Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

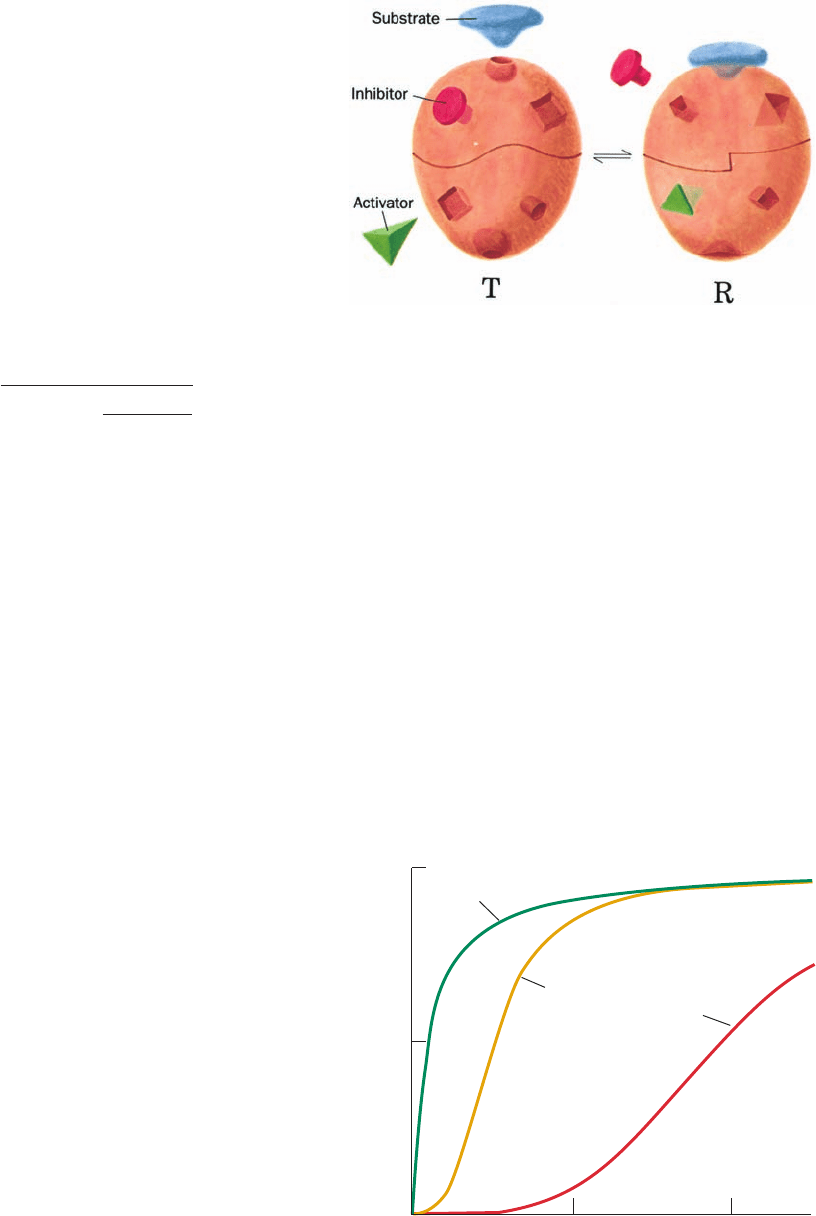
energy of ligand binding stabilizes the R state with respect to
the T state.
b. Heterotropic Interactions
The symmetry model of allosterism is also capable of
accounting for heterotropic effects. This comes about by
assuming that each protomer has specific and independent
binding sites for the three types of ligands: a substrate, S,
that for simplicity let us assume binds only to the R state
(c ⫽ 0); an activator, A, that also binds only to the R state;
and an inhibitor, I, that binds only to the T state (Fig. 10-30).
