Water and Wastewater Engineering
Подождите немного. Документ загружается.

6-16 WATER AND WASTEWATER ENGINEERING
the specifics of the product. Dry and liquid forms are available. The properties of iron with respect
to forming large complexes, dose, and pH curves are similar to those of alum. An example of the
reaction of FeCl
3
in the presence of alkalinity is
FeCl HCO H O FeOH HOs CO C
33
2
3
22
33 3 33
() () ll
(6-10)
and without alkalinity
FeCl H O FeOH HOs HCl
3
2
3
2
333 () ()
(6-11)
forming hydrochloric acid, which in turn lowers the pH.
pH and Dose
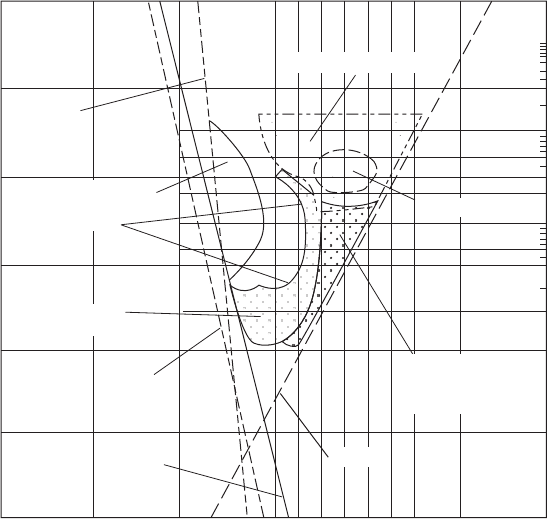
Two important factors in coagulant addition are pH and dose. The optimum dose and pH mus t
be determined from laboratory tests. The optimum pH range for alum is approximately 5.5 to
7.7 with adequate coagulation possible between pH 5 and 9 under some conditions ( Figure 6-9 a).
Al(OH)
4
Al(OH)
4
Al
3
Adsorption
destabilization
Restabilization zone
(boundaries change
with colloid)
Al
8
[OH]
Optimum sweep
20
4
Sweep coagulation
2
2
3
4
5
6
7
8
4681012
1000
300
500
100
50
30
10
5
3
0.3
log [Al], mol/L
pH of mixed solution
Combination
(sweep and
adsorption)
Alum as Al
2
[SO
4
]
3
• 14.3 H
2
O, mg/L
FIGURE 6-9a
Design and operation diagram for alum coagulation. (Source: Amirtharajah and Mills, 1982.)
COAGULATION AND FLOCCULATION 6-17
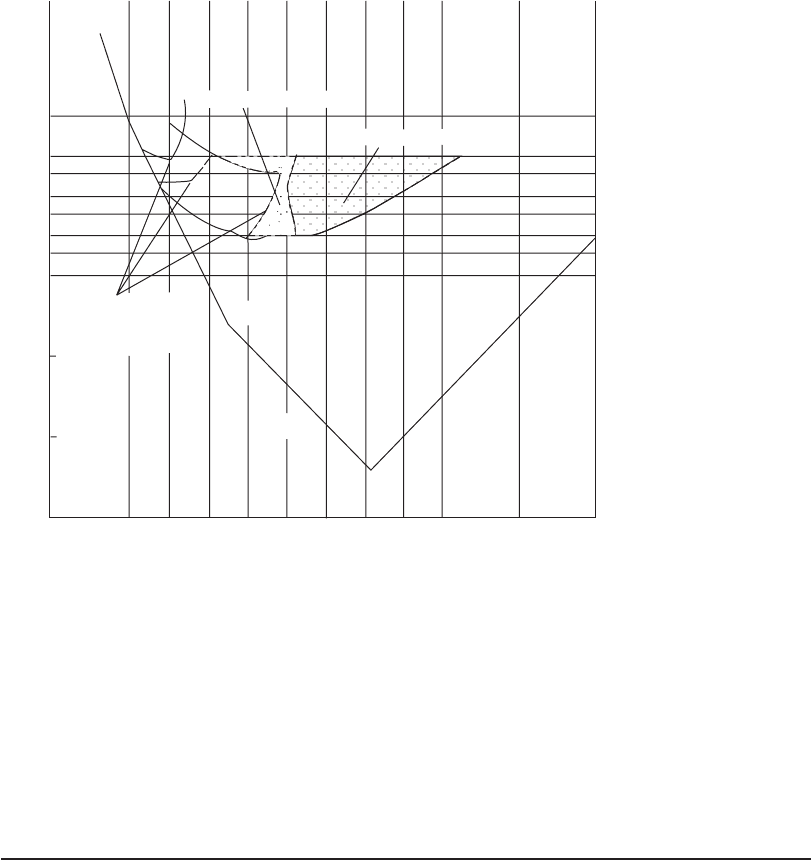
Ferric salts generally have a wider pH range for effective coagulation than aluminum, that is, pH
ranges from 4 to 9 ( Figure 6-9 b). The figures represent the alum dose and pH of the treated water
after alum has been added. Prehydrolyzed metal salts (polyaluminum chloride, polyaluminum
sulfate, and poly
iron chloride) can be used over a pH range of 4.5 to 9.5 (MWH, 2005).
