Water and Wastewater Engineering
Подождите немного. Документ загружается.

13-58 WATER AND WASTEWATER ENGINEERING
U.S. EPA (1999) Alternative Disinfectants and Oxidants Guidance Manual, U.S. Environmental
Protection Agency Publication EPA 815-R-99-014, Washington, D.C.
U.S. EPA (2006) Ultraviolet Disinfection Guidance Manual for the Final Long Term 2 Enhanced Surface
Water Treatment Rule, U.S. Environmental Protection Agency Publication EPA 815-R-06-007,
Washington, D.C.
Watson, H. (1908) “A Note on the Variation of the Rate of Disinfection with Change in Concentration of
the Disinfectant,” Journal of Hygiene, vol. 8, p. 536.
Weast, R. C, (1983) CRC Handbook of Chemistry and Physics, CRC Press, Boca Raton, Florida, pp. 16–163.
Wilczak, A., L. L Hoover, and H. H. Lai, (2003) “Effects of Treatment Changes on Chlorine Demand and
Decay,” Journal of American Water Works Association, vol. 95, no. 7, pp. 94–106.

14-1
14-10 RADIONUCLIDES
14-11 SYNTHETIC ORGANIC CHEMICALS
(SOCs) AND VOLATILE ORGANIC
CHEMICALS (VOCs)
14-12 TASTE AND ODOR (T&O)
14-13 CHAPTER REVIEW
14-14 PROBLEMS
14-15 DISCUSSION QUESTIONS
14-16 REFERENCES
REMOVAL OF SPECIFIC CONSTITUENTS
14-1 INTRODUCTION
14-2 ARSENIC
14-3 CARBON DIOXIDE
14-4 FLUORIDE
14-5 IRON AND MANGANESE
14-6 NITRATE
14-7 NATURAL ORGANIC MATTER (NOM)
14-8 PERCHLORATE
14-9 PHARMACEUTICALS AND ENDOCRINE-
DISRUPTING COMPOUNDS (EDCs)
14
CHAPTER
14-2 WATER AND WASTEWATER ENGINEERING
14-1 INTRODUCTION
The focus of the previous chapters has been on removal of broad classes of constituents: turbid-
ity, color, hardness, and pathogens. The focus of this chapter is a selected list of specific constitu-
ents that are not addressed in the previous chapters. They were selected
because of their impact
on the potability and palatability of water. These include arsenic, iron and manganese, fluoride,
nitrate, NOM, pharmaceuticals, radionuclides, taste and odor (T&O), synthetic organic chemicals
(SOCs) and volatile organic compounds (VOCs). In addition, becaus
e of its economic impact on
the softening process, treatment to remove carbon dioxide is included.
14-2 ARSENIC
A r senic is almost exclusively a groundwater contaminant. Therefore, this discussion is limited to
a few selected processes deemed reasonable for treating groundwater at a centralized facility. A
more thorough discussion may be found in the Arsenic Treatment Technology Handbook (U.S.
EPA, 2003).
Arseni
c can occur in four oxidation states in water: 5, 3, 0, 3. The most common states
are trivalent arsenite [As(III)] and pentavalent arsenate [As(V)]. Most As(III)-containing water in
the pH range of 6 to 9 will have As in the form of H
3
A sO
3
. Arsenate will be in the form HAsO
4
2
in the pH range 7 to 11.5. At pH values less than 7.0, arsenate will be in the form H
2
A sO
4
.
Treatment Strategies
Preoxidation Processes. Reduced inorganic arsenite cannot be removed effectively. Preoxida-
tion to form As(V) at the head end of all of the unit processes described in the following para-
graphs is essential. Chlorine, permanganate, and ozone are highly effective. Chlorine dioxide,
monochloramine, and UV are ineffective as stand-alone oxidants for As(III).
Side reactions with iron,
manganese, and sulfide must be accounted for in determining the
dose for oxidation (Ghurye and Clifford, 2001).
The oxidation-reduction reaction with chlorine is
HAsOOCl
HAsO
HCl
33
24
→ (14-1)
This reaction is relatively independent of pH in the range 6.3 to 8.3. In a laboratory study, at an
excess of three times the stoichiometric amount of chlorine, 95 percent of the As(III) was con-
verted to As(V) in 42 seconds (Ghurye and Clifford, 2001).
