaH2
and
C2H4.
The
abundances correlate inversely with
the
rate
coefficients
for
photolytic
destruction
(figure
5.13b).
Photolysis
of the
Ca
hydrocarbons gives rise
to
C-i
radicals,
shown
in figure
5.14d.
As
shown earlier,
the
initiation
of
all
hydrocarbon chemistry
is the
breakup
of
the
CHj
molecule.
The
rates
of the
major reactions that break
up
CH4
are
given
in
figure
5.15a.
The
principal reactions include direct photolysis,
R5—R7
(see table
5.10),
photosensitized dissociation (sum
of
R153, R155, R180,
and
R189;
see
table 5.11),
and
reactions R120:
CH +
CHt
and
R83:
H +
Cftj.
Figure
5.15a
shows that
the
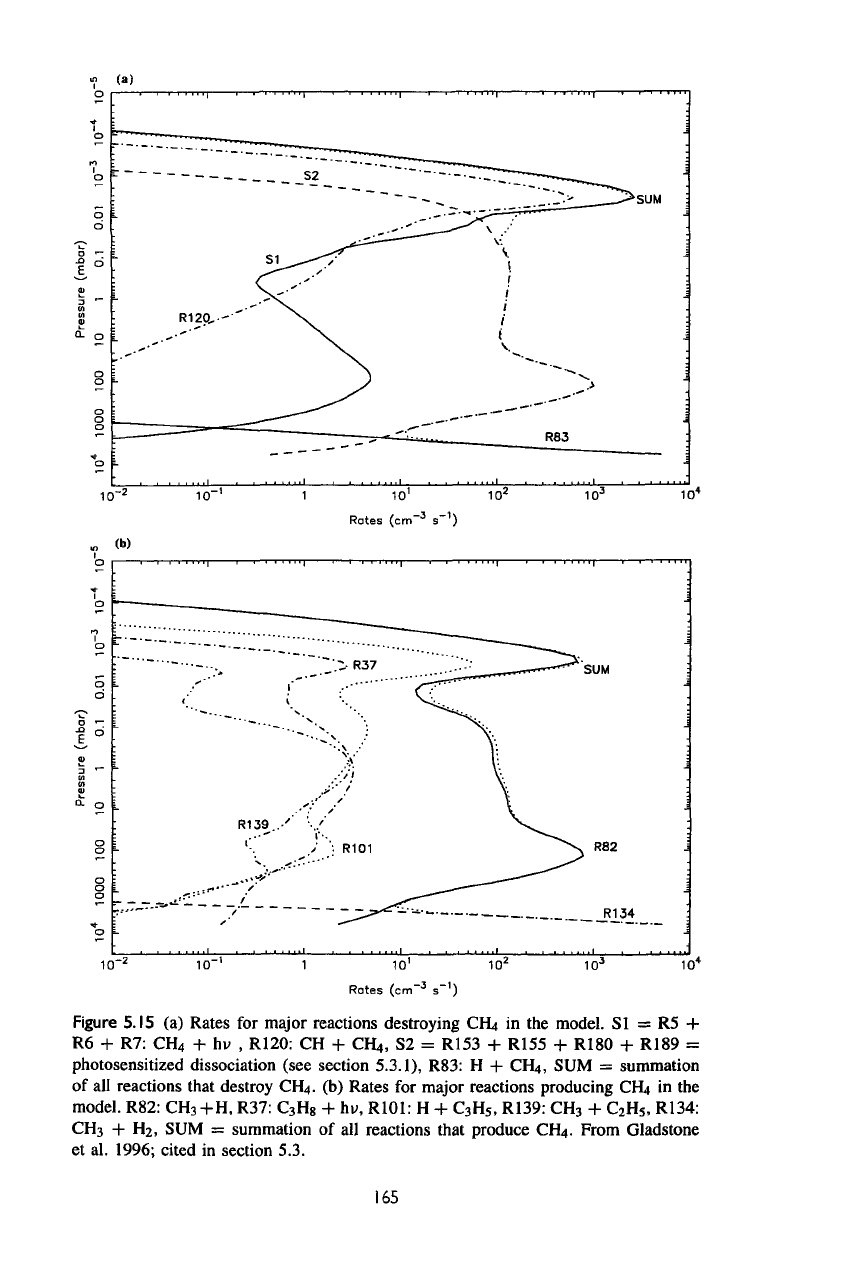
Figure
5.IS
(a)
Rates
for
major
reactions destroying
CFLt
in the
model.
SI
=
R5 +
R6
+ R7:
CH
4
+ hv
,
R120:
CH +
OH,,
S2 =
R153
+
R155
+
R180
+
R189
=
photosensitized dissociation (see section
5.3.1),
R83:
H +
CRt,
SUM =
summation
of
all
reactions
that
destroy
CFfy.
(b)
Rates
for
major
reactions producing
CUt
in the
model. R82:
CH
3
+H,
R37:
C
3
H
8
+ hv,
R101:
H +
C
3
H
5
,
R139:
CH
3
+
C
2
H
5
,
R134:
CH
3
+ H2, SUM =
summation
of all
reactions
that
produce
CH,».
From Gladstone
et
al.
1996; cited
in
section 5.3.
165
