Water and Wastewater Engineering
Подождите немного. Документ загружается.


23-28 WATER AND WASTEWATER ENGINEERING
suspended solids lost in the effluent is the product of the flow rate ( Q Q
w
) and the suspended
solids concentration ( X
e
).
E xample 23-3 illustrates the estimation of the mass of sludge to be wasted using Equation 23-38 .
Example 23-3. Estimate the mass of sludge to be wasted each day from the new activated
sludge plant at Lawrence ( Examples 23-1 and 23-2 ).
Solution:
a. U sing the data from E
xample 23-1 , calculate Y
obs
:
Y
obs
kg VSS/kg BOD removed
d
0 50
10050
5
.
.[(
1
5
5
040
)( )]d
kg/kg BOD removed.
b. The net waste activated sludge produced each day is
P
x
()( )( )0 40 12 960 84 0 11 1
333
., . .m /d g/m g/m
377 914 377 9,.g/d or kg/d of VSS
c. The total mass produced includes inert materials. Using the relationship between MLSS
and MLVSS in Example 23-2 ,
Increase in MLSS kg/d(/ . )( )1070377 9 539. .866or540 kg/d
d. The mass of solids (both volatile and inert) lost in the effluent is
()()( )( )QQ X
we
12 960 96 2 30
333
,.m /dm/d g/m
385 914 385 9,.g/d or kg/d
e. The mass to be wasted is then
Mass to be wasted or539 385 9153 15.86 . .96 44 kg/d
Comment. This mass is calculated as dry solids. Because the sludge is mostly water, the actual
mass will be considerably larger. This is discussed Chapter 15.
A more accurate prediction of sludge production can be mad
e with sufficient wastewater
characterization. The following equation accounts for heterotrophic biomass growth (Part A), cell
debris from endogenous decay (Part B), nitrifying biomass growth (Part C), and nonbiodegrad-
able VSS in the influent (Part D) (Metcalf & Eddy, 2003):
P
QY S S
k
x
dc
,vss
g/kg
Part A
()( )
()
o
3
10
1
()() ( )()(
fkQYS S
dd co
3
10 g/kg))
()
()(
1
10
k
QY
dc
n
Part B
NO
x
33
1
g/kg
Part C
)
()
k
dn c
Q()( ) ()nbVSS g/kg10
3
Part D
(23-40)

SECONDARY TREATMENT BY SUSPENDED GROWTH BIOLOGICAL PROCESSES 23-29
where NO
x
concentration of NH
4
-N in the influent that is to be nitrified, mg/L
f
d
fraction of cell mass that remains as cell debris, g VSS/g VSS
Other terms are as defined previously. In the absence of laboratory analysis, f
d
may be assumed
to be about 0.15.
To account for the total mass of solids, the total suspend ed solids (TSS) must be included.
Assuming that the VSS fraction of the total biomass is about 0.85 based on cell composition, the
production of TSS is estimated as (Metcalf & Eddy, 2003):
P
x,
...
vss
Part A Part B Part C
Par
085 085 085
ttD TSS VSSQ()
00
(23-41)
where TSS
0
influent wastewater TSS, mg/L
VSS
0
influent wastewater VSS, mg/L
The estimate of Part B becomes important if the concentration of bCOD is high. The estimate of
Part C is particularly relevant in nitrification and denitrification processes because of the potential
for washout of nitrifying bacteria. The estimate of Part D is important when nbVSS in the influent
is high. This can happen when an industrial discharge contains a high concentration of nbVSS.
Oxygen Demand
O xygen is used in reactions where substrate is degraded to produce the high-energy compounds
required for cell synthesis and respiration. For long SRT systems, the oxygen needed for cell
maintenance can be of the same order of magnitude as substrate m
etabolism. A minimum resid-
ual of 0.5 to 2 mg/L DO is usually maintained in the reactor basin to prevent oxygen deficiencies
from limiting the rate of substrate removal.
An estimate of the oxygen requirements may be made from the bCOD of the waste and
amount of biom ass wasted each da
y. If it is assumed that all of the bCOD is converted to end
products, the total oxygen demand would equal bCOD. Because a portion of waste is converted
to new cells that are wasted, the bCOD of the wasted cells must be subtracted from the total
oxygen demand. An approximation of the oxygen d emand
of the wasted cells may be made by
assuming cell oxidation can be described by the following reaction:
CHNO O CO HO NH energy
5 72 2 2 2 3
552
(23-42)
The ratio of gram molecular weights is
5 32
113
142
()
.