The oxidation-reduction reaction with permanganate is
3
2 3
2
33
424
22
HAsO
MnO H AsO
HMnOHO
→
(14-2)
Like the chlorine reaction, this reaction is relatively independent of pH in the range 6.3 to 8.3.
At a similar threefold stoichiometric excess, 95 percent of the As(III) was converted to As(V) in
36 seconds. As with chlorine oxidation, side reactions with iron, manganese, and sulfide must be
accounted
for in determining the dose (Ghurye and Clifford, 2001).
Permanganate is difficult to handle. It is commercially available as a crystal that is corrosive
and stains nearly everything purple. Manganese particles are produced as a result of permanga-
nate oxidation reactions. Therefore, postoxidation filtration is essential to prevent a
ccumulation
of deposits in the distribution system.

REMOVAL OF SPECIFIC CONSTITUENTS 14-3
The oxidation-reduction reaction with ozone is
HAsOO
HAsO
HO
333
24
2
→
(14-3)
Like the chlorine reaction, this reaction is relatively independent of pH in the range 6.3 to 8.3.
Using an excess of three times the stoichiometric dose, 95 percent of the As(III) was converted to
As(V) in 18 seconds (Ghurye and Clifford, 2001).
A comparison of preoxidation alternatives is shown in Table 14-1.
TABLE 14-1
Comparison of Preoxidation alternatives
Criteria
Liquid sodium
hypochlorite system
On-site hypochlorite
generation system
Permanganate solution
feed system Ozone generation
Safety and
regulatory issues
• HazMat regulations for
safety and handling apply
• Potential for corrosive
vapors in the presence of
moisture
• Em
ergency response plan
required with local fire
department
• Secondary containment
required
• Below 1% threshold for
hazardous classification
• Exempt from HazMat
regulations
• No secondary
containment requirements
• Solid permanganate poses
dust and inhalation hazard
• Liquid is very corrosive
• Poisonous and
reactive gas
Space
requirements
• Space requirements are
small, assuming the
Uniform Fire Code (UFC)
exempt criteria are met
• Space requirements are
large. There must be
room for salt storage,
brine tanks, hypochlorite
holding tanks, electrolytic
eq
uipment, as well as
instrumentation & control
and power.
• Space requirements
are small. Additional
space may be required
for storage of solid
permanganate.
• Space requirements
are small
Chemical
characteristics
• 5¼ or 12½% sodium
hypochlorite solution.
Degrad
es over time.
• Decay of solution creates
chlorate byproduct
• Increases pH of water
slightly
• Stable sodium
hypochlorite solution
(0.8%)
• Constant application
concentration
• Chlorate formation low
to none
• Increases pH of water
slightly
• Stable permanganate
solution, generally 3–4%
• Reacts rapidly with
dissolved organics
• Gas
• Very strong oxidizer
Chemical
delivery
• Liquid hypochlorite
delivered by tanker truck,
55-gal drum, or 5-gal pail
• Salt delivered in 50-lb
bags or 2000-lb totes
• Solid permanganate
available in 25-kg pails,
50-kg kegs, and 150-kg
drums
• N/A
Labor • Periodic delivery
• Dilution procedures
• Salt d
elivery
• Weekly loading of salt
into brine tank
• Load dry feeder
• Dilution procedures
• N/A
(continued)
14-4 WATER AND WASTEWATER ENGINEERING
Example 14-1. Estimate the stoichiometric amount of hypochlorite in mg/L required to oxidize
arsenic in groundwater with the following constituents:
A s(III) 50 g/L
Fe(II) 1.2 mg/L
Solution:
1 . The redox reaction for oxidizing As(III) is given in Equation 14-1.
HAsOOCl
HAsO
HCl
33
24
→
Note that one mole of hypochlorite reacts with one mole of arsenic.
2. The redox reaction for oxidizing Fe(II) is
2 5 24
2
2 3
FeOClHOFeOH Cl H
→ ()
Note that one mole of hypochlorite reacts with two moles of iron.
