Water and Wastewater Engineering
Подождите немного. Документ загружается.

CHEMICAL HANDLING AND STORAGE 5-21
5-9 CHAPTER REVIEW
When you have completed studying this chapter, you should be able to do the following without
the aid of your textbooks or notes:
1 . Given the plant capacity and/or the scenario for discharge, determine the number of
feeders required.
2. Describe a method for covering the range of feed capacities required when the turn-
down ratio of the feeder may not be sufficient.
3. Explain why the liquid chlorine level in chlorine tanks i
s nominally at 85 percent of the
volume of the tank.
4. Explain the difference between interruptible and noninterruptible chemicals and give
examples of each.
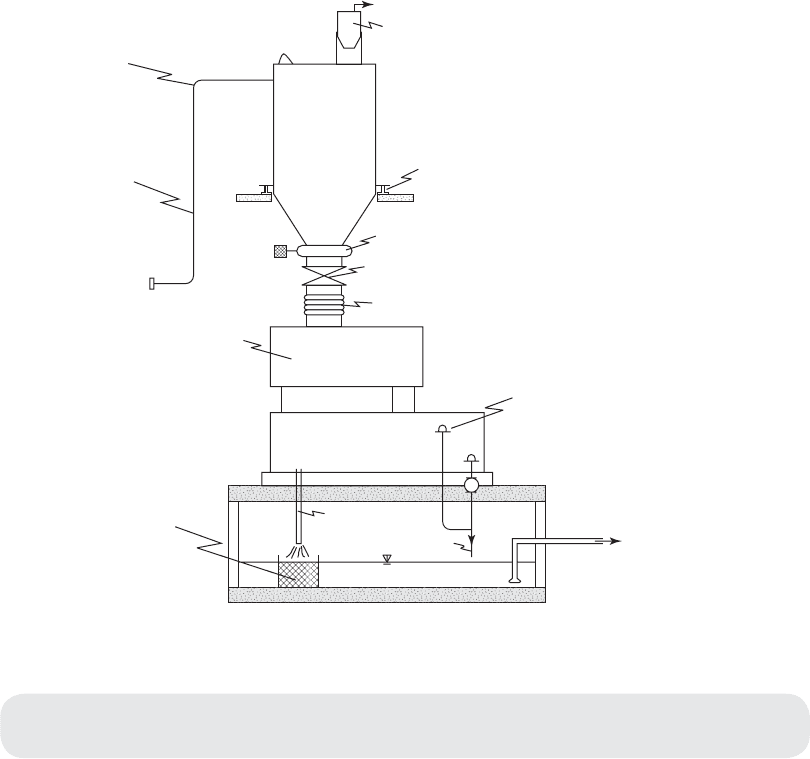
Vent
Manhole
Dust collector
Plastic milk
crate lined with
hardware cloth
Gravimetric dry
chemical feeder
10 cm dia. fill connection
to bulk delivery truck
Large radius elbow
Pneumatic
conveying pipe
Sl
aker
discharge
Drain
To venturl
eductor
Slaker
Overflow
Flexible coupling
Bin gate
Bin activator
Load cell
Cylindrical
lime
silo
FIGURE 5-6
L i me materials storage, handling, and feed system. Two feeder slaker units are required for redundancy.
Both may serve one silo. Transfer from the silo to day bins may also simplify measuring chemical usage.
Visit the text website at www.mhprofessional.com/wwe for supplementary materials
and a gallery of photos.
5-22 WATER AND WASTEWATER ENGINEERING
5. Describe a method to keep track of the inventory in a dry chemical silo.
6. Explain why the percent concentration of some liquid chemicals is important in han-
dling and storage.
7. Given the plans for secondary containment of a storage area, identify the components
that would need to be chec
ked to meet acceptable design criteria.
8. Explain the purpose of a day tank.
9. Define MSDS.
W ith the use of this text, you should be able to do the following:
10. Design a chemical storage tank or silo.
1 1 . Design a secondary containment system given the dimensions of a storage tank or silo.
12. Examine a set of drawings to verify
safety features for a chlorine gas feed and storage
system.
13. Select an appropriate feeder for a given chemical.
14. Given a chemical and material or two chemicals, use appropriate charts to determine
whether or not they are incompatible.
5-10 PROBLEMS
5-1. Design a storage silo for lime for a water treatment plant with a design average day
capacity of 0.23 m
3
/ s. The maximum dose is estimated to be 133 mg/L as CaO.
The local supplier has current contracts with other municipalities that specify
85% purity and an average bulk density of 960 kg/m
3
. Shipping time is
normally two weeks. Provide a dimensioned drawing of the silo with recom-
mended appurtenances.