Then, through the derivation in Section B of the Appendix
to this chapter, we obtain a more general equation for the
symmetry model that describes heterotropic interactions
as well as homotropic interactions:
[10.23]
where ␣⫽[S]/k
R
as before and, analogously, ⫽[I]/k
I
and ␥⫽[A]/k
A
.
Note that this equation differs from Eq. [10.22] for c ⫽ 0
only in that the second term in the denominator is modu-
lated by terms related to the amounts of activator and in-
hibitor bound to the oligomer.
Figure 10-31 indicates the consequences of effector
binding to a tetramer that follows this model:
1. Activator binding (␥⬎0) increases the concentra-
tion of the substrate-binding R state (the second term in
the denominator of Eq. [10.23] decreases) because it is the
only state capable of binding activator. The presence of ac-
tivator therefore increases the protein’s substrate-binding
affinity (a positive heterotropic effect), although it de-
creases the protein’s degree of substrate-binding coopera-
tivity (compare Curves 1 and 2 in Fig. 10-31). (Note: There
is nothing in the derivation of Eq. [10.23] that differentiates
the roles of substrate and activator; consequently, the sub-
strate and the activator each bind to the protein with a
positive homotropic effect as well as being positive het-
erotropic effectors of each other.)
2. The presence of inhibitor ( ⬎ 0), which only binds to
the T state, reduces the binding affinity for substrate (a neg-
ative heterotropic effect) by increasing the concentration
of the T state (the second term in the denominator of Eq.
[10.23] increases). Therefore, since substrate must “work
harder” to convert the oligomer to the substrate-binding R
state, inhibitor increases the cooperativity of substrate
binding (compare Curves 2 and 3 of Fig. 10-31), as well as
that for activator binding.
The model derived here is a rather simple one.In a more
realistic but algebraically much more complicated symme-
try model, all types of ligands would bind to both confor-
mational states of the oligomer. Nevertheless, this model
demonstrates that both homotropic and heterotropic effects
can be explained solely by the requirement that the molecu-
lar symmetry of the oligomer be conserved rather than by
the existence of any direct interactions between ligands. In
Section 10-4D, we compare the theoretical predictions of
Y
S
⫽
␣(1 ⫹␣)
n⫺1
(1 ⫹␣)
n
⫹
L(1 ⫹)
n
(1 ⫹␥)
n
the symmetry model with our experimentally based model
of hemoglobin oxygen binding.
C. The Sequential Model
The symmetry model provides a reasonable rationalization
for the ligand-binding properties of many proteins. There
are, however, several valid objections to it. Foremost of
these is that it is difficult to believe that oligomeric symmetry
is invariably preserved in all proteins so that there are never
any hybrid conformations such as R
n⫺2
T
2
. Furthermore,
Section 10-4. Allosteric Regulation 351
Figure 10-30 Heterotropic interactions in the symmetry model
of allosterism. Heterotropic effects arise when substrates and
activators bind exclusively (or at least preferentially) to the R
state (right), and inhibitors bind exclusively (or at least
preferentially) to the T state (left).The binding of substrate
and/or activator to the oligomer therefore facilitates the further
binding of substrate and activator. Conversely, the binding of
inhibitor prevents (or at least inhibits) the oligomer from binding
substrate or activator.
1.0
0.5
0
0 1.0 2.0
Y
S
α
γ
β
= 100
= 0
γ
β
= 0
= 0
γ
β
= 0
= 2.5
= 1000
= 4
L
n
1
2
3
Figure 10-31 The effects of allosteric activator (␥ ⫽ [A]/k
A
)
and inhibitor ( ⫽ [I]/k
I
) on the shape of the fractional
saturation curve for substrate (␣ ⫽ [S]/k
R
) according to Eq.
[10.23] for tetramers. [After Monod, J., Wyman, J., and
Changeux, J.-P., J. Mol. Biol. 12, 94 (1965).]