Thus the oxygen demand of the waste activated sludge may be estimated as 1.42 ( P
x
).
The mass of oxygen required may be estimated as:
M kg/g
O
2
.
QS S
ox
()( ) ()10 1 42
3
P
(23-43)
where M
O
2
mass of oxygen, kg/d
Q wastewater flow rate into the aeration tank, m
3
/ d
S
o
influent bCOD, g/m
3
S effluent bCOD, g/m
3
P
x
waste activated sludge produced, kg/d (see Equation 23-38 )

23-30 WATER AND WASTEWATER ENGINEERING
Note that the definition of S and S
o
has been changed from readily biodegradable soluble COD
(rbsCOD) to biodegradable COD (bCOD).
Where nitrification is included in the process, the oxygen requirement must include a term to
account for ammonia and organic nitrogen oxidation:
M kg/g NO
O x
2
QS S Q
o
()( ) () ()10 1 42 4 33
3
..P
x
(23-44)
where NO
x
is the amount of TKN oxidized to nitrate.
A nitrogen balance that accounts for the influent TKN, nitrogen removed for biomass s yn-
thesis, and unoxidized effluent nitrogen is used to determine NO
x
. Assuming the biomass com-
position may be described as C
5
H
7
NO
2
, then synthesis nitrogen is estimated as 0.12 g N/g of
biomass. The nitrogen mass balance is
Nitrogen oxidized Nitrogen in influent Nitroogenineffluent Nitrogen in cell tissue
Q(NNO TKN
x
)( )QQN P
ex0
012.
NO TKN
x
0
012N
P
Q
e
x
.
⎛
⎝
⎜
⎞
⎠
⎟
(23-45)
where NO
x
nitrogen oxidized, mg/L
T KN
0
influent total Kjeldahl nitrogen, mg/L
N
e
effluent NH
4
-N, mg/L
Other terms are as previously defined.
If the process includes denitrific ation, the amount of oxygen supplied by nitrate decreases
the amount of oxygen that must be supplied by aeration. This “oxygen credit” amounts to 2.86 g
O
2
/g NO
3
-N.
The volume of air to be supplied must take into account the percent of air that is oxygen and
the transfer efficiency of the dissolution of oxygen into the wastewater.
E xample 23-4 illustrates the estimation of the mass of oxygen to be supplied.
Example 23-4. Estimate the mass of oxygen to be supplied (kg/d) for the new activated sludge
plant at Lawrence ( Ex
amples 23-1 and 23-3 ). Assume that BOD
5
rbsCOD and that it is 68%
of the bCOD.
Solution:
a . Using the data from Examples 23-1 and 23-3 , convert the rbsCOD to bCOD.
bCOD
rbsCOD
068.
and
S
S
o
84 0
068
123 53
11 1
068
3
3
3
.
.
.
.
.
g/m
g/m
g/m
16 32
3
.g/m
SECONDARY TREATMENT BY SUSPENDED GROWTH BIOLOGICAL PROCESSES 23-31
b. Estimate the mass of O
2
as
M kg/g
M
O
O
2
3
2
10 1 42
12 960
QS S P
ox
()( ) ()
(
.
, mm/d g/m g/m kg/g
3333
123 53 16 3210
1
)( )( )..
.. .
,. . .
42 377 9
1 389 4 5 366 8528
()kg/d of VSS
or kg/d of oxygen850
Comment. This is the theoretical amount of oxygen required. For aeration design, the fraction
of air that is oxygen and the oxygen transfer efficiency must be taken into account.