5-2. Design a storage silo for soda ash for a water treatment plant with a design average day
capacity of 0.23 m
3
/ s. The maximum dose is estimated to be 106 mg/L as Na
2
CO
3
. The
local supplier has current contracts with other municipalities that specify 99% purity
and an average bulk density of 800 kg/m
3
. Shipping time is normally 10 working days.
Provide a dimensioned drawing of the silo with recommended appurtenances.
5-3. Mule Shoe is to provide fluoride to augment the natural fluoride in the water supply.
The natural fluoride concentration is 0.25 mg/L. The design concentration is 1.0 mg/L.
The flow rate i
s 0.057 m
3
/ s. The municipal water authority has decide to use
45 kg polyethylene kegs of fluorosilicic acid (H
2
SiF
6
) provided by the chemical
supplier as their storage system. Commercial strength is 40% H
2
SiF
6
. Estimate the
number of kegs they must store if delivery is once a month.
5-4. For safety and security reasons, the city of Alum Rock has decided to replace its chlo-
rine gas disinfection system with a sodium hypochlorite (NaOCl)
system. You have
been tasked with the design of the storage tank(s) for sodium hypochlorite that is to
replace the gas cylinders. The existing storage system consists of twelve 900 kg
CHEMICAL HANDLING AND STORAGE 5-23
chlorine gas cylinders. These are housed in a room that is 11.5 m 7 m 3.3 m.
Assume that the chlorine gas is 100% pure and that commercial NaOCl will provide
12% available chlorine. Specify the dimensions of the tank(s) and the materials for
constructing an equivalent sodium hy
pochlorite storage system. Provide a dimensioned
drawing of the tank(s) with recommended appurtenances and show how they will fit in
the existing chlorine room. Assume the density of 12% NaOCl is 1,210 kg/m
3
and that
a 1.0 m clearance between the top of the tank and the ceiling is required.
5-5. Determine the size (in m
3
/hour) and number of diaphragm pumps to feed ferric chlo-
ride for a 3,800 m
3
/ d water treatment plant. The optimum dose selected is 50 mg/L.
Ferric chloride may be obtained in a liquid form that is 40% pure. The density of this
solution is 1.415 kg/L.
5-6. Black Gold is to expand their water treatment plant because of a major increase in
population due to the discovery
of oil in the county. Lime is used to adjust the pH of
their coagulation process. The estimated dosage range from the opening of the plant
until it reaches its design life is 3 to 150 kg/h. The bulk density of lime is approxi-
mately 960 kg/m
3
. Select an appropriate type of feeder or combination of feeders
from the list below.
Feeder type Model
Capacity
m
3
/h
Turn-down
ratio
Loss-in-weight A-1 0.06 100:1
A-2 2.0 100:1
Continuous belt B-1 0.03 10:1
B-2 0.06 10:1
5-7. A chlorinator needs to be selected to complete the design of the disinfection facili-
ties for Camp Verde. The average chlorine dose required is estimated to be 2.0 mg/L.
The maximum dose required is estimated to be 10 mg/L. The average flow rate is
0.23 m
3
/ s. The available chlorinators are listed below. Each rotameter has a turn-
down ratio of 20:1. Select the appropriate model and rotameter(s).
Model
Capacity,
kg/d
Rotameter
rating, kg/d
V-1 225 45
90
135
180
225
V-2 900 45
115
225
450
5-24 WATER AND WASTEWATER ENGINEERING
5-11 DISCUSSION QUESTIONS
5-1. A small batch coagulation plant did not receive the expected shipment of alum on
time. However, they have lime (CaO) available, if they could use it. Can they use it
as a substitute? Explain why or why not. Mention any safety precautions.
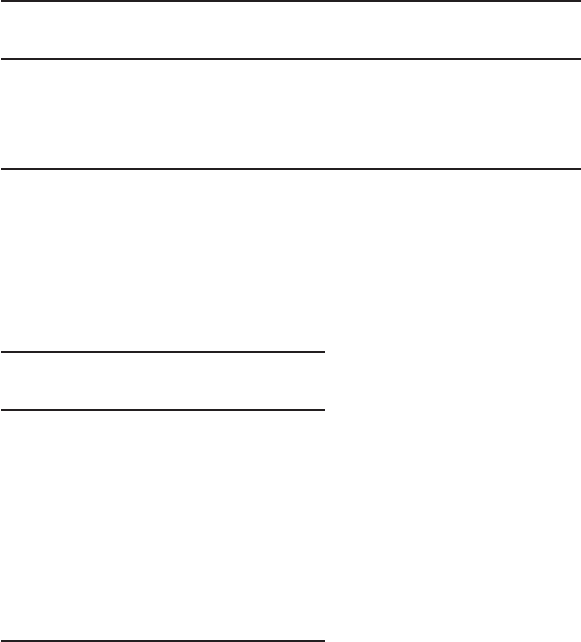
5-2. In Figure P-5-2 , identify design items that are either wrong or that are m
issing.
