Water and Wastewater Engineering
Подождите немного. Документ загружается.

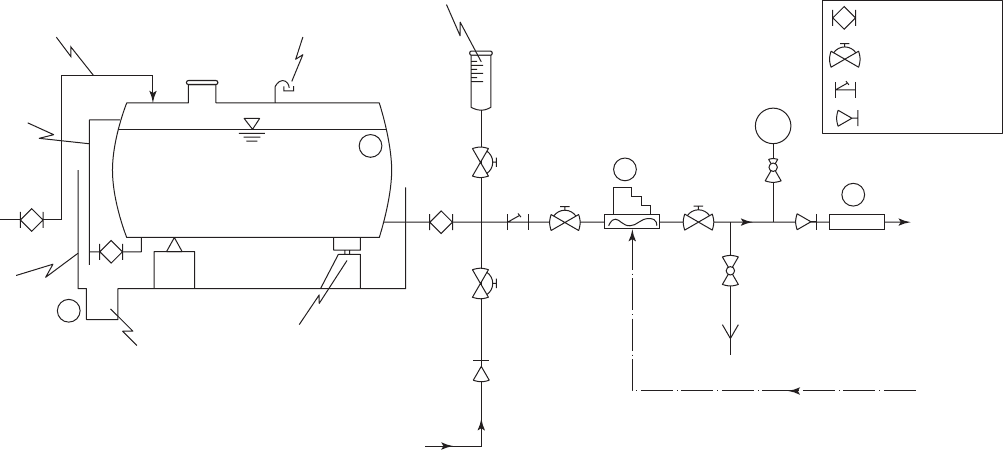
5-11
5 cm schedule 80 pvc fill line
7.5 cm over
flow line
Secondary
containment
enclosure
wall
Load cell
Liquid
chemical storage
Max level
10 cm
vent
Calibration
column
Pulsation
dampener
Sampling tap
Drain
To injection
point
Eccentric plug valve
Isolation valve
Strainer
Backflow preventer
Feedback
signal
transmitter
Utility
water
Sump
3
2
1
4
FIGURE 5-4
L i q uid chemical feed system.
Notes:
1. Tanks, horizontal or vertical.
2. Flow pacing—variable speed motor. Provide two in parallel.
3. Volume of enclosure around storage tank shall be 100% of liquid volume of tank 10% freeboard.
4. Magnetic flowmeter for monitoring (optional).

5-12 WATER AND WASTEWATER ENGINEERING
Type of feeder Application
Capacity
m
3
/h
Turn-down
ratio Remarks
Proportioning pump
Peristaltic Most solutions 10
6
to 10
3
10:1 Flow rate very sensitive to changes in head
Positive displacement
Piston at low feed Most solutions, light
slurries
3 10
4
to 5
10:1
a
Higher turn-down ratio leads to inaccuracy
Diaphragm at low feed Most solutions 1 10
4
to
4 10
3
10:1 Higher turn-down ratio leads to inaccuracy
Rotating dipper Most solutions or slurries3 10
3
to 0.8 100:1
Nonpositive displacement
Rotameter Clear solutions 1 10
4
to
5 10
3
10:1 Calibrated valve
Loss-in-weight Most solutions 6 10
5
to
6 10
3
30:1 Tank with control valve
a
Although manufacturers sometimes claim the capability of high turn-down ratios (i.e., 100:1) by using a combination of stroke length and speed, pumps
should be sized so that the turn-down ratio does not exceed 10:1 to ensure accuracy at low feed rates (Anderson, 2005).
TABLE 5-3
Liquid feeder characteristics
Peristaltic Pumps. These pu mps use a rotating cam to create successive waves of c ontrac-
tion on a flexible tube to move the fluid. They are particularly well suited to small flow rates of
chemical on the order of a few milliliters per minute up to about 1 L per minute.
Gas Feed System
A conventional gas feed system for chlorine, called a chlorinator, i s shown in Figure 5-5 . It
consists of an inlet pressure reducing valve, a rotameter, a metering control orifice, a vacuum
differential regulating valve, and a venturi injector. The vacuum created by the chlorine injector
moves the gas from the storage cylinder
s to the injection system. Evaporators may be used on
very large systems.
