Water and Wastewater Engineering
Подождите немного. Документ загружается.

13-38 WATER AND WASTEWATER ENGINEERING
Example 13-8. Continue the design of the ozone disinfec tion system for Stillwater ( Example
13-5 ). The pH and temperature selected for the design analysis are 7.0 and 5 C respectively. For
the design of the contact chamber assume t
10
/ t
0
0.65. From bench-scale test data, the second
order rate constant was determined to be 3.5 L/mol · s. Assume a transferred dose of 2.0 mg/L.
Solution:
a. The difference between the removal/inactivation required and the physical removal cred-
its is the required inactivation. F
rom Example 13-5 for the Noir River:
Required log
removal/inactivation
Treatment log
removal credit
Disinfectant
log inactivation
required to meet
standard
Giardia cysts
3 2.5 0.5
Viruses 422
Cryptosporidium oocysts
532
The last column in this table (Disinfectant log inactivation required to meet standard) is
the difference between the valu es found in the first two columns, that is, (Required log
removal/inactivation) (Treatment log removal credit). The “Required log removal/
inactivation” for Cryptosporidium i s based on the raw water concentration of 1.1 to
2.0 oocys
ts/L given in Example 13-5 , and the additional log-inactivation requirements
given in Table 13-4 . In the footnote to Table 13-4 it is noted that the additional treat-
ment reflects a credit of 3 log credit for conventional treatment. Therefore, 2 log is
added to the 3 log credit given in the footnote for a total of 5 log requ ired.
b. From Example 13-5 , the primary disinfectant is
ozone.
c. Using the EPA’s Ct tables in Appendix D , the Ct to achieve the required log inactivation
for each microorganism at a temperature of 5 C is
Giardia cysts
1.9 mg · min/L
Viruses
4 log inactivation will occur at the Ct of 1.9 mg · min/L
Cryptosporidium oocysts
32 mg · min/L
Therefore, the Cryptosporidium Ct governs.
d. Determine the required hydraulic residence time. With the bench-scale test dose of
2.0 mg/L transferred dose, the required t
10
i s
Ct
C
32
20
16
mg min/L
mg/L
min
.
DISINFECTION AND FLUORIDATION 13-39
a n d if the ozone concentration remains constant throughout the contact chamber, the
theoretical hydraulic detention time with the assumed t
10
/ t
0
of 0.65 is
t
tt
t
10
00
0
16
065
16
065
24 6
min
min
min
.
.
.
e . Because ozone generating capacity is expensive and the energy consumption is high,
Rakness (2005) recommends an optimized design that takes the decay into account by
numerical integration. To perform the numerical integration, the concentration leaving
eac
h chamber must be estimated. This requires the decay rate constant in compatible
units for Ct calculation. The conversion is
()
()
35
48 000
7
.
,
L/mole s
mg/mole of ozone
..
.
29 10
438 10
5
3
L/mg s
or L/mg min
f . An iterative solution is required. Based on Rakness (2005), assume 10 cells. The spread-
sheet solution is shown below. Note that this is a solution, not the first trial. The first
trial with 2.0 mg/L did not achieve the desired Ct of 32 mg · min/L.
Cell no.
Concentration at cell
influent,
mg/L
HDT,
min
Residual at cell
effluent,
mg/L
t
10
,
min
Ct,
mg-min/L
12.30 2.46 2.24 1.60 N/A
2 2.24 2.46 2.19 1.60 3.50
3 2.19 2.46 2.14 1.60 3.42
4 2.14 2.46 2.09 1.60 3.35
5 2.09 2.46 2.05 1.60 3.27
6 2.05 2.46 2.00 1.60 3.20
7 2.00 2.46 1.96 1.60 3.13
8 1.96 2.46 1.92 1.60 3.07
9 1.92 2.46 1.88 1.60 3.01
10 1.88 2.46 1.84 1.60 2.95
11 1.84 2.46 1.81 1.60 2.89
Sum 31.79 or 32
E xplanation of computations:
For the first cell, 2.30 is the initial dose; 2.46 min is the hy draulic detention time
(HDT) based on a total HDT of 24.6 min calculated in step d divided into 10 cells
that provide contact (the need for the 11 cells shown is discussed below); 2.24 is the
calculated
concentration of the influent dose after 2.46 min using the second order decay

13-40 WATER AND WASTEWATER ENGINEERING
equation from Example 13-3 ; t
10
i s the effective contact time using t
10
/ t
0
0.65 for the
efficiency of contact; Ct i s the product of 2.24 1.60. Using the first cell, the residual at
the cell effluent is calculated as follows:
C
230
1 4 38 10 2 46
3
.