3. Calculate the molar concentrations of arsenic and iron:
Moles/Lof As
g/ L
g/mole g/
50
74 92 10
6
()(. gg
moles/L
Moles/Lof Fe
mg/L
)
667 10
12
7
.
.
(()()55 85 10
215 10
3
5
.
.
g/mole mg/g
moles/L
4. The required stoichiometric addition is
667 10 05 215 10
7 5
...
moles/L moles/L()( )11 141 10
5
.
moles/L of OCl
5. In mg/L
()()1 141 10 5145
5
..
moles/L of OCl g/mole 55 871 10 0 59
4
..
g/L or mg/L
TABLE 14-1 (continued)
Comparison of Preoxidation alternatives
Criteria
Liquid sodium
hypochlorite system
On-site hypochlorite
generation system
Permanganate solution
feed system Ozone generation
Operation and
maintenance
• Low day-to-day O&M.
Long-term material
maintenance could be
a problem because of
corrosive effects of liqu
id
hypochlorite.
• Moderate O&M,
mainly associated with
salt handling. Change
electrode cells every five
years.
• Low day-to-day O&M for
automated systems
• Stains everything purple
• Low day-to-day
O&M
Off-normal
operation
• A temporary bleach
solution can be mixed in
the storage tank
• A temporary bleach
solution can be mixed in
the day tank
• N/A • N/A
Community
relations
• HazMat signage required • No HazMat regulations.
Hydrogen byproduct
vented to atmosphere.
• N/A • N/A
Adapted from U.S. EPA, 2003.

REMOVAL OF SPECIFIC CONSTITUENTS 14-5
Comment. According to Ghurye and Clifford (2001), three times this dose is required to oxidize
95 percent of the arsenic in less than one minute.
Ion Exchange. Limitations to the use of ion exchange are the concentration of total dissolved
solids (TDS) and sulfate. If the TDS is less than 500 mg/L (Wang et al., 2000) and the sulfate
concentration is less
than 50 mg/L (U.S. EPA, 2003), strong-base ion exchange is effective and
economical. Iron, manganese, or a combination of the two, should not exceed 0.3 mg/L because
the oxidized forms will plug the ion exchange resin (GLUMRB, 2003).
Designs generally employ empty bed contact times (EBCTs) of 1.5 to 3
minutes. Downflow
cocurrent regeneration has been shown to be more effective than countercurrent regeneration
(Clifford, 1999).
Activated Alumina. Activated alumina (AA) is a porous, granular material with ion exchange
properties. It is a mixture of amorphous and gamma aluminum oxide prepared by low- temperature
(300 to 600 C) dehydration of Al(OH)
3
. The media is placed in columns that are designed and
operated in the same fashion as ion exchange columns.
The pH must be tightly controlled in the range 5.5 to 6.0. The alumina capacity is signif-
icantly reduced in the presence of sulfate ions. Arsenic is diffi
cult to remove from alumina.
A 4 percent NaOH solution is recommended for regeneration (Clifford, 1999). Because of the
high pH of the regeneration process approximately 2 percent of the media dissolves during each
regeneration cycle.
Iron-Based Sorbents (IBS). Adsorption on IBS is considered an emerging technology by the
EPA. The processes are proprietary. They
are based on sorption on iron-impregnated media.
The process is described as irreversible chemis orption. The m edia is placed in columns that are
designed in the same fashion as ion exchange columns. The media is discarded when it becomes
s
aturated. Appropriate media disposal procedures are discussed in Chapter 15.
Reverse Osmosis (RO). Conventional RO design and operation results in highly effective
arsenic removal. While chlorine m ay be used to control biological growth, it is not necessary to
preoxidize the raw water to remove significant amounts of ar
senic. Some membranes are dam-
aged by chlorine and may be damaged by other oxidants as well. RO is capable of achieving
over 97 percent removal of As(V) and 92 percent removal of As(III) (NSF, 2001a and 2001b).
Because As(III) removal is incons istent, U.S. EPA (2002) recommends preoxidation. Careful
s
election of the membrane material is essential if chlorine preoxidation is considered a necessary
part of the process. Manufacturers should be consulted to obtain information on material war-
ranty requirements.