Oxygen Transfer
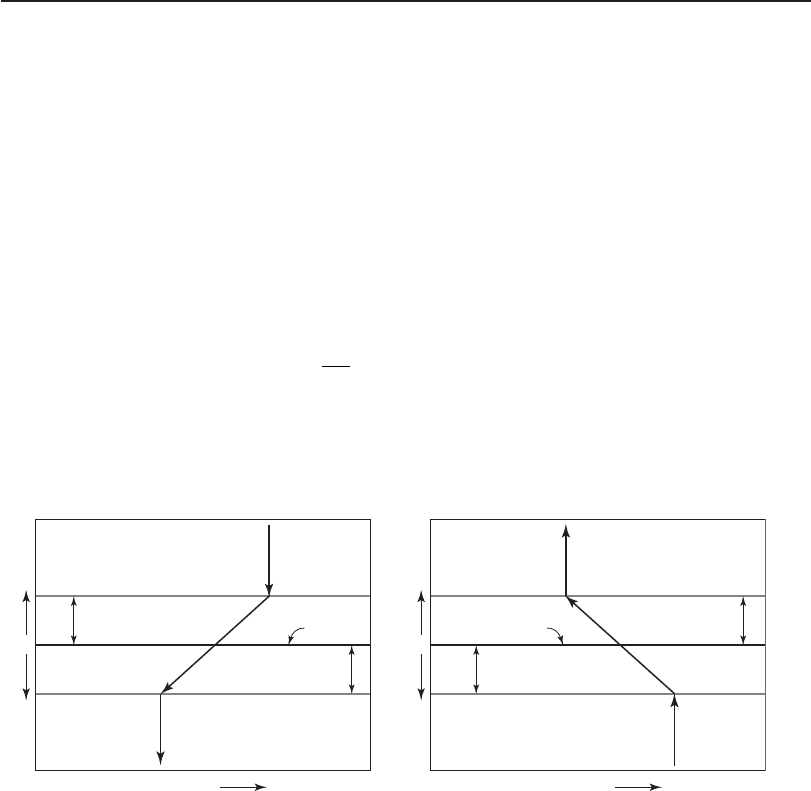
In 1924, Lewis and Whitman postulated a two-film theory to describe the mass transfer of gases.
According to their theory, the boundary between the gas phase and the liquid phase (also called
the interface ) is composed of two distinct films that serve as a barrier between the bulk phases
( Figure 2
3-11 ). For a molecule of gas to go into solution, it must pass through the bulk of the gas,
the gas film, the liquid film, and into the bulk of the liquid ( Figure 23-11a ). To leave the liquid,
the gas molecule must follow the reverse course ( Figure 23-11b ). The driving force causing the
gas to move, and hence the ma
ss transfer, is the concentration gradient: C
s
C. C
s
i s the satura-
tion concentration of the gas in the liquid, and C i s the actual concentration. When C
s
i s greater
than C, the gas will go into solution.
The rate of mass transfer is given by
dC
dt
KaC C
Ls t
()
(23-46)
where K
L
a i s the volumetric mass transfer coefficient with units of s
1
and C
t
i s the concen-
tration in the bulk liquid at time t. Integrating Equation 23-46 between the limits C C
0
and
Gas film
Bulk gas
Bulk liquid
C
s
C
t
Liquid film
0
Distance from interface
Gas film
Liquid film
Bulk gas
Bulk liquid
C
s
C
t
Interface Interface
Concentration
(a)
Concentration
(b)
0
Distance from interface
FIGURE 23-11
Two-film model of the interface between gas and liquid: ( a ) absorption mode and ( b ) desorption mode.
( Source: Davis and Cornwell, 2008.)
23-32 WATER AND WASTEWATER ENGINEERING
C C
t
and t 0 and t t, where C
0
i s the initial concentration and C
t
i s the concentration at
time t, yields
CC
CC
Ka t
st
s
L
0
exp[ ( )( )]
(23-47)
The effects of mixing intensity and tank geometry must be considered in the design process.
In most cases aeration devices are rated by manufacturers based on clean water. A correction fac-
tor is used to estimate
K
L
a in the actual system:
Ka
Ka
L
L
wastewater
clean water
()
()
(23-48)
Typical values of are 0.2 to 0.5 for conventional BOD oxidation, 0.4 to 0.7 for nitrification
only, and 0.5 to 0.75 for nitrification-denitrification (Rosso and Stenstrom, 2007).
A second correction factor i s used to correct the oxygen transfer rate for differences in
oxygen solubility due to constit
uents in the water such as salts, particulate matter, and surface
active substances:
C
C
s
s
()
()
wastewater
clean water
(23-49)
Values of range from about 0.7 to 0.98. A typical value for wastewater is 0.95.