JWCL281_c10_323-358.qxd 2/24/10 1:58 PM Page 351
there are well-established instances of negative homotropic
effects (e.g., in the GroEL–GroES complex, the binding of
ATP to the cis ring of GroEL prevents ATP from binding
to the trans ring; Section 9-2Cd), although the symmetry
model, which permits only positive homotropic effects, is
unable to account for them.
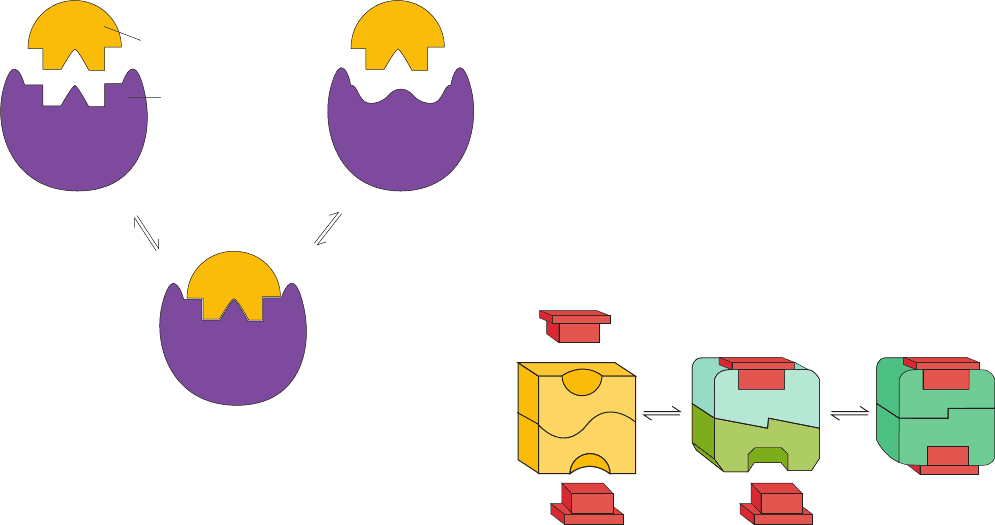
The symmetry model implicitly assumes Emil Fischer’s
“lock-and-key” model of ligand binding in which ligand-
binding sites of proteins are rigid and complementary in
shape to their ligand (Fig. 10-32, left).A more sophisticated
extension of the lock-and-key model, known as the
induced-fit hypothesis, postulates that a flexible interaction
between ligand and protein induces a conformational
change in the protein, which results in its increased ligand-
binding affinity (Fig. 10-32, right).The observation, through
X-ray crystal structure analysis, that such conformational
changes occur in numerous proteins has established the va-
lidity of the induced-fit hypothesis.
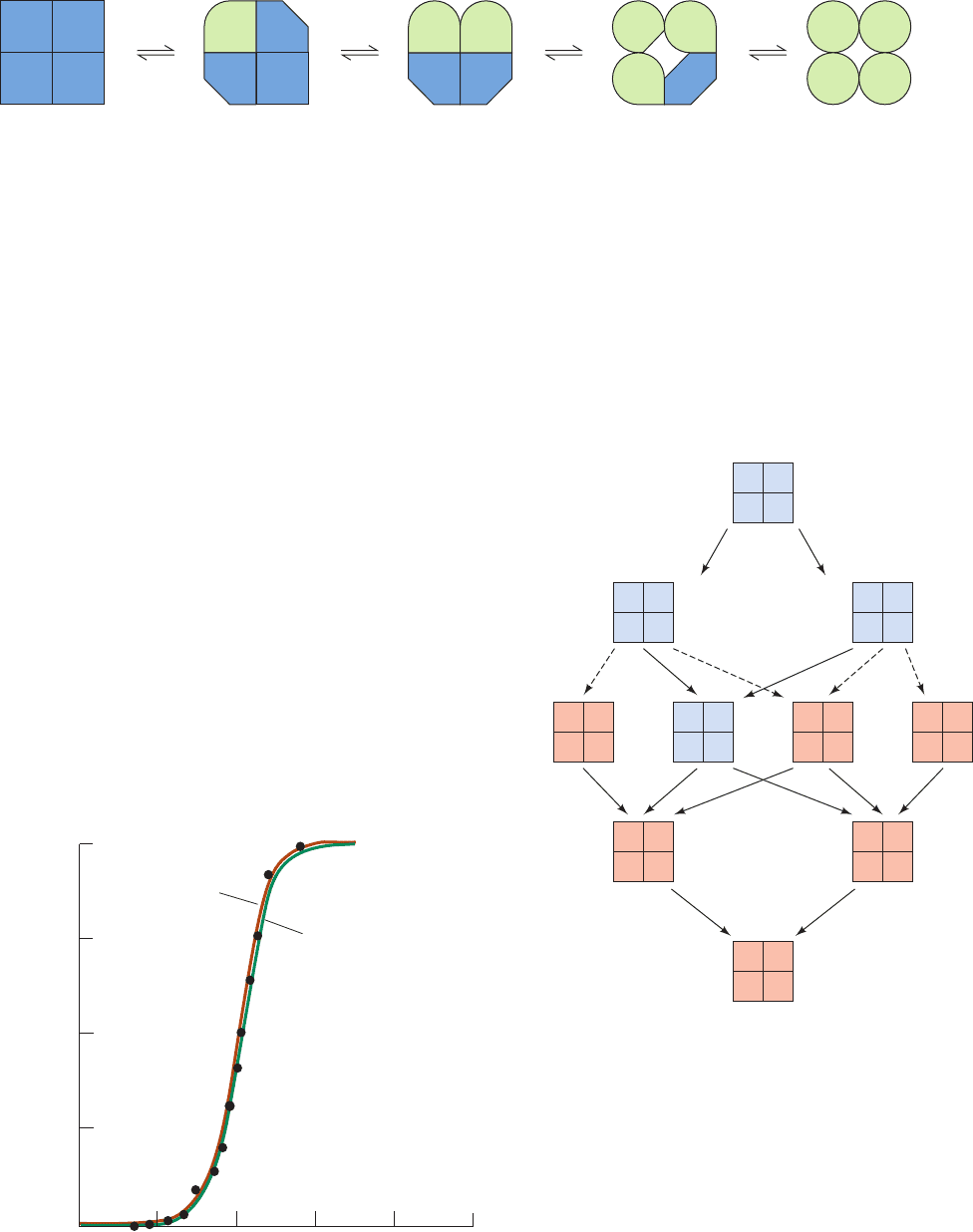
Daniel Koshland, George Némethy, and David Filmer
adapted the induced-fit hypothesis to explain allosteric ef-
fects. In the resulting sequential model (alternatively, the
induced-fit or KNF model), ligand binding induces a confor-
mational change in a subunit; cooperative interactions arise
through the influence that these conformational changes
have on neighboring subunits (Fig. 10-33). If, for example,
they increase the neighbor’s ligand-binding affinity, then
ligand binding is positively cooperative. The strengths of these
interactions depend on the degree of mechanical coupling
between subunits. In the limit of very strong coupling, con-
formational changes become concerted, so that the
oligomer maintains its symmetry (the symmetry model).
With looser coupling, however, conformational changes
occur sequentially as more and more ligand is bound
(Fig. 10-34). Thus, the essence of the sequential model is that
a protein’s ligand-binding affinity varies with its number of
bound ligands, whereas in the symmetry model this affinity
depends only on the protein’s quaternary state.
The degree of coupling between oligomer subunits de-
pends on how these subunits are arranged, that is, on the
protein’s symmetry. Consequently, in the sequential model,
the fractional saturation has a different algebraic form for
each oligomeric symmetry.The form of the Adair equation
(Eq. [10.17] for a tetramer) similarly depends on the num-
ber of subunits in the protein. In fact, the sequential model
of allosterism may be considered an extension of the Adair
model that provides a physical rationalization for the val-
ues of its microscopic dissociation constants, k
i
.