Becaus e of the num ber and complexity of coagulant reactions, the actual dose and pH for a
given water on a given day is generally determ ined empirically from a laboratory test. The test
procedure is called a “jar test” based on the configuration of the test apparatus ( Figure 6-10 ). It is
illu
strated in the next example.
Example 6-3. Six beakers are filled with the raw water, and then each is mixed and flocculated
uniformly by identical paddle stirrers driven by a single motor (a gang stirrer ). A typical test is
conducted by first dosing each jar with the same alum do
se and varying the pH in each jar. The
test is then repeated in a second set of jars by holding the pH constant at the optimum pH and
varying the coagulant dose.
In the example set of data below, two sets of such jar tests were conducted on a raw water
containing 15 NTU and a
HCO
3
alkalinity concentration of 50 mg/L expressed as CaCO
3
.
The turbidity was measured after the mixture was allowed to settle for 30 minutes. The objective
is to find the optimal pH, coagulant dose, and the theoretical amount of alkalinity that would be
consumed at the optimal dose.
Fe[OH]
2
Fe
3+
2
3
4
5
6
27
100
270
10
2.7
1.0
0.27
Restabilization zone
(changes with colloid
surface area)
FeOH
2
Sweep coagulation
Adsorption-destabilization
Fe[OH]
4
Log [Fe], mol/L
8
10
12
2 4 6 8 10 12
pH
Fe Cl
3
.
6H
2
O, mg/L
FIGURE 6-9b
Design and operation diagram for Fe(III) coagulation. (Source: Johnson and Amirtharajah, 1983.)

6-18 WATER AND WASTEWATER ENGINEERING
FIGURE 6-10
Jar test apparatus with turbid water (a) and three samples during flocculation (b).
(Source: Mackenzie L. Davis.)
(a)
(b)
Jar test I
Jar numbers
12 3 4 5 6
pH 5.0 5.5 6.0 6.5 7.0 7.5
Alum dose (mg/L) 10 10 10 10 10 10
Turbidity (NTU) 11 7 5.55.7 8 13
COAGULATION AND FLOCCULATION 6-19
Jar test II
Jar numbers
123 4 5 6
pH 6.0 6.0 6.0 6.0 6.0 6.0
Alum dose (mg/L) 5 7 101215 20
Turbidity (NTU) 14 9.55 4.5 613
Solution:
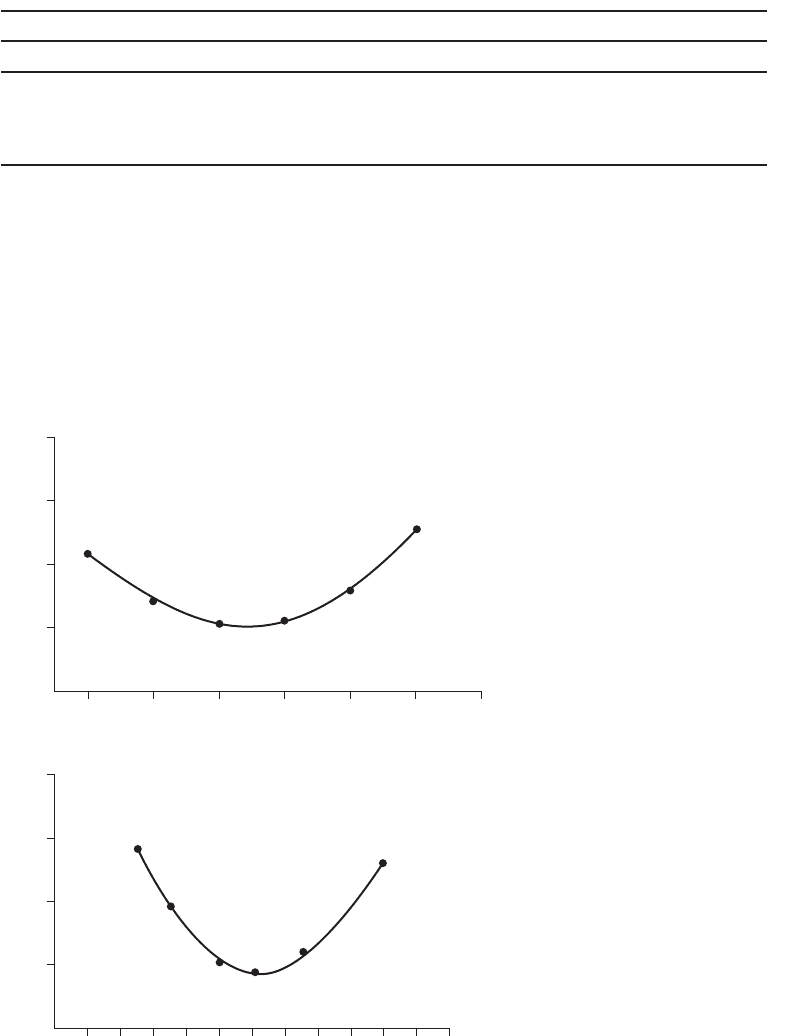
a . The results of the two jar tests are plotted in Figure 6-11 . In the first test, the optimal pH