The interrelationship between these factors and temperature, elevation above sea level, and
the depth of diffusers is expressed as follows (Metcalf & Eddy, 2003):
AOTR SOTR
avg
()( )
()(
CC
C
L
s
T
20
20
1 024
⎛
⎝
⎜
⎞
⎠
⎟
. )( )F
(23-50)
where AOTR actual oxygen transfer rate, kg O
2
/h
SOTR standard oxygen transfer rate in tap water at 20 C and zero DO, kg O
2
/h
C
avg
average dissolved oxygen saturation concentration in clean water in aeration
tank at temperature T and elevation H, mg/L
C
L
operating oxygen concentration, mg/L
C
s, 20
dissolved oxygen saturation in clean water at 20 C and 1 atm, mg/L
T operating temperature, C
F fouling factor
The average dissolved oxygen saturation concentration in clean water (C
avg
) in an aeration tank
at temperature T and elevation H is defined as
CC
P
P
O
sT H
d
H
t
avg
atm
()()
,,
,
.0 5
21
⎛
⎝
⎜
⎞
⎠
⎟
where C
s,T,H
oxygen saturation concentration in clean water at temperature T and elevation
H, mg/L
P
d
pressure at depth of air release, kPa
P
atm,
H
atmospheric pressure at elevation H, kPa
O
t
percent oxygen concentration leaving tank
(21%)(1 %O
2
absorbed)

SECONDARY TREATMENT BY SUSPENDED GROWTH BIOLOGICAL PROCESSES 23-33
The atmospheric pressure at elevation H i s computed from the ratio of pressure at elevation
H divided by the pressure at sea level:
PP
gM z z
RT
HSL
HSL
/exp
()( )
⎡
⎣
⎢
⎤
⎦
⎥
where g acceleration due to gravity, 9.81 m/s
2
M mole of air 28.97 kg/kg - mole
z
H
elevation H, m
z
SL
elevation of sea level, m
R universal gas constant, 8.314 N · m/kg · mole · K
T temperature, K
The percent oxygen absorbed may range from 5 to 14 percent. Typically it is assumed to be 8 percent.
This results in an O
t
of about 19 percent. The fouling factor is typically 0.65 to 0.9. For mechanical
aeration C
avg
C
s,T,H
.
Other terms used to rate aeration systems are Standard Oxygen Transfer Efficiency (SOTE, %)
and Standard Aeration Efficiency (SAE, kg O
2
/kW-h). SAE is preferred in evaluating alternatives
because it takes energy consumption into account.
T ypical clean water transfer efficiencies are given in Table 23-6 .
E xample 23-5 illustrates the use of SOTR and AOTR in determining the number of aerators.
Example 23-5. Estimate the required air flow rate for the new activated sludge plant at Lawrence
( Examples 23
-1 , 23-3 , and 23-4 ). Use the following assumptions in preparing the estimate:
Clean water correction, 0.50
Salinity correction, 0.95
Fouling factor 0.9
S ummer wastewater temperature 22 C
TABLE 23-6
Typical clean water oxygen transfer efficiencies
Diffuser type
and placement
Air flow rate/diffuser,
m
3
/d
SOTE, % at 4.5 m
submergence
SAE,
kg O
2
/kW · h
Diffused air
Porous grid 100–160 13–45 1.9–6.6
Nonporous,
single spiral roll
400–1,400 9–12 1.3–1.9
Jet, side header 2,000–12,000 15–24 2.2–3.5
Mechanical surface
Radial flow, low speed
N/A
a
N/A 1.5–2.1
Axial flow, high speed N/A N/A 1.1–1.4
Horizontal rotor N/A N/A 1.5–2.1
a
Not applicable.
Sources: Metcalf & Eddy, 2003; WEF, 1998.
23-34 WATER AND WASTEWATER ENGINEERING
A t mospheric pressure 101.325 kPa
Elevation 100 m
Depth of aerator 5.6 m
Operating DO 2.0 mg/L
% oxygen leaving aeration tank 19%
Manufacturer ’ s SOTR 650 kg/d
Manufacturer ’ s air flow rate at standard conditions 50 m
3
/ d · aerator
Solution:
a . From Example 23-4 , the required AOTR is 850 kg/d. This will be designated AOTR
req
for this problem.
b. Solve Equation 23-50 for SOTR. This is the required SOTR (SOTR
req
).