D. Hemoglobin Cooperativity
Hemoglobin’s fractional saturation curve is closely approx-
imated by both the symmetry model and the sequential
model (Fig. 10-35). Clearly such curves cannot by them-
selves be used to differentiate between these two models,
if, in fact, either is correct. It is of interest, however, to com-
pare these models with the mechanistic model of Hb we
developed in Section 10-2C.
Hb, of course,is not composed of identical subunits, as the
symmetry model demands. At least to a first approximation,
however, the functional differences of Hb’s closely related ␣
and  subunits may be ignored (although their structural dif-
ferences are essential to the molecular mechanism of Hb co-
operativity). To this approximation, Hb largely follows the
symmetry model, although it also exhibits some features of
the sequential model. The quaternary T S R conformation
change is concerted as the symmetry model requires.Yet lig-
and binding to the T state does cause small tertiary struc-
tural changes as the sequential model predicts.This phenom-
enon is evident in the X-ray structure of crystals of human
Hb whose ␣ subunits are fully oxygenated and whose  sub-
units are unliganded. This particular crystal form constrains
352 Chapter 10. Hemoglobin: Protein Function in Microcosm
Figure 10-32 Models of ligand binding. In the lock-and-key
mechanism of ligand binding (left), proteins are postulated to
have preformed ligand-binding sites that are complementary in
shape to their ligand. Under the induced-fit mechanism, a protein
does not have this complementary binding site in the absence of
ligand (right). Rather, the ligand induces a conformational
change at the binding site that results in the complementary
interaction.
Figure 10-33 The sequential model of allosterism. Substrate
binding to the low-affinity T state induces conformational
changes in unliganded subunits that give them ligand-binding
affinities between those of the low-affinity T state and the
high-affinity R state.
Lock and key Induced fit
Ligand
Protein
Protein–Ligand
complex
++
Substrate
T
T
R'
T'
R
R
JWCL281_c10_323-358.qxd 2/24/10 1:58 PM Page 352
the partially liganded Hb to remain in the T state. Neverthe-
less, its ␣ subunit Fe’s are 0.15 Å closer to the still domed
porphyrins than they are in deoxyHb (25% of the total dis-
tance moved in the T S R transition). Such tertiary struc-
tural changes are undoubtedly responsible for the buildup of
strain that eventually triggers the T S R transition.
A more telling but more difficult question to answer is
does ligand-binding cooperativity in Hb arise solely from
the T S R transition, in agreement with the symmetry
model, or do the T and R states themselves exhibit at least
some degree of cooperativity, in accord with the sequential
model? Put another way, does the ligand-binding affinity of
Hb subunits depend only on Hb’s quaternary state (symme-
try model) or does this affinity vary with the number of lig-
ands bound to Hb (sequential model)? Although this matter
has been under experimental scrutiny and a subject of spir-
ited debate for over four decades, it is still not fully resolved.
For example, the determination, by Andrea Mozzarelli and
Eaton, of the Hill plot of the above-described single crystals
of T-state Hb via sophisticated optical spectroscopic tech-
niques is indicative of noncooperative ligand binding, in ac-
cord with the symmetry model. Likewise, time-resolved
spectroscopic measurements covering the picosecond to
microsecond time scales after ligand binding was initiated
indicate that the conformational changes that Hb initially
undergoes on binding ligand are consistent with the symme-
try model. However, detailed thermodynamic analysis, by
Gary Ackers, of the interactions associated with the formation
of each of Hb’s 10 different ligation microstates (Fig. 10-36)
indicates that the Hb tetramer undergoes a T S R transition
Section 10-4. Allosteric Regulation 353
Figure 10-34 Sequential binding of ligand in the sequential
model of allosterism. Ligand binding progressively induces
conformational changes in the subunits, with the greatest changes
occurring in those subunits that have bound ligand.The coupling
Figure 10-35 The sequential and the symmetry models of
allosterism can provide equally good fits to the measured
O
2
-dissociation curve of Hb. [After Koshland, D.E., Jr., Némethy,
G., and Filmer, D., Biochemistry 5, 382 (1966).]