Enhanced Lime Softening. For those water systems employing lime softening, the adjustment
of the pH to values above 10.5 is effective in removing As(V) by coprecipitation.
Oxidation/Filtration. For those water systems employing oxidation and filtration for iron
removal, As(V) adsorbs onto the iron hy droxide precipitate. The removal efficiency is highly
dependent on the initial iron concentration and ratio of iron to arsenic. In general, if the Fe:As
mass ratio is greater than 20:1, the removal efficiency
will be on the order of 80 to 95 percent
(U.S. EPA, 2003).
14-6 WATER AND WASTEWATER ENGINEERING
Modified Iron Removal (MIR). Several alternatives are available when the iron-to-arsenic
ratio is less than 20:1. These systems work in a fashion similar to conventional iron removal
systems but use either a proprietary media or add iron (in the Fe
3
form) to maximize the iron
removal efficiency. These systems use either manganese greensand or other manganese dioxide
based systems.
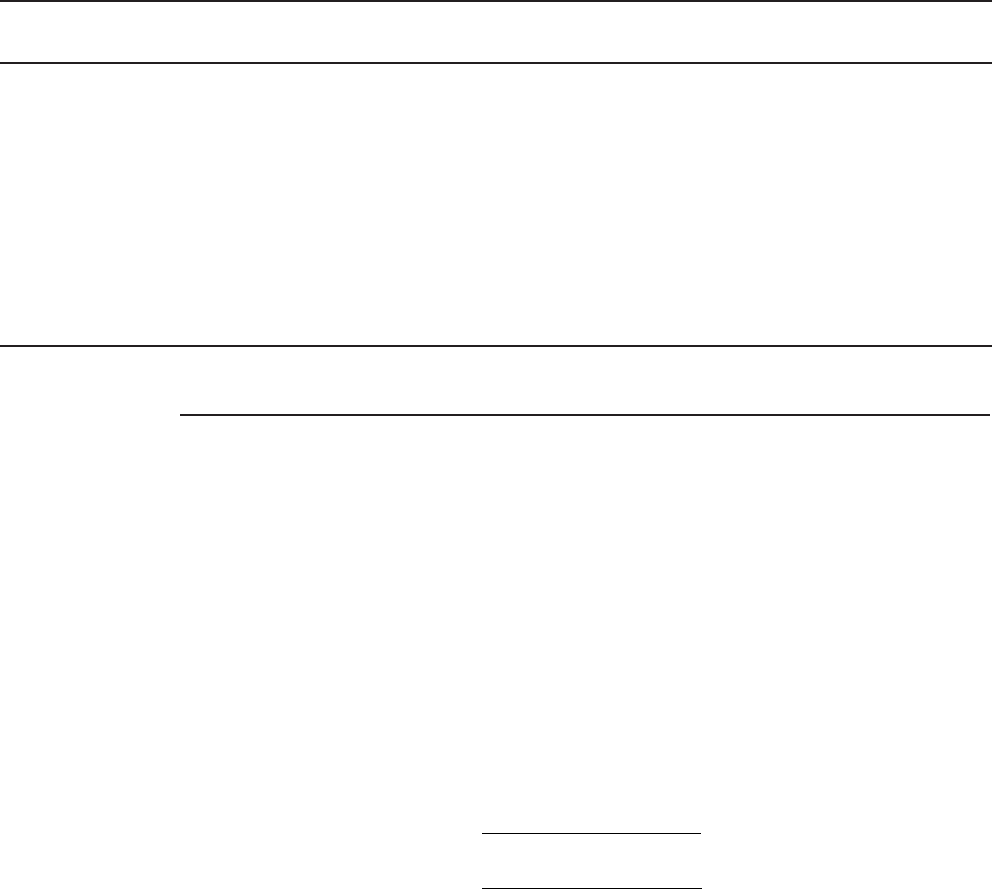
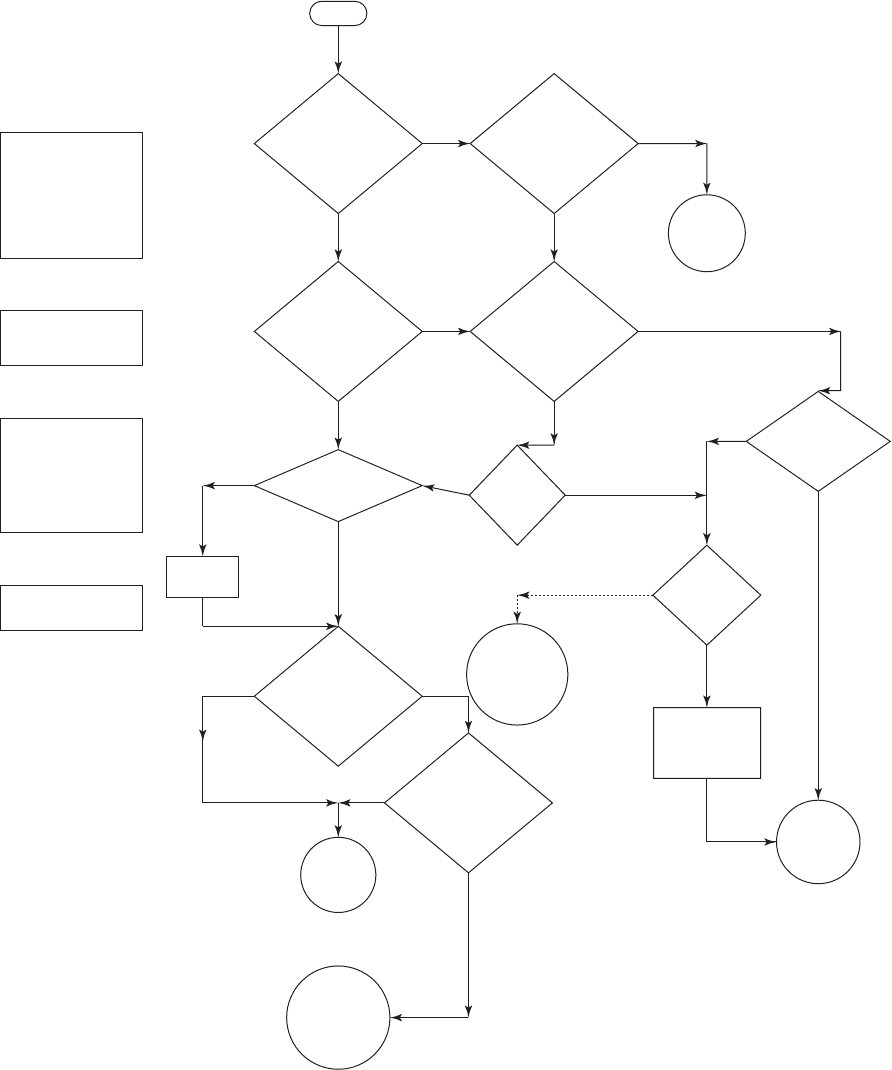
Decision Trees and Technology Comparison. Figure 14-1 is an adaptation of EPA’s decision
trees for selecting a new treatment technology for arsenic removal. Figure 14-2 is an a
dditional
aid in process selection. Tables 14-2 and 14-3 on pages 14-9, 14-10, and 14-11 summarize a com-
parison of the technologies and some typical design and operating parameters.
14-3 CARBON DIOXIDE
R e moving CO
2
will improve neither the potability nor the palatability of the water. Its removal
is an economic issue in the lime-soda softening process and in posttreatment of NF/RO treated
water. As noted in Chapter 7, CO
2
and H
2
CO
3
in water are essentially the same. Carbon dioxide
is the principal acid present in unpolluted, naturally occurring water. Raw water CO
2
concentra-
tions in surface water are generally negligible. In groundwater, concentrations ranging from a
few mg/L to nearly 100 mg/L have been reported (AWWA, 1990). Concentrations in the range of
20 to 40 mg/L are not uncommon. Carbon dioxide must be removed or neutralized before the pH
can be raised to precipitate hardness. When the concentration exceeds 10 mg/L as CO
2
(22.7 mg/L
as CaCO
3
or 0.45 meq/L), the economics of removal by aeration ( stripping ) are favored over
removal by lime neutralization (AWWA, 1990). In NF/RO treated water, air stripping in con-
junction with other treatment is used to increase the stability of the permeate.