SOTR
AOTR
req
req
a
()()()
()(
,
1 024
20
20
.
T
s
F
C
C
vvg
C
L
)
⎛
⎝
⎜
⎞
⎠
⎟
c. From Appendix A, find C
s,T,H
= 9.17 mg/L or 9.17 g/m
3
at 20 C.
d. P
d
i s the pressure at the depth of air release. P
d
= P
atm, H
P
water
. Converting P
atm, H
to
meters of water,
P
Hatm
Atmospheric pressure
Spec ific weight
,
oof air
kN/m
kN/m
m
101 325
98
10 34
2
3
.
.
.
From the assumed depth of the aerator,
P
d
10 34 5 615 9...mm m
e. Find C
s,T,H
= 8.83 mg/L from Appendix A at 22
C and calculate C
avg
.
C
avg
mg/L
m
m
()()883 0 5
15 9
10 34
19
21
..
.
.
⎛
⎝
⎞
⎠
10 8. mg/L
f. Calculate SOTR
req
.
SOTR
kg d
req
850
1 024 0 5009
22 20
/
⎛
⎝
⎜
⎞
()()()...
⎠⎠
⎟
⎛
⎝
⎜
⎞
⎠
917
095 10 8 2 0
.
.. .
mg/L
mg/L mg/L()( )
⎟⎟
⎛
⎝
⎞
⎠
⎛
⎝
⎜
⎞
⎠
⎟
850
047
917
8 36
kg/dmg/L
mg/L.
.
.
1983 7 1 980, . ,or g/dk
g. Calculate the ratio of SOTR
manuf
/SOTR
req
.
SOTR
SOTR
kg/d
kg/d
manuf
req
650
1 980
0 328
,
.
SECONDARY TREATMENT BY SUSPENDED GROWTH BIOLOGICAL PROCESSES 23-35
h. The required air flow rate is found from the following relationship:
AOTR
Density of air Mass %O inair
req
()( )
2
⎛
⎝
⎜⎜
⎞
⎠
⎟
⎛
⎝
⎜
⎞
⎠
⎟
SOTR
SOTR
req
manuf
The density of air at standard conditions is 1.185 kg/m
3
. Air contains about 23.2%
oxygen on a mass basis. The required air flow rate is
850
1185 0232
1
0 328
941
3
kg/d
kg/m()()..
.
,
⎛
⎝
⎞
⎠
88 9 400
3
or m /d,
i. The number of aerators required is
9 400
50
188
3
3
, m /d
m /d aerator
aerators
Comments:
1 . When elevation above mean sea level is important, for example in Denver, Colorado,
then corrections for the standard atmospheric pressure may be in order.
2. If SOTE is provided by the manufacturer, the ratio SOTR
manuf
/SOTR
req
can be replaced
with SOTE provided that a correction is made for depth if it is not the same as that in the
design.
Food-to-Microorganism Ratio (F/M)
The food-to-microorganism ratio was developed in the 1950s and 1960s. The “food” is substrate.
It is still widely used. It is intuitive, conceptually easy to explain, and relies on measurements that
are routinely taken.
In equation form, the food-to-microorganism ratio (F/M) is
F/M
QS
X
o
V
(23-51)
where Q wastewater flow rate into the aeration tank, m
3
/ d
S
o
influent readily biodegradable soluble COD (rbsCOD), mg/L
volume of aeration tank m,
3
V
X microorganism concentration (mixed-liquor volatile suspended solids or MLVSS) in
the aeration tank, mg/L
The units of F/M are
mgBOD /d
mgMLVSS
mg
mg d
5
The F/M ratio has some basis in theory, but the values used in practice are derived from empirical
observations. They serve as a check on design calculations. Typical F/M ratios for various modi-
fications of the activated sludge process range from 0.04 to 2.0 m
g/mg · d.