Bolted
manhole
Pressure
transmitter
Batch meter
Flushing
connection,
typical
Alum storage tank
Provide
cap for
future
connection
Calibration reservoir
and valves, typical
Pulsation dampener
and
pressure gauge,
typical
Sample drain,
typical
Flushing
connection, typical
Operations buildin
g
chemical feed room
Utility water
Bulk chemical storage area
Mixing pump
Quick
connect,
typical
Type 304
stainless
S
G
G
FIGURE P-5-2
CHEMICAL HANDLING AND STORAGE 5-25
5-12 REFERENCES
Anderson, J. L. (2005) “Chemicals and Chemical Handling,” in E. E. Baruth (ed.), Water Treatment Plant
Design, McGraw-Hill, New York, pp. 15.1–15.53.
GLUMRB (2003) Recommended Standards for Water Works, Great Lakes–Upper Mississippi River
Board of State and Provincial Public Health and Environmental Managers, Health Education
Services, Albany, New York.
H udson, H. E. (1978) “Chemical Handling and Feeding,” in R. L. Sanks (ed.), Water Treatment Plant
Design for the Practi
cing Engineer, Ann Arbor Science, Ann Arbor, Michigan, pp. 125–130.
H udson, H. E. (1981) Water Clarification Processes, Van Nostrand Reinhold, New York.
Kawamura, S. (2000) Integrated Design and Operation of Water Treatment Facilities, 2nd ed., John
Wiley & Sons, New York, pp. 358–361, 367–372.
Metcalf & Eddy (2003) Wastewater Engineering: Treatment and Reuse, 4th ed., McGraw-Hill, Boston,
MA, pp. 532–540.
NIOSH (2003) NIOSH Pocket Guide to Chemical Hazards, Nostrand Reinhold, New York.
U.S. EPA (1974) Design Criteria for Mechanical, Electrical, and Fluid Systems and Component
Reliability, Supplement to Federal Guidelines: Design, Operation, and Maintenance of Wastewater
Treatment Facilities, U.S. Environmental Protection Agency Report No. 430-99-74-001,
Washington, D.C.

6-1
CHAPTER
6
COAGULATION AND FLOCCULATION
6-1 INTRODUCTION
6-2 CHARACTERISTICS OF PARTICLES
6-3 COAGULATION THEORY
6-4 COAGULATION PRACTICE
6-5 FLOCCULATION THEORY
6-6 MIXING THEORY
6-7 MIXING PRACTICE
6-8 OPERATION AND MAINTENANCE
6-9 CHAPTER REVIEW
6-10 PROBLEMS
6-11 DISCUSSION QUESTIONS
6-12 REFERENCES
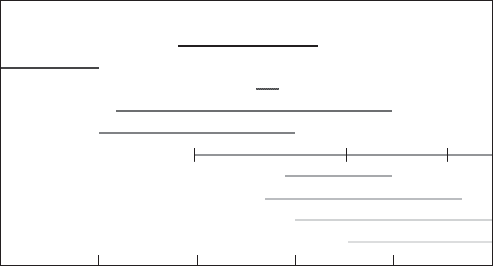
6-2 WATER AND WASTEWATER ENGINEERING
Particle size, m
Fog MistRain
0.01 0.1 1 10 100 1000
Screen mesh
Visible to eye
Human hair
Pollens
Bacteria
Algae
Cryptospordium oocysts
Viruses
Giardia cysts
FIGURE 6-1
Particulates in water and miscellaneous other reference sizes.
6-1 INTRODUCTION
Coagulation and flocculation are essential components of conventional water treatment systems
that are designed to
• Remove infectious agents,
• Remove toxic compounds that have adsorbed to the surface of particles,
• Remove precursors to the formation of disinfection byproducts, and
• Make the water palatable.
S urface water supplies contain organic and inorganic particles. Organic particles may include
algae, bacteria, cysts of protozoa, oocysts, and d etritus from vegetation that has fallen into the
water. Erosion produces inorganic particles of clay, silt, and mineral oxides. Surface water will
als
o include particulate and dissolved organic matter, collectively referred to as natural organic
matter (NOM), that is a product of decay and leaching of organic detritus. NOM is important
because it is a precursor to the formation of disinfection byproducts.