The chlorine passes through the rotameter that measures the gas flow rate, then through a
metering or control orifice. A vacuum differential regulator is mounted across the control orifice
to stabilize the flow for a particular setting of the orifice. Current design practic
e is to locate the
vacuum regulators as close as possible to the storage containers to minimize the amount of pres-
surized gas piping in the plant.
T ypically, the control orifice has a range of 20 to 1, and the vacuum differential regulator
has a range of about 10 to 1. The overall range is thus about 200 to 1. Because rotameter ranges
are generally limited to about 20 to 1, their s
election controls the actual operating range without
changing rotameters (Anderson, 2005).
To maintain an inventory of the chemical remaining in a cylinder, it is placed on a scale be-
fore being put into service. The weight is noted periodically.
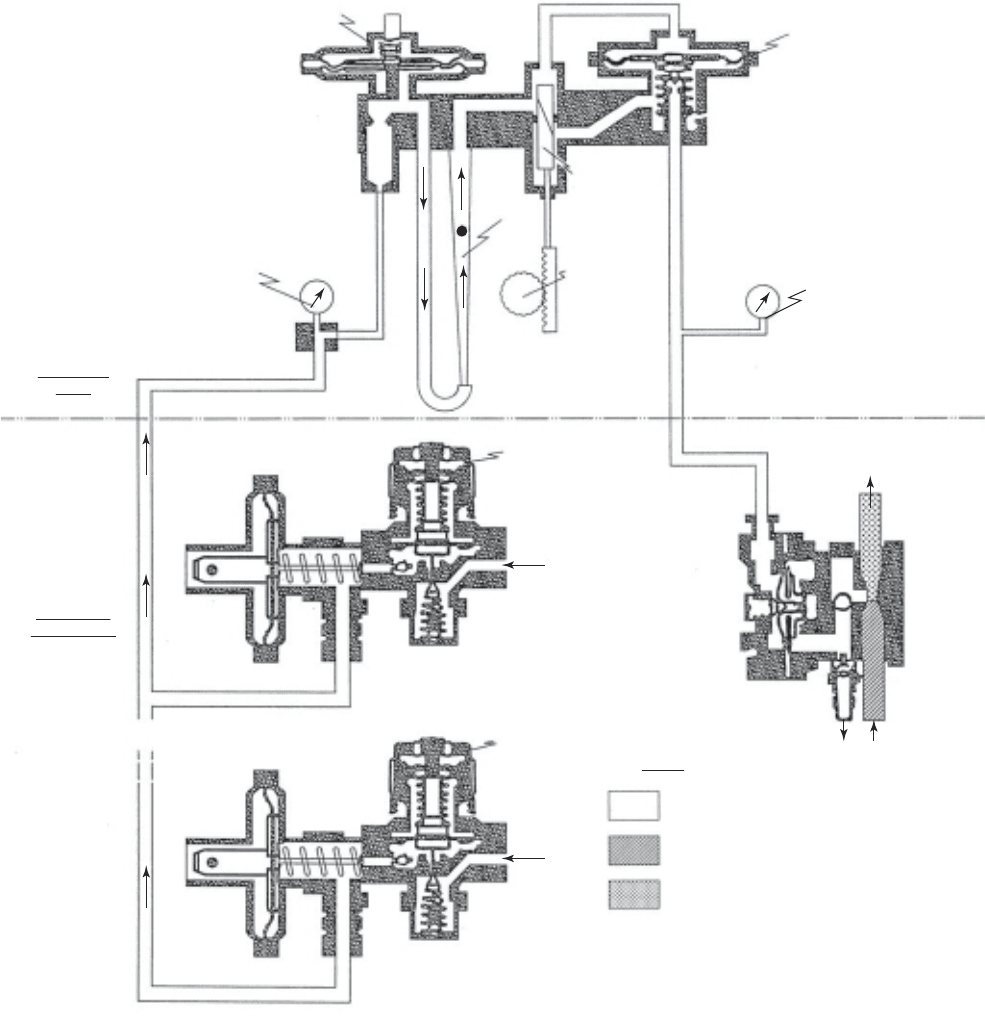
CHEMICAL HANDLING AND STORAGE 5-13
Pressure relief valve
(relieves at 25 to 50 cm water
pressure)
Legend
Gas
Water
Solution
Vacuum gauge
(30 to 100 cm water vacuum)
Chlorine gas
feeder
Standard vacuum
regulator
check unit
Solution
discharge
Gas
supply
Remote from
control module
Automatic
switchoever
vacuum
regulator
check unit
(used in pairs)
Gas
supply
Injector
Drain
relief
Injector
treated water
supply
Differential regulating
valve
Rotameter
V-notch
variable
or
ifice
Injector vaccum
gauge
Manual
feed rate
adjuster
FIGURE 5-5
Flow diagram for conventional chlorinator.