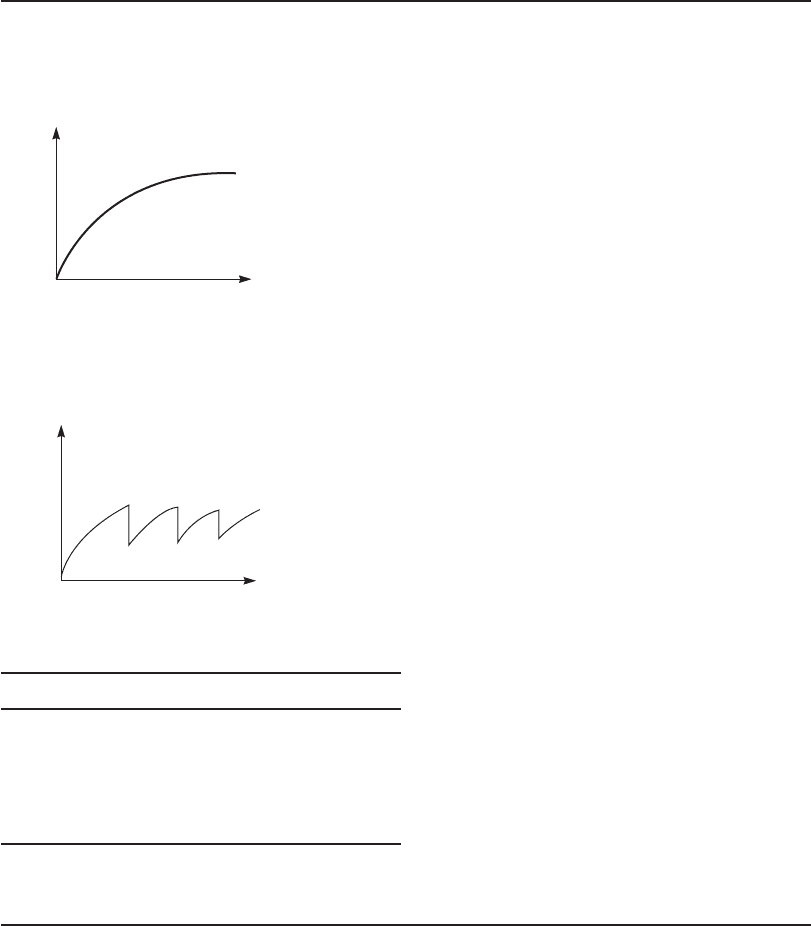
23-36 WATER AND WASTEWATER ENGINEERING
Example 23-6. Two “fill and draw,” batch-operated activated sludge tanks are operated as follows:
Tank A is settled once each day, and half the liquid is removed with care not to disturb the
slu dge that settles to the bottom. This liquid is replaced with fresh settled sewage. A plot of
MLVSS concentration versus tim
e takes the shape shown below.
Time
Bacterial
concentration,
MLVSS, mg/L
Tank B is not settled. Once each day half the mixed liquor is removed while the tank is being
violently agitated. The liquid is replaced with fresh settled sewage. A plot of MLVSS concentra-
tion versus time is shown below.
Time
Bacterial
concentration,
MLVSS, mg/ L
A comparison of the operating characteristics of the two systems is shown in the following table.
Parameter Tank A Tank B
F/M Low High
c
Long Short
Sludge volumeSmall Large
Oxygen required High Low
Power High Low
The optimum choice is somewhere between these extremes . A balance must be struck between
the cost of sludge disposal and the cost of power to provide oxygen (air).
Specific Denitrification Rate
The specific denitrification rate (SDNR) must be determined for preanoxic nitrate removal
processes. The best method for determining the SDNR is based on simulation modeling. Mass
balances of biomass, NO
3
-N, rbCOD, and bpCOD are coupled with internal recycle rates to
determine SDNR. The following paragraphs describe a desktop design approach that makes use
of plots generated from simulation modeling (Metcalf & Eddy, 2003).