Stripping
Air stripping of CO
2
may be accomplished by a variety of devices: spray aerators, multiple-tray
aerators, cascade aerators, cone aerators, and packed columns. Of these types, multiple-tray aera-
tors have found wide use in stripping CO
2
. The multiple-tray aerators consist of a series of trays
with slatted, perforated, or wire-mesh bottoms. The raw water is distributed at the top, flows
down over a series of trays, and is collected in a basin at the base of the unit. Although natural
ventilation may be used, artificial ventilation is more common because the u
nits are housed in
buildings. This is especially true in colder climates where freezing temperatures occur. Blowers
force air from the bottom of the tray system countercurrent to the flow of water. Scroll panels
provide good cross-ventilation, and the roof is open except directly over the distributing trays
.
Design Equation. The following empirical equation may be used to estimate the number of
trays (Scott, 1955):
CC
n
kn
0
10 (14-4)
where C
n
concentration of carbon dioxide after passing through n trays, mg/L
C
0
raw water concentration of carbon dioxide, mg/L
n number of trays including distribution tray at top
k coefficient dependent on ventilation, temperature, turbulence, installation
The value of k ranges from 0.12 to 0.16.
REMOVAL OF SPECIFIC CONSTITUENTS 14-7
Start
SO
4
2
50 mg/L
NO
3
5 mg/L as N
TDS 500 mg/L
pH 6.5 and 9
Fe Mn 0.3 mg/L
Turbility 0.3 NTU
Fe 0.5 mg/L
Mn 0.05 mg/L
Cl 250 mg/L
F
2 mg/L
SiO
2
30 mg/L
SO
4
2
360 mg/L
TDS 1,000 mg/L
TOC 4 mg/L
Fe 1.0 mg/L
Mn 1.0 mg/L
TBLL technology based local
limit for discharge of brine
IBS iron-based sorbants
IX ion exchange
MIR modified iron removal
RSF rapid sand filter
Key
Yes
Yes Yes
Yes
No
No
Yes
Yes
Ye
s
Yes
No
Yes
No
No
No
No
No
Yes
NoNo
Does
source water
meet water quality
criteria in
Box A
?
Adjust
pH
Evaluate
IBS
or
RO
Adjust
pH to
5.5–6.0
Use
IX
Does
source water
meet water quality
criteria in
Box B
Does
source water
meet water quality
criteria in
Box D
?
Is
Fe: As
20:1
?
Is pH 5.5–8.5
?
Can
local TBLL
be met
?
Does
source water
meet water quality
criteria in
Box C
Is
PO
4
1.0
mg/L
?
Is
pH
6.0–8.5
?
Is pH 5.5–6.0?
Use
activated
alumina
Use
MIR
Use
RSF
or
greensand
Box A
Box B
Box C
Box D
3
FIGURE 14-1
Decision tree for treatment to remove arsenic.
( Note: The tree assumes oxidation to As(V) before all of the selections.)
(Adapted from U.S. EPA, 2003.)
14-8 WATER AND WASTEWATER ENGINEERING
0.0
0
5
10
15
20
25
30
35
40
Fe Secondary Maximum Concentration Limit
45
Arsenic, g/L
Iron, mg/L
or above
As MCL
50
C
B
A
0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0
Media adsorption
Iron coag/filt
Ion exchange
Iron removal (M)
RO / NF
Modified iron removal process
Iron removal process
(optimized for maximium as removal)
20:1 F
e/A
s
r
atio
FIGURE 14-2
Arsenic treatment process selection guide.
( Source: Cook, 2005.)
Design Practice. Multiple-tray aerators are typically designed with three to nine trays. These are
spaced about 300 to 750 mm apart. The footprint of the structure ranges from 2 to 6 m
2
/1,000 m
3
of design capacity. The water application rates range from 60 to 75 m
3
/ d · m
2
(Dyksen, 2005).