5-14 WATER AND WASTEWATER ENGINEERING
5-5 CHEMICAL COMPATIBILITY
It is not intended here to present an exhaustive list of incompatible chemicals but rather to
highlight combinations that may lead to violent reactions . Many others used in water and waste-
water require care. In particular, the design should provide sufficient piping and storage vessels
that the potential for residues from
prior-use chemicals reacting with new-use chemicals is
minimized.
Because there is a tendency for water of crystallization from alum to slake lime, it is impera-
tive that mixture of these two chemicals be avoided. In a closed container, this combination ma
y
lead to a violent explosion. For the s ame reason, ferric sulfate and lime should not be mixed
(Anderson, 2005). Mixtures of potassium permanganate and ferric chloride will form toxic chlo-
rine gas (Kawamura, 2000).
An abbreviated list of incompatible chemicals is pre
sented in Table 5-4 . A rigorou s search
for chemical combinations not shown in Table 5-4 is recommended as part of the design
process.
5-6 MATERIALS COMPATIBILITY
For very corrosive chem icals s uch as ferric salts, sod ium hypochlorite, and sodium hydrox-
ide, nonmetallic materials are preferred. These include fiberglass-reinforced plastic (FRP)
and various forms of polyethylene (PE) such as high-
density, cross-linked polyethylene
(HDXLPE).
Polyvinyl chloride (PVC), polypropylene, rubber-lined steel and type 316 stainless steel are
used for alum. In warm climates where the temperature of liquid alum may exceed 50 C, chlori-
nated polyvinyl chloride (CPVC) is
recommended.
Lime and soda ash can be stored in concrete or mild steel silos.
S uitable materials for storage containers for other chemicals are listed in Appendix A. Rec-
ommended materials for piping are given in Table 5-5 .
ChemicalsKeep out of contact with: Remarks
Activated carbon Oxidizing agents such as chlorine,
hypochlorites, potassium permanganate,
sufuric acid
Potential for fire
Alum Lime, milk of lime—Ca(OH)
2
Violent exothermic reaction
Ammonia Concentrated chlorine and
chlorine compounds
Violent exothermic reaction
Ferric chloride Potassium permanganate Formation of chlorine gas
Fluorine compounds All chemicals Etches glass
Sulfuric acid Strong bases
, light metal compounds
containing potassium and sodium
Violent exothermic reaction
TABLE 5-4
A short list of incompatible chemicals used in water and wastewater treatment

5-15
Piping material
Chemical
Iron
or
steel
Type
316
stainless
Type
304
stainless Copper PVC—type 1
Fiberglass-reinforced
polyester
(FRP) Polypropylene
Rubber
tubing Glass
Activated carbon (slurry)X X X
Alum NR S NR S X X X
Ammonia, aquaSX
Calcium hydroxide (slurry)SXX X XX
Calci
um hypochlorite X X X
Carbon dioxide (dry)SXXXXXXX
Chlorinated copperas XX
Chlorine (dry gas)S XNR NR
Chlorine solution NR NR NR S X X
Chlorine dioxide (3% soln.) X X
Coagulant aids Consult manufacturer—generally not corrosive
Copper sulfate X S X X X
Dolomitic lime (slurry)XXX X XX
Ferric chlorideNRNRNRNRSXXXX
Fluos
ilicic acid NR NR NR X X NR
Hydrochloric acid NR NR NR NR X X X X
Potassium permanganate (2% soln.) X X X X X
Sodium carbonate (soln.) S X X X X
Sodium chlorideXXXXX
Sodium chlorite X X X X
Sodium fluoride (1% to 5% soln.) X X X X X
Sodium hexametaphosphate (soln.) X X X X
Sodium hydroxide (to 50% s
oln.) X X X X X X X
Sodium hypochlorite (to 16% soln.) S X X X
Sodium silicate S X X X X X X
Sodium silicofluorideXX X
Sulfur dioxide (dry gas)XXX X
Sulfur dioxide (soln.) X
Sulfuric acid (conc.) S
Sulfuric acid NR S X X X X
TABLE 5-5
Recommended materials for piping
Key: S Industrial standard or excellent for handling
X Suitable for handling
NR Not recommended
Source: Anderson, 2005.