Figure 10-36 Free energy penalties for binding O
2
to various
ligation states of Hb tetramers relative to O
2
-binding to
noncooperative Hb ␣ dimers. Only the ten unique liganding
states are diagrammed (an additional six states are related to
those shown by Hb’s 2-fold symmetry). Penalties (in kJ ⴢ mol
⫺1
)
for individual binding steps are shown beside the arrows.
Cumulative free energy penalties are shown to the left of each
Hb tetramer state. Ligation states that predominantly assume the
T state are blue and those that predominantly assume the R state
are red.The preferred pathways, those in which the free energy
penalty of ligand binding progressively decreases with each
successively bound ligand (those with all solid arrows), pass
through the T state in which O
2
is bound to both sites on one ␣
dimer before its conversion to the R state. Note that the T S R
transition predominantly occurs via pathways in which at least
one subunit in each ␣ protomer is liganded. [Based on data
from Ackers, G.K., Adv. Prot. Chem. 51, 193 (1998).]
S S S S
SS
SS
S SS SS
S
between subunits is not necessarily of sufficient strength to
maintain the symmetry of the oligomer as it is in the symmetry
model.
1.00
0.75
0.50
0.25
0
–1 0 1 2 3 4
Symmetry
model
Sequential
(induced-fit)
model
log pO
2
Y
O
2
α
1
11.7 11.7
0
–2.5–2.5
β
2
β
1
α
2
O
2
26.4
O
2
O
2
O
2
O
2
11.7 11.7
O
2
O
2
28.9
O
2
O
2
O
2
28.9
O
2
O
2
27.6
O
2
O
2
O
2
28.0
O
2
O
2
20.9
9.2
16.7 15.9
16.3
9.216.3
8.0
0.4 1.3
0.9
0.9 8.0
O
2
O
2
28.5
O
2
JWCL281_c10_323-358.qxd 2/24/10 1:58 PM Page 353

A. Homotropic Interactions—Equation [10.22]
The fractional saturation Y
S
for ligand binding is
expressed:
Y
S
⫽
[10.21]
Defining ␣⫽[S]/k
R
and c ⫽ k
R
/k
T
, and using Eq. [10.20] to
substitute [R
n⫺1
] for [R
n
], [R
n⫺2
] for [R
n⫺1
] etc., the terms
enclosed by the first set of parentheses in the numerator of
Eq. [10.21] are reduced to
and similarly, the terms in the first set of parentheses of the
denominator of Eq. [10.21] become
Likewise, the terms in the second set of parentheses of the
numerator and the denominator of Eq. [10.21] assume the
respective forms
and
Accordingly,
which, on cancellation of terms, yields the equation
describing the symmetry model for homotropic interactions:
[10.22]Y
S
⫽
␣(1 ⫹␣)
n⫺1
⫹ Lc␣(1 ⫹ c␣)
n⫺1
(1 ⫹␣)
n
⫹ L(1 ⫹ c␣)
n
Y
S
⫽
[R
0
]␣n(1 ⫹␣)
n⫺1
⫹ L[R
0
]c␣n(1 ⫹ c␣)
n⫺1
n5[R
0
](1 ⫹␣)
n
⫹ L[R
0
](1 ⫹ c␣)
n
6
[T
0
](1 ⫹ [S]>k
T
)
n
⫽ L[R
0
](1 ⫹ c␣)
n
[T
0
]([S]>k
T
)n(1 ⫹ [S]>k
T
)
n⫺1
⫽ L[R
0
]c␣n(1 ⫹ c␣)
n⫺1
[R
0
] e1 ⫹ n␣⫹
p
⫹
n!␣
n
n!