SECONDARY TREATMENT BY SUSPENDED GROWTH BIOLOGICAL PROCESSES 23-37
The amount of nitrate removed in the anoxic tank is described by the following equation:
NO SDNR MLVSS
anox
r
()()( )
V
(23-52)
where NO
r
nitrate removed, g/d
anox
anoxic tank volume m
,
3
V
SDNR specific denitrification rate, g NO
3
-N/g MLVSS · d
MLVSS mixed liquor volatile suspended solids, mg/L
An important design parameter is the amount of BOD needed to provide a sufficient amount
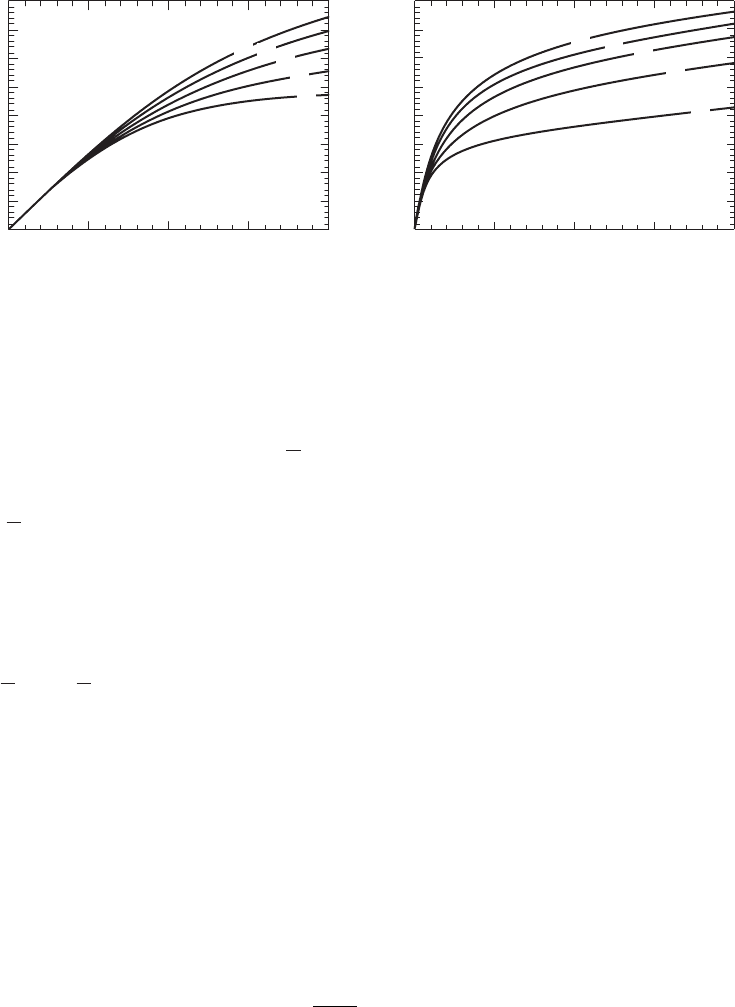
of electron donor for nitrate removal. In the desktop technique, this is addressed by graphs
relating SDNR to the F/M ratio ( Figure 23-12 ). Equation 23-
51 is used to calculate the F/M ratio
using
anox
.
V
V
T o a chieve typical effluent limits for nitrate, a portion of the aerobically treated wastewater
must be recycled to the anoxic tank. This is called internal recycle. A mass balance accounts for
the nitrate produced in the aerobic zone. As a conservative design approach, all of the influent
TKN is assumed to be biodegradable and the effluent soluble organi
c nitrogen is ignored. The
mass balance is expressed as
kg of nitrate produced in aerobic zone nitr aate in effluent nitrate in internal recycl ee
nitrate in RAS
QNQQRQ
e
( ) [ ( )( ) ( )( )]NO IR
x
(23-53)
Solving for the internal recycle ratio:
IR
NOx
N
R
e
10.
(23-54)
where IR internal recycle ratio
internal recycle/influent flow rate
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0 5 10 15 20
F/M
b
, g BOD/g biomass . dF/M
b
, g BOD/g biomass . d
0
0.05
0.1
0.15
0.2
0.25
0.3
0.35
0.4
0
0.5 11.5 2
SDNR, g NO
3
-N/g biomass . d
SDNR, g NO
3
-N/g biomass . d
10
20
30
40
50
10
20
30
40
50
FIGURE 23-12
Plot of specific denitrification rates (SDNR
b
) based on biomass concentration at 20
C versus food to biomass (F/M
b
) ratio
for various percentages of rbCOD relative to the biodegradable COD of the influent wastewater. ( Source: Metcalf & Eddy,
2003.)