was chosen as 6.0, and this pH was used for the second jar test. From the second jar test,
the optimal alum dose was estimated to be about 12.5 mg/L. In actual practice, the labo-
ratory tec
hnician would probably try to repeat the test using a pH of 6.25 and varying the
alum dose between 10 and 15 to pinpoint the optimal conditions.
From Figure 6-11 , the optimum pH was estimated to be 6.0 and the optimum dose
was estimated to be 12.5 mg/L.
20
20
5.0 5.5 6.0 6.5
pH
Alum dose, mg/L
(b)
(a)
7.0 7.5 8.0
15
24681012141618202224
Turbidity remaining, NTU Turbidity remaining, NTU
10
5
15
10
5
FIGURE 6-11
Results of jar test. (a) Cons tant alum dose,
(b) constant pH.

6-20 WATER AND WASTEWATER ENGINEERING
b. The amount of alkalinity that will be consumed is found by using Equation 6-8 , which
shows that one mole of alum consumes six moles of
HCO
3
. With the molecular weight
of alum equal to 594, the moles of alum added per liter is:
12 5 10
594
21 10
3
5
.
.
g/L
g/mole
moles/L
which will consume
6 2 1 10 1 26 10
5 4
()..
moles/L moles/L HCO
3
The molecular weight of is 61, so
()()()126 10 61 10 7
4 3
..
moles/L g/mole mg/g 77 mg/L HCO
3
are consumed, which can be expressed as CaCO
3
:
()77
3
.
..
..
mg/L HCO
E W CaCO
EW HCO
3
3
()77
50
61
. mg/L HCO
g/equivalent
g/equival
3
eent
mg/L HCO as CaCO
3
6 31
3
.
A s noted earlier, the lack of sufficient alkalinity will require the addition of a base to adjust
the pH into the acceptable range. Lime (CaO), calcium hydroxide Ca(OH)
2
, sodium hydroxide
(NaOH), and sodium carbonate (Na
2
CO
3
), also known as soda ash, are the most common chemi-
cals used to adjust the pH. Table 6-3 illustrates the neutralization reactions.
TABLE 6-3
Neutralization reactions
To neutralize sulfuric acid with
Lime: H
2
SO
4
CaO CaSO
4
H
2
O
Calcium hydroxide: H
2
SO
4
Ca(OH)
2
CaSO
4
H
2
O
Sodium hydroxide: H
2
SO
4
NaOH NaSO
4
2H
2
O
Soda ash: H
2
SO
4
Na
2
CO
3
Na
2
SO
4
H
2
O CO
2
To neutralize hydrochloric acid with
Lime: 2HCl CaO CaCl
2
H
2
O
Calcium hydroxide: HCl Ca(OH)
2
CaCl
2
H
2
O
Sodium hydroxide: HCl NaOH NaCl H
2
O
Soda ash: HCl Na
2
CO
3
NaCl H
2
O CO
2
Note: a stoichiometric reaction will yield a pH of 7.0.