f⫽ [R
0
](1 ⫹␣)
n
⫽ [R
0
]␣n(1 ⫹␣)
n⫺1
⫽ [R
0
]␣n e1 ⫹
2(n ⫺ 1)␣
2
⫹
p
⫹
n(n ⫺ 1)!␣
n⫺1
n(n ⫺ 1)!
f
[R
0
] en␣⫹
2n(n ⫺ 1)␣
2
2
⫹
p
⫹
n n!␣
n
n!
f
([R
1
] ⫹ 2[R
2
] ⫹
p
⫹ n[R
n
]) ⫹ ([T
1
] ⫹ 2[T
2
] ⫹
p
⫹ n[T
n
])
n5([R
0
] ⫹ [R
1
] ⫹
p
⫹ [R
n
]) ⫹ ([T
0
] ⫹ [T
1
] ⫹
p
⫹ [T
n
])6
B. Heterotropic Interactions—Equation [10.23]
For an oligomer that binds activator A and substrate S to
only its R state, and inhibitor I to only its T state, the frac-
tional saturation for substrate, Y
S
, the fraction of substrate-
binding sites occupied by substrate, is expressed:
Here the subscripts i, j, and k indicate the respective
numbers of S, A, and I molecules that are bound to one
oligomer; that is, R
i, j
⬅ RS
i
A
j
and T
k
⬅ TI
k
.Then defining
␣⫽[S]/k
R
and following the foregoing derivation of
Eq. [10.22]:
where
In analogy with the definition of ␣, we define ⫽[I]/k
I
and
␥⫽[A]/k
A
, and again follow the derivation of Eq. [10.22]
to obtain
and
so that
The symmetry model equation extended to include het-
erotropic effects is therefore expressed
[10.23]Y
S
⫽
␣(1 ⫹␣)
n⫺1
(1 ⫹␣)
n
⫹
L(1 ⫹)
n
(1 ⫹␥)
n
L¿ ⫽
L(1 ⫹)
n
(1 ⫹␥)
n
a
n
j⫽0
[R
0, j
] ⫽ [R
0, 0
](1 ⫹␥)
n
a
n
k⫽0
[T
k
] ⫽ [T
0
](1 ⫹)
n
L¿ ⫽
a
n
k⫽0
[T
k
]>
a
n
j⫽0
[R
0, j
]
Y
S
⫽
a
a
n
j⫽0
[R
0, j
]b ␣n(1 ⫹␣)
n⫺1
n ea
a
n
j⫽0
[R
0, j
]b(1 ⫹␣)
n
⫹
a
n
k⫽0
[T
k
]f
⫽
␣(1 ⫹␣)
n⫺1
(1 ⫹␣)
n
⫹ L¿
Y
S
⫽
a
n
i⫽1
a
n
j⫽0
i[R
i, j
]
n a
a
n
i⫽0
a
n
j⫽0
[R
i, j
] ⫹
a
n
k⫽0
[T
k
] b
APPENDIX Derivations of Symmetry Model Equations
only when at least one liganding site on each of its ␣ pro-
tomers is occupied.This heretofore unrecognized symmetry is
inconsistent with the symmetry model. Moreover, as Takashi
Yonetani has shown, heterotropic effectors such as BPG can
decrease Hb’s oxygen affinity without changing its quaternary
state. Evidently,cooperativity arises both from concerted qua-
ternary switching (as called for by the symmetry model) and
from sequential modulation of ligand binding within each
quaternary state through ligand-induced alterations in terti-
ary structure (in accord with the sequential model).