5-16 WATER AND WASTEWATER ENGINEERING
The following example illustrates the complete design of the storage and handling system for one
chemical.
Example 5-4. Design the coagulant chemical handling and storage system for Boiling Water,
Arizona using the following design data:
Average daily design flow rate 38,000 m
3
/ d
Coagulant ferric chloride
M a ximum dosage 50 mg/L as FeCl
3
Shipping time 1 week
S ummer temperature frequently exceeds 40 C
Solution:
a . Off-loading piping
From Table 5-5, select a 100 mm diameter schedule 80 PVC pipe with a notation to
check manufacturer’s data for temperature limitations.
b. Storage tank
(1) From Appendix A select FRP for tank material. The tank
should be located indoors
in a cool location.
(2) From Appendix A, note that ferric chloride is shipped as a 40% solution with
100% active ingredient. At the maximum dosage, the d aily mass of ferric chloride
us ed is
()( )()(50 38 000 10 10
3336
mg/L m /d L/m kg/mg,
))1 900, kg/d
and the mass of solution required is
1 900
040
4750
,
.
,
kg/d
kg/d
(3) Noting, from Table 5-1 , that coagulants are noninterru ptible, the volum e to be held
in two tanks for redundancy is 30-days supply plus two times the shipping time.
( )( ( )( ))4750 30 2 7 209 0000,,kg/dd d kg
(4) U sing the density of ferric chlorid e from Appendix A, the volume of solution to be
stored is
209 000
1 440
145 14 150
3
3
,
,
.
kg
kg/m
or m
c. Feeder
Two feeder pumps are required to meet redundancy requirements.
From Table 5-3 , a piston metering pump with PVC or PE coated piston is s elected.
Checking the capacity
CHEMICAL HANDLING AND STORAGE 5-17
()4750
1
24
1
1 440
3
,
,
kg/d
h/d
kg/m
⎛
⎝
⎜
⎞
⎠
⎟
⎛
⎝
⎜
⎞
⎠
⎟
014
3
. m /h
where 1,440 kg/m
3
i s the density of ferric chloride from Appendix A. This is in the op-
erating range of 3 10
4
to 5 m
3
/h.
d. Transfer piping
From Table 5-5 , select a 50 to 100 mm diameter schedule 80 PVC pipe.
e. The arrangement of the system is shown in Figure 5-4 .
5-7 DESIGNING FOR SAFETY AND HAZARDOUS CONDITIONS
Table 5-6 provides a general overview of safety requ irements and protective measures for han-
dling chemicals. Many of thes e measures are to be implemented by the operators, but s everal
require design provisions. Material Safety Data Sheets (MSDS) provided by the manufacturer of
the chemical provide
more detailed information on its s afe handling. Another general reference
for chemical safety, expos ure limits, and incompatibilities is NIOSH Pocket Guide to Chemical
Hazards (NIOSH, 2003).