..
mg/L
L/mg min mi()(
nnmg/L)( )230
224
.
.
The Ct for this cell is not counted because the influent water has no ozone. The effluent
ozone concentration is a first approximation based on the decay assuming the ozone con-
centration at the inlet was 2.30 mg/L. A more rigorous solution requ ires integration of
the ozone profile based on the kinetics of ozone dissolving into solution and the
decay.
For the second and subsequent cells, the calculations are identical except the influent
concentration is the effluent concentration from the previous cell. The 11th cell is added
to achieve the required Ct of 32 mg · min/L.
g. Design the contact chamber.
The volume of the
chamber is calculated from the hydraulic detention time and the design
flow rate ( Example 13-4 ).
tQ
0
3
24 6 18 500
1
1 440
()( ).,
,
min m /d
min/d
⎛
⎝
⎜⎜
⎞
⎠
⎟
316 04 316
3
.or m
V
U sing the Henry and Freeman (1996) optimum ratios, a depth of 6.0 m and an assumed
H 4 L:
L
H
4
6
4
1 5. m/cell
HL ()( )( )(/cell number of cells wid th of celll
mmdeep m long/cell cel
)
()( )(316 6 1 5 10 .lls width of cell)( )
V
width of cell
m
mm/cell ce
316
615 10
3
()( )(. llls
or m
)
351 35..
The final contact chamber dimensions must include 11 chambers to account for the first
chamber. Therefore, the final dimensions are 6 m deep 3.5 m wide 16.5 m long.
Comments:
1 . Two contact chambers of the size designed must be provided for red
undancy.
2. Because the initial estimate of t
0
was based on a dose of 2.0 mg/L rather than 2.3 mg/L
the calculated t
0
i s a little high. However, the effluent concentration from the first cell is
an approximation, so the extra detention time provides a small safety factor.
3. The effluent concentration used for contact assumed that it was constant while the water
passed through the cell. This is conservative because the average was slightly higher.

DISINFECTION AND FLUORIDATION 13-41
Multiple Contact Reactors. When sequential contactors are used to provide the required con-
tact time to meet the Ct requirements, the calculation procedure is modified by the following
stepwise procedure:
1 . Calculate the Ct value at the exit of each sequential reactor using the residual disinfec-
tant
concentration at that point.
2. Find the the 3 log (99.9%) or 4 log (99.99%) Ct required from the appropriate EPA Ct
table based on water temperature and pH.
3. Compute the inactivation ratio, that is, Ct
calc.
/ Ct
99.9
or Ct
calc.
/ Ct
99.99
.
4. Calculate the estimated log inactivation by multiplying the ratio computed in step 3 by 3
for Giardia and by 4 for viruses because of the requirement for 3 log and 4 log inactiva-
tions, respectively.
5. Sum the segment inactivations to determine the total system log inac
tivation.
This process is demonstrated in Example 13-9 .
Example 13-9. Estimate the total log inactivation for Giardia for disinfection contact in a con-
tact basin followed by a pipeline as described below. The water temperature is 5 C and the pH is
7.5 for both reactors.
Reactor
t
10
contact time
Chlorine residual, mg/L
Clearwell 67 min 1.0
Pipe 53 min 0.6
The chlorine residual was measured at the exit from the reactor.
Solution:
a . Calculate Ct for the clearwell.
Ct
calc
mg/L min
.
. mg min / L()()1 0 67 67
b . F ind the Ct
99.9
for Giardia from Appendix D . At a temperature of 5 C, pH 7.5, and
C 1.0 mg/L, Ct
99.9
is 179 mg · min/L.
c. Calculate Ct
calc.
/ Ct
99.9
.
Ct
Ct
calc
mg min/L
mg min/
.
.
L
99 9
67
179
0
.. .374 0 37or
d . Calculate Ct for the pipe.
Ct
calc
mg/L min mg min/L
.
..()()06 53 318

13-42 WATER AND WASTEWATER ENGINEERING
e . Find the Ct
99.9
for Giardia from Appendix D . At a temperature of 5 C, pH 7.5 and
C 0.6 mg/L, Ct
99.9
is 171 mg · min/L.
f. Calculate Ct
calc.
/ Ct
99.9
.
Ct
Ct
calc
mg min/L
mg min/L
.