Groundwater treated to remove hardness
, or iron or manganese, by precipitation contains
finely divided particles.
Both the precipitates and the surface water particles may, for practical purposes, be classi-
fied as suspended and colloidal. Suspended particles range in size from about 0.1 m up to abo
ut
100 m in diameter ( Figure 6-1 ). Colloidal particles are in the size range between dissolved sub-
stances and suspended particles. They are in a solid state and c an be rem oved from the liquid by
physical means such as very high-forc e centrifugation or by pa
ssage of the liquid through filters
with very small pore spaces. Colloidal particles are too small to be removed by sedimentation or
by sand filtration processes.
The object of coagulation (and subsequently flocculation) is to turn the small particles into
larger particles called flocs, either as precipitates
or suspended particles. The flocs are readily
removed in subsequent processes such as settling, dissolved air flotation (DAF), or filtration.
For the purpose of this discussion coagulation means the addition of one or more chemicals to

COAGULATION AND FLOCCULATION 6-3
condition the small particles for subsequent processing by flocculation. * Flocculation i s the
process of aggregation of the destabilized particles and precipitation products.
6-2 CHARACTERISTICS OF PARTICLES
Electrical Properties
The most important electrical property of the colloidal and suspended particles is their surface
charge. This charge c auses the particles to remain in suspension without aggregating for long
periods of time. Surface water particle suspensions are thermodynamically unstable and, given
enough time, they will flocculate and settle. However, the aggregation process is very slow, and
the particles cannot be removed by sedimentation in a reasonable amount of time, that is, a short
enough time that would allow production of a sufficient amount of water for a community of
more than a few people.
For most particles in water the sign of the c
harge is negative (Niehof and Loeb, 1972; Hunter
and Liss, 1979). This charge arises in four principal ways (Stumm and Morgan, 1970):
• Ionization. For example, silica has hydroxyl groups on its exterior surfac e. Depending on
the pH, these can accept or donate protons:
Si OH Si OH Si O
2
pH 2pH 2pH 2
• Adsorption. In this case, a solute becomes bound to the solid surface, for example, a humic
acid or natural color on a silica surface. These large macromolecules have carboxylic acid
groups that dissociate at pH values greater then 5
to form negative ions.
• Isomorphous replacement. Under geologic conditions, the metal in a metal oxide is re-
placed by a metal atom with a lower valence. For example, if, in an array of solid SiO
2
tetrahedra, an Si atom is replaced by an Al atom (Al
3
has one less electron than Si
4
), the
lattice becomes negatively charged.
• Structural imperfections. In the formation of the mineral crystal, bonds are broken on the
edge of the crystal. These lead to development of surface charge.
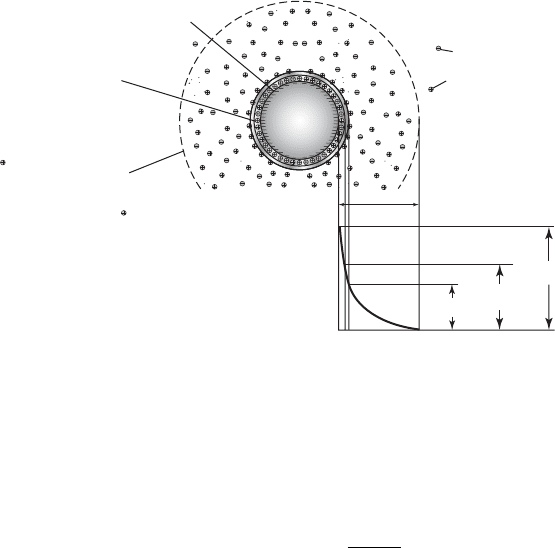
Electrical Double Layer. A colloidal dispersion in solution does not have a net charge. This is
because the negatively charged particle
s accumulate positive counterions on and near the particle
surface. Thus, as shown in Figure 6-2 , a double layer forms. The adsorbed layer of cations (known
as the Helmholtz layer or the Stern layer) is bound to the particle surface by electrostatic and adsorp-
tion forces. It is about 0.5
nanometers (nm) thick. A loose diffuse layer forms beyond the Helmholtz
layer. The double layer (Helmholtz plus diffuse) has a net negative charge over the bulk solution.
Depending on the solution characteristics, it can extend up to 30 nm into the solution (Kruyt, 1952).