The Emergency Planning and Community Right-to-Know Act (EPCRA), also known as
Title III of the Superfund Amendments and Reauthorization Act (SARA), requires facilities
with chemicals above the thresholds given in Table 5-7 to report this to the State Emergency
Response Commission (SERC) and coordinate with the appropriate Local Emergency Planning
Commission (LEPC). Con
struction of a new facility exceeding these amounts requires that the
owner notify the SERC and LEPC. Operating and maintenance manuals should address these
issues.
I n many communities, chlorine gas is the most hazardous substance in substantial q
uan-
tity in the community. Not only is it a hazard because of potential accidental release from
delivery through application to the water supply, but it also is a security hazard. Although it
is more expensive, sodium hypochlorite (NaOCl) is being used to replace gaseous chlorine to
reduce the hazar
d that gaseous chlorine poses. Many water treatment plants are using alterna-
tive disinfectants, su ch as ultraviolet (UV) radiation and ozone, to reduce the need for large
amounts of chlorine. Wastewater treatment plants have implemented the use of UV for the
same reason.
5-8 OPERATION AND MAINTENANCE
The major issues in operation and maintenance are safety programs and training, preventive
maintenance, good housekeeping, and good record keeping.
Because the concentrated chemicals used in water and wastewater treatment are for the most
part harmful to human health, formal safety programs are essential. This includes period
ic hands-
on training, provision of appropriate safety equipment in accessible locations, and provision of
personal protective equipment (PPE).

5-18
Protective equipment required
Chemical (D = dry;
L = liquid; G = gas)
Positive
ventilation
Protective
clothing
Neck cloths
Gloves
Rubber boots
Rubber gloves
Goggles
Face shields
Rubber aprons
Respirator
Gas mask
Avoid skin
contact
Safety shower
and eye baths
Remarks
Activated alumina (D)
■
Store away from gasoline, mineral or
vegetable oils, calcium hypochlorite (HTH),
lime, sodium chlorite, or potassium
permanganate
Activated carbon
Powder (D)
■■ ■■ ■■ ■
Granulate (D)
■■ ■
Alum sulfate (D)
■■ ■■ ■ ■ ■
Similar to other acids
Alum sulfate (L)
■■ ■
Ammonium hyd
roxide (L)
■ ■■
Moist NH
3
reacts with many metals and
alloys—liquid contact produces burns
Ammonium sulfate (D)
■■ ■■ ■ ■ ■ ■
See alum sulfate above
Anhydrous ammonia (G)
■■ ■ ■ ■■■ ■
Fire sprinklers and water hoses effective in
removing gas
Bauxite (D)
■■■ ■
Bentonite (D)
■
Calcium carbonate (D)
■
Calcium h
ypochlorite (D)
■■■ ■■
Carbon dioxide (G)
■■■
Chlorine (G)
■■■■■■
Avoid contact with hydrogen or organic
compounds or other flammable materials
Chlorine dioxide (G)
■ ■ ■■ ■■ ■■■ ■
Solution is corrosive
Copper sulfate (D)
■■ ■■ ■ ■ ■ ■
Very corrosive
Ferric chloride (D)
■ ■ ■ ■■■■■ ■ ■
Very corrosive
Ferric sulfate (D)
■ ■ ■ ■■■■■ ■ ■
Ferrous sulfate (D)
■■ ■■ ■ ■ ■
Ferrous sulfate (L)
■■■■■■
Fluorosilicic acid (L)
■ ■■■■■■■ ■ ■ ■
Have lime slurry on hand
Fluorspar (D)
■■ ■ ■ ■■■ ■ ■
Etches glass when moist
TABLE 5-6
Protective measures for water & wastewater treatment chemicals

5-19
Protective equipment required
Chemical (D = dry;
L = liquid; G = gas)
Positive
ventilation
Protective
clothing
Neck cloths
Gloves
Rubber boots
Rubber gloves
Goggles
Face shields
Rubber aprons
Respirator
Gas mask
Avoid skin
contact
Safety shower
and eye baths
Remarks