.
.
99 9
31 8
171
0 186 0 19..or
g . The sum of Ct
calc.
/ Ct
99.9
is 0.37 0.19 0.56. The equivalent log reduction is then
()( )()()330 5617
99 9
log / log
calc
Ct Ct G
..
..iiardia inactivation.
Comment. Note that the time is t
10
and not the hydraulic residence time.
13-3 EMERGENCY DISINFECTION
When disasters such as floods, tornadoes, and hurricanes occur or when the water treatment plant
disinfection system fails, emergency precautions are required to prevent widespread disease.
In the case of disruption of the water treatment plant disinfection system, loss of water pres-
sure due to a break in the water main, or similar circumstances, the water utility will announce a
boil water advisory notice. This means that water to be used for consumption, food preparation,
and brushing teeth should be boiled vigorously (rolling boil) for 5 minutes.
In the case of natural disasters, the boil water advi
sory should also be given. In addition, treat-
ment of other water for washing hands and utensils is also recommended. Clear water may be
obtained by filtering through clean cloth. Disinfection of the clear water can be accomplished with
household bleach. In general, commercial household bleach contains 5.2 percent (5
2,000 mg/L)
NaOCL. Two to four drops of household bleach per liter of water will provide a measure of protec-
tion. Boiled water should be stored in containers cleaned with boiled water or disinfected water.
For extended durations without public water s u pply , ad vis ories should also include in
struc-
tions to bury fecal waste and to wash hands in disinfected or boiled water. Epidemics that follow
natural disasters often claim more lives than the disaster because these simple measures are not
implemented.
13-4 FLUORIDATION
Introduction
When the concentration of naturally occurring fluoride is too low to prevent tooth decay, it is added
into the water supply. When it produces mottling because it is too high, it is removed from the water.
The discussion in this chapter is focused on increasing the concentration to prevent tooth decay.
Fluoridation Chemistry
The three m ost commonly used fluoride compounds are sodium flu oride (NaF), fluorosilic ic
acid (H
2
SiF
6
), and sodium fluorosilicate (Na
2
SiF
6
). The American Water Works Association
(AWWA) standards for these compounds are:
• AWWA Standard B701 for sodium fluoride.
DISINFECTION AND FLUORIDATION 13-43
• AWWA Standard B702 for sodium fluorosilicate.
• AWWA Standard B703 for fluorosilicic acid.
Sodium Fluoride. When added to water, NaF dissociates into sodium and fluoride ions:
NaF Na F
(13-32)
At temperatures commonly found in water treatment, its solubility is 4 g/100 mL of water. Stan-
dard grade NaF has a solution pH of approximately 7.6. Commercial grade NaF has a nominal
purity of 98 percent (AWWA, 2004).
Fluorosilicic Acid. The most common concentration of H
2
SiF
6
used in water treatment is
23–25 perc ent aqueous solution. In water it dissoc iates virtually 100 percent to form hydroflu-
oric acid and silicon tetrafluoride:
HSiF HF SiF
26 4
2
(13-33)
H ydrofluoric acid strongly dissociates:
HF H F
(13-34)
At high concentration, SiF
4
will volatilize out of solution. At normal water treatment doses it
reacts with water to form silicic acid (H
2
SiO
3
) or silicon dioxide (SiO
2
):
SiF HO HF HSiO
42 23
3 4
(13-35)
SiF HO HF SiO
42 2
24
(13-36)
All solutions of fluorosilicic acid exhibit a pH of approximately 1.2. Commercial grade fluo-
rosilicic acid has a nominal purity of 23 percent and a freezing point of 16C (AWWA, 2004).
Sodium Fluorosilicate. When dissolved in water, Na
2
SiF
6
dissociates:
Na SiF Na SiF
626
2
(13-37)
The most common reaction of SiF
6
is hydrolysis:
SiF HO H F SiO
6
246
22
(13-38)
An alternate pathway is the slow dissociation to form F
and silicon tetrafluoride:
SiFFSiF
6
2
4
(13-39)
The pH values of the solutions are generally about 3.6. Commercial grade sodium fluorosili-
cate has a nominal purity of 98.5 percent (AWWA, 2004).