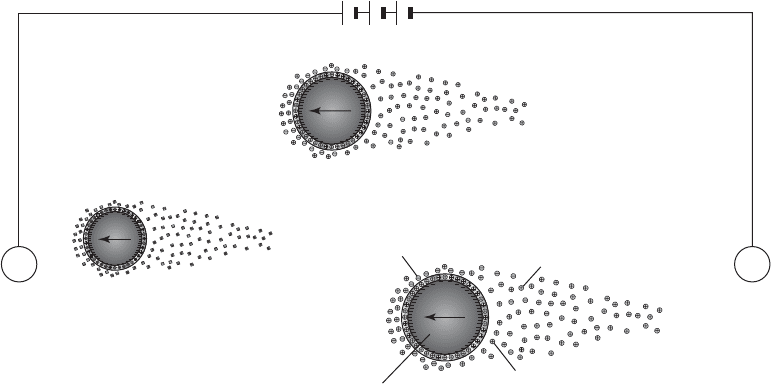
Zeta Potential. When a charged particle is plac
ed in an electric field, it will migrate to the pole
of opposite charge. This movement is called electrophoresis. A s the particle moves, a portion of
the water near the surface moves with it. This movement displaces the ion cloud and gives it the
*Although the conditioning of colloidal and suspended matter is the primary function of the coagulation process, the precipita-
tion of dissolved NOM is a concurrent objective.
6-4 WATER AND WASTEWATER ENGINEERING
shape shown in Figure 6-3 . The electric potential between the shear plane and the bulk solution is
called the zeta potential. It is noted in Figure 6-2 . The zeta potential is calculated as
Z
vk
z
0
0
(6-1)
where Z zeta potential, mV
v
0
electrophoretic mobility, ( m/s)/(V/cm) v
E
/ E
v
E
electrophoretic velocity of migrating particle, m/s
E electric field at particle, V/cm
k
z
shape constant of 4 or 6
dynamic viscosity of water, Pa · s
permitivity relative to vacuum
78.54 for water
0
permitivity in vacuum
8.854188 10
12
N / V
2
The values for electrophoretic mobility for particles in natural water vary from about 2 to
2 ( m/s)/(V/cm). The constant k
z
i s 4 if the extent of the diffuse layer is small relative to
the curvature of the particle. It is 6 where the particle is much smaller than the thickness of the
double layer (MWH, 2005).
E mpirically, when the absolute value of the zeta potential is reduced below about 20 mV,
rapid flocculation occurs (Kruyt, 1952).
Particle Stability. Particles in nat
ural waters remain stable when there is a balance between the
electrostatic force of the charged particles and attractive forces known as van der Waals forces.
Because the particles have a net negative charge, the principal mechanism controlling stability is
electrostatic repulsion.
Negatively charged
particle surface
Diffuse
ion layer
Negative ion
Fixed charge
(Stern) layer
Ions in equilibrium
with bulk solution
Double layer
Zeta
potential
Distance from particle surface, nm
Electrostatic potential
Nernst
potential
Helmholtz
potential
Positive counterion
0
0.5
30
FIGURE 6-2
Surface charge on a particle in water.
COAGULATION AND FLOCCULATION 6-5
Van der Waals forces arise from magnetic and electronic resonance when two particles
approach one another. Because the double layer extends further into solution than the van der
Waals forces, an energy barrier is formed that prevents particles from aggregating.
The theory of particle to particle interaction is based on the interaction of the attractive and
repulsive forces as two particles approach each other. The theory is known as the DLVO theory after
the individuals who developed it (Derjaguin and Landau, 1941; Verway and Overbeek, 1948).
The DLVO model concept is illustrated in Figure 6-4 . The left and right ordinate represent the
respective surfaces of two particles. The diagrams show the force
s acting on the particles as they move
toward each other. Two cases are shown. The van der Waals attractive force is the same in both cases. In
case (a), the repulsive force from the electrostatic force exceeds the attractive force, and the net energy
is repulsive. If the particles aggregate at all, it will be a loose aggregation at a distance of 4/ k,
where k i s
the double layer thickness. This aggregation can be ruptured easily because the net force holding them
together is weak. The particles will not aggregate strongly because of the energy barrier. In case (b), the
repulsive force is less and the resultant net energy is zero. The particles will aggregate strongly because
the resultant attrac
tive forces become stronger as the particles close on one another.
6-3 COAGULATION THEORY
Coagulants
Inorganic coagulants used for the treatment of potable water exhibit the following characteristics:
• They are nontoxic at the working dosage.
Negatively
charged
ion
Particle with high
negative surface
charge moves
towards positive
pole
Positively charged
counter ions
attracted to negative
pole
Diffuse ion cloud
travels with particle
CathodeAnode
FIGURE 6-3
Schematic illustration of electrophorsis. Charged particle movement in an applied electric field. Note that each particle
drags a cloud of ions with it.