COAGULATION AND FLOCCULATION 6-21
E xample 6-4 illustrates the impact of the lack of alkalinity on the solution pH and the method
for estimating the amount of base to bring the solution to a pH range that is satisfactory for
coagulation.
Example 6-4. Estim ate the pH that results from the addition of 100 mg/L of alum to a water
with no alkalinity, and estimate the amount of sodium hydroxi
de (NaOH) in mg/L required to
bring the pH to 7.0.
Solution:
a . From Example 6-2 , the number of moles of alum added is 1.68 10
4
moles/L.
b. From Equation 6-9, note that 3 moles of sulfuric acid are produc ed for each mole of
alum added. Therefore, the moles/L of sulfuric acid is
3 168 10 5 04 10
44
()..
moles/L moles/L
c. Sulfuric acid dissociates to form two moles of H
for each mole of acid
HSO H SO
4
2
24
2
so the moles/L of H
formed is
2 5 04 10 1 01 10
4 3
()..
moles/L moles/L
d. The estimated pH is
pH log H log moles/L
[] [ ]101 10 3 00
3
..
e. From Figure 6-9 a, it is evident that this is out of the range of coagulation with alum.
f. Using NaOH to neutralize the sulfuric acid, the reaction is
HSO NaOH NaSO HO
24 24 2
22
Therefore, 2 moles of sodium hydroxide are required to neutralize each mole of sulfuric acid
2 1 01 10 2 02 10
33
()..
moles/L moles/L
g. Converting to mg/L
()()()202 10 40 10 80
33
.
moles/L g/mole mg/g ..64 81or mg/L
Comments:
1 . To determine if base needs to be added when alkalinity is present, estimate the amount of
alkalinity present and calculate the amount of alkalinity “destroyed,” as in Example 6-2 .
If the amount destroyed exceeds the amount present, estimate the exc ess alum and
use
this amount to estimate the amount of base to add.

6-22 WATER AND WASTEWATER ENGINEERING
2. Because of the d iversity of species that occur when alum and/or ferric chloride are hy-
drolyzed, and because natural waters will contain ions that will react with the base, it is
not practical to calculate the dose. In actual practice, the dose is determined by titration
of a water sa
mple.
6-4 COAGULATION PRACTICE
The selection of the coagulant and the coagulant dose is a function of the characteristics of the
coagulant (including its price), the concentration and type of particles, characteristics of NOM,
water temperature, and other constituents of the raw water such as alkalinity and phosphorus.
There is no formal approac
h to incorporate this collection of variables in the selection process.
Jar test experiments and experience play a large role in the selection process. Some of the factors
to be considered in the decision process are discussed in the following paragraphs.
Overview
High tu rbidity, high alkalinity water is the easiest to coagulate. Alum, ferric chloride, and high
molecular weight polymers have been used successfully for these waters.
Control of the pH is of utmost importance in coagulating high turbidity, low alkalinity water.
Polymers function well. Addition of a base may
be required for alum and ferric chloride.
Alum and ferric chloride at high doses can coagulate low turbidity, high alkalinity waters.
A combination of alum followed by polymer often works well. For this system, that is, low tur-
bidity and high alkalinity, polymers cannot work alone. Coagulant aids
may be required.
Low turbidity, low alkalinity waters are the most difficult to coagulate. Neither polymers
nor alum/ferric chloride work alone when the turbidity and alkalinity are low. pH adjustment is
required. Direct filtration should be considered for this type of water.
Coagulation of color is very pH
dependent. Alum, ferric chloride, and cationic polymers are effec-
tive at pH values in the range of 4 to 5. The floc that are formed in coagulating color are very fragile.