13-44 WATER AND WASTEWATER ENGINEERING
Available Fluoride Ion. The available fluori de ion is the weight fraction of fluoride in the
compound:
AFI
GMW F
GMW of Compound
(13-40)
The available fluoride as a percent of the commercial grade compound is
Available AFIPurity%% ()( )
(13-41)
Fluoridation Practice
Dosage. The dosage is the amount of fluoride chemical to achieve the optimum flu orid e level
to prevent tooth decay. Initially, the level was obtained by examination of the teeth of thousands
of children living in various places with different fluoride levels. Early in the investigation the
variation was linked to the local air temperature, which had a direct bearing on the amo
unt of
water children consumed at different ages (Reeves, 1999). The Division of Oral Health of the
Centers for Disease Control and Prevention (CDC) developed an optimum scheme based on the
five-year annual average of maximum daily temperatures. The following ex pression, when cal-
culated to one decimal point, summarizes the tabular presentation that may
be found in AWWA
Manual M4:
Dosage
810 5 82 10 1 7432
42 2
() ()TT..
(13-42)
where Dosage fluoride concentration, mg/L
T annual average of maximum daily temperatures, C
The range of acceptable conc entrations is from 0.1 mg/L below to 0.4 mg/L above the dosage
(AWWA, 2004). The applied dosage is the dose calculated using Equation 13-40 min
us the natu-
rally occurring fluoride concentration.
Feed Systems. The simplest fluoridation system is based on fluorosilicic acid. The acid is sup-
plied in carboys. These are set on a platform sc ale, which is used to monitor the dose. Piston,
diaphragm
, or peristaltic pumps made of polyvinyl chloride (PVC) or polypropylene are used to
inject the fluorosilicic acid into the main water flow. A vacuum breaker is used to prevent water
from the main being siphoned into the feed system.
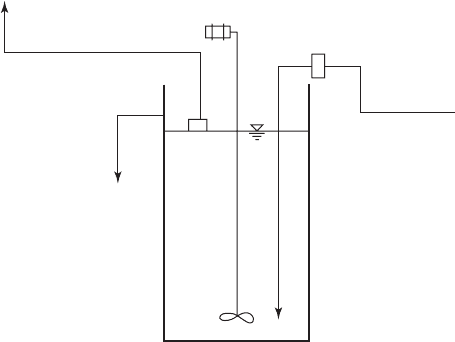
A sodium fluoride saturator ( Figure 13-15 ) is
a simple system that may be used for plants up
to 50,000 m
3
/ d. Sodium fluoride from bags is transferred into the tank and dissolved. The pump
selection and use of a vacuum breaker is the same as that for fluorosilicic acid. The mixing tank
must be corrosion resistant. The water supply will have to be softened because precipitation of
the fluorid
e as CaF will reduce the dose.
Dry feeding of sodium fluorosilicate or sodiu m fluoride may be by either gravimetric or
volumetric feeder to a dissolving tank. The solution is then transported to the main water flow by
either gravity or a pump.
For the smallest plants ( 3,000 m
3
/ d), solution feed from carboys is usually selec ted. For
plants in the intermediate range (3,000 to 10,000 m
3
/ d), manual or automatic fluoride solution
preparation in a saturator is generally selected. Dry feed systems are generally found in plants
DISINFECTION AND FLUORIDATION 13-45
having higher flow rates. Volumetric feeders will provide satisfactory service for flows as low as
500 m
3
/d but are more commonly employed when sodium fluorosilicate is the source of fluoride
and the flow rates are over 10,000 m
3
/ d. For large plants (10,000 m
3
/ d) using sodium fluoride,
gravimetric feeders are appropriate (AWWA, 2004).
Feed Point. Fluoride compounds must be fed after conventional filtration or softening. Feed-
ing fluoride compounds upstream of coagulation/flocculation, settling, and filtration results in
a significant decreas
e in TOC removal, clarifier performance, overloading of the filter and loss
of up to 40 percent of the applied fluoride dose ((Pommerenk and Schafran, 2002). In soften-
ing plants the fluoride will precipitate as CaF if it is introduced before filtration of the softened
water.
Safety Precautions. The greatest chance for exposure to dry fluoride chemicals com es from
the inhalation of
dust generated when feeder hoppers are being filled. The fumes from fluo-
rosilicic acid are ex tremely toxic. During filling operations, operators must wear a respirator
approved by the National Institute for Occupational Safety and Health (NIOSH), splash-proof
safety goggles, an apron, and rubber gloves. A deluge shower and eyewash shoul
d be installed
in the room where fluoridation chemicals are used or stored. Air exhausted from the fluoride
handling equipment shall discharge through a dust filter to the outside atmosphere of the building
(GLUMRB, 2003).