Hydrated lime (D)
■■ ■■ ■■ ■ ■ ■
Can burn eyes or skin
Hydrochloric acid (L)
■■ ■■ ■■ ■ ■
Iron-exchange resins (D)
■■■ ■ ■
Hydrogen cation resins are acidic
Ozone (G)
■ ■■
Potassium permanganate (D)
■■ ■■■ ■ ■ ■
Large quantities present fire hazard
Quicklime (D)
■■ ■■ ■■ ■ ■
Can burn eyes or skin
Sodium aluminate (D)
■■■ ■
Sodium aluminate (L)
■■■ ■
Sodium bisulfate (D)
■■■
Sodium carbonate (D)
■■ ■ ■ ■
Sodium chloride (D)
■■ ■ ■ ■ ■
Can dehydrate skin
Sodium chlorite (D)
■■ ■ ■ ■ ■
Rinse any spills immediately with water
Sodium fluoride (D)
■■ ■■ ■ ■ ■ ■
Sodium polyphosphate, glassy (D)
■■ ■■ ■ ■
Sodium hydroxide (D)
■ ■ ■ ■■■■■ ■
Sodium hydroxide (L)
■■■■■■■
Sodium hypochlorite (L)
■ ■ ■■■■ ■ ■ ■
Sodium silicate (D)
■
Sodium fluorosilicate (D)
■■ ■■ ■■ ■ ■
Sodium sulfite (D)
■■■ ■
Sodium dioxide (G)
■■■■■
Sulfuric acid (L)
■■ ■■■■■ ■ ■
Adapted from Anderson, 2005.
5-20 WATER AND WASTEWATER ENGINEERING
Preventive maintenance includes regularly scheduled times for equipment to be taken out
of service for replacement of worn parts, calibration, and so on. Frequently, this type of work
is scheduled in the winter to take advantage of low flows. In addition, feeders, feed lines, and
instrumentation are to be checked routinely during ea
ch shift.
Good housekeeping includes prompt cleaning of spills and removal of chemical dust.
Although regulatory agencies will dictate that certain records be kept, operation of the plant
often requires more information than is reported. For example, the status of the chemical inven-
tory and the operating performance of each piec
e of the chemical handling and feeding equip-
ment should be logged and conveyed to the next shift operator (Kawamura, 2000).
Hints from the Field. Operation and maintenance personnel who have to live with the results
of the engineer’s design have offered the following suggestions:
• S chedule 80 PVC and CPCV are the most commonly used materials for s
odium hypochlo-
rite piping. Early installation of these systems failed because of leaks at the solvent welded
joints. Special glues designed for use with NaOCl must be used to guard against this type of
failure.
• Q uicklime storage silos should always be c
ylindrical. Because of its hygroscopic nature,
lime will invariably cake in the silo. In one case, the working volume of a 200 Mg square
silo was effectively reduced to 35 Mg. Vibration and other attempts to loosen the caked
material were ineffective. The additional expense of a cylindrical silo will be repaid many
times by the redu
ced O&M costs of trying to loosen the caked lime.
• As shown in Figure 5-6 , place the slaker directly beneath the lime storage silo to minimize
dust in transporting the lime to the slaker.
• Grit in the lime can be removed after slaking by the simple expedient of placing a milk
crate lined with hardware cloth in the exit stream ( Figure 5-6 ).
• Transport the slaked lime to the mixing device with an eductor and flexible hose ( Figure 5-6 ).
Pumps will cake with lime, and rigid pipes will clog. The eductor elim
inates moving parts,
and the flexible pipe makes it easy to spot blockages and either break them in place or quickly
replace a section for out-of-service cleaning.
TABLE 5-7
EPCRA threshold planning quantities
Chemical
Threshold planning
quantity, kg
Chlorine 45
Chlorine dioxide Not listed
Anhydrous ammonia 225
Aqua ammonia Not listed
Hydrogen peroxide (52%) 450
Sulfuric acid 450
Ozone 45