Coagulant Selection
Metal Salts. A s noted previously, the most common coagu lants are alum, ferric chloride, and
ferric sulfate. The predominant choice of coagulant is alum, followed by ferric chloride and fer-
ric sulfate, respectively. While cost may be the overriding factor, the operating region, as noted
in F
igure 6-9 , plays a significant role in coagulant selection. Ferric chloride is effec tive over a
broader range. Polyaluminum chloride (PACl) is less sensitive to pH and can be used over a pH
range from 4.5 to 9.5 (MWH, 2005).
The metal salt hydrolysis products react with
SO
4
2
, NOM, F
, and
PO
4
3
to form both
soluble and insoluble products. This will result in a requirement for increased dosage to achieve
the desired destabilization.
T ypical dosages of alum range from 10 to 150 mg/L. Ferric chloride and ferric sulfate dos-
ages range from 5 to 150 mg/L and
from 10 to 250 mg/L respectively (MWH, 2005).
NOM removal is a means of reducing disinfection byproducts. In the regulatory language of
the U.S. EPA, enhanced coagulation i s a recommended technique for removing NOM. Because
NOM binds with metal ion coagulants, this is a consideration in selecting a coagu
lant and the
dose to be applied. Of the metal salts and prehydrolyzed metal salts, the most effective for the
removal of NOM, in order of increasing effectiveness, are iron, alum and PACl (MWH, 2005).
COAGULATION AND FLOCCULATION 6-23
Polymer. In rare instances, usually when the turbidity and alkalinity are high, cationic polymers
(poly-DADMAC and epi-DMA) have been used as primary coagulants, but their use typically
has been in conjunction with a metal salt. The main advantage of using polymers in conjunction
with metal s
alts is the ability to reduce the metal salt concentration and resulting sludge produc-
tion by 40 to 80 percent.
The epi-DMA dose generally decreases as the pH increases. The dose for poly-DADMAC is
only slightly affected by pH. Typical dosages are on the order of 1 to 10 mg/L.
Polymers are not effective in re
moving NOM.
Coagulant Aids
Insoluble particulate materials such as clay, sodium silicate, pure precipitated calcium carbonate,
diatomite, and activated carbon have been used as coagulant aids. They are used in waters that have
low concentrations of particles and, thus, have few nuc
leating sites to form larger floc. Because
their density is higher than most floc particles, floc settling velocity is increased by the addition of
coagulant aids. The dosage must be carefully controlled to avoid lowering the water quality.
Flocculant Aids
Uncharged and negatively charged polymers are used as flocculant aids. Their purpose is to
build a stronger floc. They are added after the coagulants are added and the particles are already
destabilized.
A ctivated silica and sodium silicate are comm
on flocculant aids. In processes where these are
added, called ballasted flocculation, micro-sand is added after chemical coagulation but before
flocculation to act as a nucleus for floc formation. The sand has a higher density than the floc and
increases its settling velocit
y (Willis, 2005).
6-5 FLOCCULATION THEORY
S moluchowski (1917) observed that small particles undergo random Brownian motion due to
collisions with fluid molecules and that these motions res ult in particle to particle collisions.
Langelier (1921) observed that stirring water containing particles created velocity gradients that
brought about particle c
ollisions. These observations provide the basis for describing the mecha-
nisms of flocculation.
Microscale Flocculation
The flocculation of small particles (less than 0.1 m in diameter) is caused by diffusion. The rate
of flocculation is relative to the rate at which the particles diffuse. Thus, the primary mechanism
of aggregation is through Brownian motion. This aggregation is called microscale flocculation or
perikinetic flocculation. After a period
of seconds, the microflocs range in s ize from 1 to about
100 m in diameter.
Macroscale Flocculation
Mixing is the major flocculation mechanism for particles greater than 1 m in diameter. This
mechanism is known as macroscale flocculation or orthokinetic flocculation. Mechanical mixing
is employed to achieve orthokinetic flocculation.
6-24 WATER AND WASTEWATER ENGINEERING
Mechanical m ixing cau ses u nequal shearing forces on the floc, and some of the floc are
broken up. After some period of mixing, a steady state distribution of floc sizes is achieved and
formation and breakup become nearly equal.
Differential Settling
Because the floc particles are of different size, they settle at different rates. Differences in the
settling velocities cause the particles to collide and flocculate.
Chemical Sequence
The addition of multiple chemicals to improve flocculation is common practice. The order of
addition is important to achieve optimum results at minimum cost. Typically, the addition of
a polymer after the addition of hydrolyzing metal salts is most effective. Ideally, the polymer
addition
should be made 5 to 10 minutes after the addition of the hydrolyzing metal salt. This
allows for the formation of pinpoint floc that is then “bridged” by polymer. In conventional water
treatment plant design this is rarely possible because of space limitations.