14-4 FLUORIDE
When the raw water fluoride concentration exceeds the recommended limits (see Chapter 13
for limits), fluoride removal is required. Activated alumina is an excellent medium for fluoride
removal. It is far superior to synthetic organic ion exchange resins (Clifford, 1999). The design
fundam
entals are similar to those u sed for ion exchange columns with sy nthetic resins. Unlike
typical ion exchange column behavior, the breakthrough curve is not sharp and the design must
account for substantial leakage. Although countercurrent regeneration is the mos t efficient way
to minimize the leakage problem, most plant
s are designed with cocurrent regeneration because
of its simplicity.
A typical fluoride-removal plant utilizing activated alumina consists of two or more adsorp-
tion beds. The raw water pH is adjusted to 5.5 to 6.0, and it is passed downward through a 1 to
1.5 m deep bed of me
dium. The medium is specified by mesh size. For example 8 30 mesh
means that the activated alumina particles will pass a number 8 sieve and will be retained on a
number 30 sieve. Activated alumina medium is generally 28 48 mesh.
Following e
xhaustion, the medium is backwashed and then treated in a two-s tep regenera-
tion. Backwashing with raw water is to remove entrained particles, break up clumps of alumina,
14-9
TABLE 14-2
Arsenic treatment technologies summary comparison
Sorption processes Membrane processes
Factors Ion exchange Activated alumina
a
Iron-based sorbents Modified iron removal
Nanofiltration or reverse
osmosis
IX AA IBS MIR NF/RO
USEPA BAT
a
Yes Yes
No
c
No Yes
USEPA SSCT
b
Yes Yes
No
c
No Yes
System size
b,d
25–10,000 25–10,000 25–10,000 25–10,000 501–10,000
Removal efficiency
95%
e
95%
e
Up to 98%
e
Up to 95%
95%
e
Total water loss 1–2% 1–2% 1–2% 5%15–75%
Preoxidation required
f
Yes Yes
Yes
g
Yes
Likely
h
Optimal water quality
conditions
pH 6.5–9
e
5 mg/L NO
3
1
50 mg/L SO
4
2 1
500 mg/L TDS
k
0.3 NTU Turbidity
pH 5.5–6
i
pH 6–8.3
l
250 mg/L Cl
1
2 mg/L F
1
360 mg/L SO
4
2 k
30 mg/L Silica
m
0.5 mg/L Fe
3 i
0.05 mg/L Mn
2 i
1,000 mg/L TDS
i
4 mg/L TOC
k
0.3 NTU Turbidity
pH 6–8.5
1 mg/L PO
4
3
0.3 NTU Turbidity
pH 5.5–8.5
0.3 NTU
turbidity
No particulates
Operator skill required Medium
Low
a
Low Low Medium
Waste generated Spent resin, spent brine,
backwash water
Spent media, backwash water Spent media, backwash
water
Spent media, backwash
water
Reject water, CIP water
Other considerations Possible pre & post pH
adjustment
Prefiltration required
Potentially hazardous brine waste
Nitrate peaking
Carbonate peaking affects pH
Possible pre & post pH adjust
ment
Prefiltration may be required
Modified AA available
Media may be very
expensive.
o
Prefiltration may be
required
Media may be expensive.
Prefiltration may be
required
High water loss
(15–75% of feed water)
Centrulized costMedium Medium Medium Medium High
a
Activated alumina is assumed to operate in a nonregenerated mode.
b
U.S. EPA, (2002). Implementation Guidance for the Arsenic Role, EPA Pub. No 816K02018.
c
IBS’s track record in the United States was not established enough to be considered as Best Available Technology (BAT) or Small System Compliance Technology (SSCT) at the
time the rule was promulgated.
d
Affordable for systems with the given number of people served.
e
U.S. EPA, 2000.
f
Preoxidation only required for As(III).
g
Some iron based sorbents may catalyze the As(III) to As(V) oxidation and therefore would not require a pre-oxidation step.
h
RO will remove As(III), but its efficiency is not consistent and pre-oxidation will increase removal efficiency.
i
AwwaRF, 2002.
j
Kempic, 2002.
k
Wang, et al., 2000.
l
AA can be used economically at higher pHs, but with a significant decrease in the capacity of the media.
m
Ghurye and Clifford, 2001.
n
Tumab, 2002.
o
With increased domestic use. IMS cost will significantly decrease.
Adapted from U.S. EPA, 2003.