Fluorosilicic acid should not be
stored out of doors. Exposure to the sun will cause build up
of pressure in the containers. Exposure to temperatures below 16C will result in freezing and
potential container rupture.
The state solid waste division should be consulted for proper procedures for dispo
sal of
empty fluoride containers and/or bags. Fluorosilicic acid containers should not be reused
(AWWA, 2004).
Pump suction
line
Overflow line
Floating
strainer
Mixer
Vacuum breaker
Water inlet
FIGURE 13-15
Sodium fluoride saturator. NaF is added manually from bags to prepare
the solution. The Polyethylene tank has a volume of about 200 L.
( Source: AWWA, 2004.)

13-46 WATER AND WASTEWATER ENGINEERING
Example 13-10. Determ ine the chemical feed rate for sodium fluorosilicate in g/min as the
compound and in mL/min of the saturated solution for the following conditions:
Average maximum daily air temperatue 18C
Naturally occurring fluoride concentration 0.2 mg/L
C o mm
ercial purity of Na
2
SiF
6
95%
Solubility 0.762 g/100 mL
Flow rate 158 m
3
/h
Solution:
a. Determine the optimum fluoride level using Equation 13-42 .
Dosage
810 18 5 82 10 18 1 743 209
42 2
() () ...555 10or . mg/L
b. Determine the dosage to be added to the natural background concentration.
Dosage mg/L mg/L mg/L10 02 08...
c . Calculate the available fluoride ion in Na
2
SiF
6
.
AFI
g/mole
g/mole g/mole
()( )
()( )
619
223 28
(()( )619
114
188
0 606 0 61
g/mole
or..
d . Calculate the mass feed rate.
Feed rate
mg/L m /h L/m
()()( )
(
08 158 1 000
33
.,
0061 095
218 119 218 3 6
..
,.
)( )
mg/h or g/h or g/min
e . The solution feed rate:
Solution feed rate
g/min
g/mL
3 6
762 10
3
.
.
4472m L/min
Comments:
1 . The factor of 0.95 in step (d) is the purity.
2. The solution feed rate is the mass feed rate divided by the solubility of sodium fluorosilicate.
13-5 OPERATION AND MAINTENANCE
The following O&M activities are typical:
• Routine measurement of residuals both in the plant and the distribution system to ensure
compliance with applicable regulations.
• Daily, weekly, and monthly preventive maintenance is essential as the chemicals are cor-
rosive and materials failure can res
ult in catastrophic injury and damage to facilities.

DISINFECTION AND FLUORIDATION 13-47
• Corrective action drills and maintenance of response equipment and materials for chemical
leakage.
• Periodic “hands-on” safety training.
Visit the text website at www.mhprofessional.com/wwe for supplementary materials
and a gallery of photos.
13-6 CHAPTER REVIEW
When you have completed studying this chapter, you should be able to do the following without
the aid of your textbooks or notes:
1 . Identify the chemical species that are included in the terms free available chlorine,
chloramine, and total chlorine.
2. Explain why sodium hypochlorite is replacing chlorine as a disinfectant and why it is
equally effective.
3. Explain why light in the ultraviolet range of wavelengths is effective in disinfection
while light in other wavelengths is not.
4. Discuss the role of NOM and each of the common disinfectants (Cl
2
, chloramines,
ClO
2
, O
3
, and UV) in forming disinfection byproducts.
5. Explain the implications of the Chick-Watson law in the design of disinfection facilities.
6. Identify the target microorganisms used by the U.S. Environmental Protection Agency
to establish regulations for disinfection.
7. Identify the four factors that bou
nd the selection of a primary disinfectant.
8. Select an appropriate reactor configuration to provide superior contacting performance.
9. Explain why temperature is used to govern the concentration of fluoride in drinking water.
10. Select an appropriate chemical form of fluoride given the design water flow rate for a
community.
W ith the use of thi
s text, you should be able to do the following:
11. Given the pH and chlorine or hypochlorous acid concentration in water, determine the
fractions that are HOCl and OCl
.
12. Calculate the masses of ammonia and Cl
2
or HOCl required to achieve a given concen-
tration of monochloramine for a given water plant flow rate.
13. Calculate the percent available chlorine or the relative oxidation potential of com-
pounds given a table of half-reactions.
14. Determine the decay rate constant for first and second order reactions of a disinfectant
from a set of data.
15. Determine the decay rate con
stant for a Chick-Watson reaction given a set of Ct data
and an assumption about the value of n.