6-6 MIXING THEORY
The crux of efficient coagulation is the efficiency of mixing the coagulant with the raw water.
Efficient flocculation requires mixing to bring the particles into contact with one another.
The following discussion includes the theoretical considerations in mixing coagulants, floc-
culation, and the practical aspects of sele
cting a mixing device. Many aspects of this discussion
also apply to pH adjustment, softening (Chapter 7), and disinfection (Chapter 13).
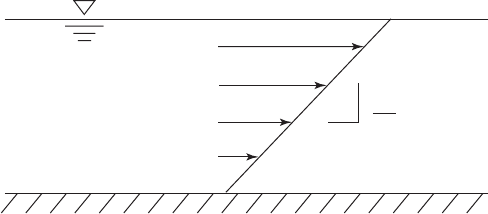
Velocity Gradient
In the 1940s Kolmogorov (1941) and Camp and Stein (1943) independently developed a method of
quantifying the energy dissipation in a vessel. Camp and Stein further proposed that the root-mean-
square (RMS) of the velocity gradient ( G ) of the fluid, that is dv / dy in Figure 6-12 , be used to esti-
mate energy dissipation. They further propos
ed that the rate of flocculation is directly proportional
to G. S ubsequent research demonstrated that the proportionality also applied to coagulation with
both metal ion coagulants and polymers (Harris, et al., 1966; Birkner and Morgan, 1968).
dv
dy
FIGURE 6-12
Velocity gradient.

COAGULATION AND FLOCCULATION 6-25
The velocity gradient may be thought of as the amount of shear taking place; that is, the
higher the G value, the more violent the mixing. The velocity gradient is a function of the power
input into a unit volume of water. The RMS velocity gradient may be estimated as
G
P
12/
⎛
⎝
⎜
⎞
⎠
⎟
V
(6-12)
where G global RMS velocity gradient, s
1
P
power of mixing input to vessel, W
dynamic viscosity of water, Pa · s
volume of liquid m,
3
V
Different velocity gradients are appropriate for different processes. Coagulation requires very
high velocity gradients. Flocculation requires a velocity gradient high enough to cause particle
contact and to keep the flocs from settling but low enough to prevent the flocs from tearing apart.
In addition, different chemicals require different velocity gradient
s.
Mixing Time
E xperimental work has revealed that coagulant reactions are very fast. Alum hydrolyzes to
Al(OH)
2
within 10
5
s (Base and Mesmer, 1976). Hahn and Stumm (1968) found the time
to form m ono- and polynuclear hydroxide species was on the order of 10
3
s, and the time of
formation of polymer species was on the order of 10
2
s.
This work along with field observations implies that nearly instantaneous and intense mixing
of metal salts is of critical importance. This is especially true when the metal salts are being used
to lower the surface charge of the particles (adsorption and destabilization in Figure 6-9 ). Mixing
tim
es of less than 1 s are recommended in this case. The formation of the aluminum-hydroxide-
precipitate is slower and occurs in the range of 1 to 7 s. Thus, in sweep coagulation ( Figure 6-9 )
the extremely short mixing times are not as critical (Amirtharajah and Mills, 1982).
The time requirement
s for flocculation are more dependent on the requirements of d own-
stream processes. For conventional treatment where settling follows flocculation the flocculation
time ranges from 20 to 30 minutes. If direct filtration is to follow flocculation, shorter times on
the order of 10 to 20 minutes are often selected (MWH, 2005).
For these time-depen
dent reactions, the time that a fluid particle remains in the reactor
affects the degree to whic h the reaction goes to completion. In ideal reactors the average time
in the reactor (the theoretical detention time also known as hydraulic detention time, hydraulic
residence time, or detention time) is defined as
t
Q
=
V
(6-13)
where t theoretical detention time, s
volume of fluid in reactor m
,
3
V
Q flow rate into reactor, m
3
/ s
Theoretically, given the desired detention time and the design flow rate, the liquid volume of
the vessel to achieve the design detention time may be calculated. However, real reactors do not
behave as ideal reactors because of density differences due to temperature or other causes, s
hort