354 Chapter 10. Hemoglobin: Protein Function in Microcosm
JWCL281_c10_323-358.qxd 2/24/10 1:58 PM Page 354

References 355
1 Hemoglobin Function The heme group in myoglobin
and in each subunit of hemoglobin reversibly binds O
2
. In de-
oxyHb, the Fe(II) is five-coordinated to the four pyrrole nitro-
gen atoms of the protoporphyrin IX and to the protein’s prox-
imal His. On oxygenation, O
2
becomes the sixth ligand of
Fe(II). Mb has a hyperbolic fractional saturation curve (Hill
constant, n ⫽ 1). However, that of Hb is sigmoidal (n ⬇ 2.8) as
a consequence of its cooperative O
2
binding: Hb binds its
fourth O
2
with 100-fold greater affinity than its first O
2
.The
variation of O
2
affinity with pH, the Bohr effect, causes Hb to
release O
2
in the tissues in response to the binding of protons
liberated by the hydration of CO
2
to . Hb facilitates the
transport of CO
2
, both directly, by binding CO
2
as N-terminal
carbamate, and indirectly, by increasing the concentration of
through the Bohr effect. The presence of BPG in ery-
throcytes, which only binds to deoxyHb, further modulates the
O
2
affinity of Hb. Short-term high-altitude adaptation results
from an increase of BPG concentration in the erythrocytes,
which increases the amount of O
2
delivered to the tissues by
decreasing hemoglobin’s O
2
affinity.
2 Structure and Mechanism The ␣ and  subunits of Hb
consist mostly of seven or eight consecutive helices arranged to
form a hydrophobic pocket that almost completely envelops
the heme. Oxygen binding moves the Fe(II) from a position
⬃0.6 Å out of the heme plane on the side of the proximal His
to the center of the heme, thereby relieving the steric interfer-
ence that would otherwise occur between the bound O
2
and
the porphyrin.The Fe(II) pulls the attached proximal His after
it in a motion that can only occur if its imidazole ring reorients
so as to avoid collision with the heme. In the T S R conforma-
tional transition, the symmetry equivalent ␣
1
C–
2
FG and
␣
2
C–
1
FG contacts simultaneously shift between two stable
positions. Intermediate positions are sterically prevented, so
that these contacts act as a two-position conformational switch.
The Perutz mechanism of O
2
binding proposes that the low
O
2
affinity of the T state arises from strain that prevents the
Fe(II) from moving into the heme plane to form a strong
Fe¬O
2
bond. This strain is relieved by the concerted 4º shift
of the Hb molecule to the high O
2
affinity R state. The quater-
nary shift is opposed by a network of salt bridges in the T state
that involve the C-terminal carboxyl groups and that are rup-
tured in the R state.The stability of the R state relative to the T
state increases with the degree of oxygenation as a result of the
strain of binding O
2
in the T state. The existence of this strain
has been demonstrated through the breakage of the
Fe(II)–proximal His bond on hemoglobin’s simultaneous bind-
ing of IHP, a tight-binding BPG analog that forces Hb into the
T state, and NO, a strong ligand that forces Hb into the R state.
Conversely, mutagenically detaching the proximal His from
the protein eliminates most of hemoglobin’s cooperativity.
HCO
⫺
3
HCO
⫺
3
The Bohr effect results from increases in the pK’s of the ␣
N-terminal amino group and His 146 on forming the T-state
salt bridges.Surface-exposed His residues also participate in the
Bohr effect. BPG binding occurs in the central cavity of T-state
Hb through several salt bridges.The distal His residue protects
deoxyHb from autooxidation by taking up the protons that
would otherwise catalyze the oxidation of the heme Fe.
3 Abnormal Hemoglobins Over 1000 mutant varieties of
Hb are known.About half of them are innocuous because they
result in surface residue changes. However, alterations of inter-
nal residues often disrupt the structure of Hb, which causes he-
molytic anemia.Changes at the O
2
-binding site that stabilize the
Fe(III) state eliminate O
2
binding to these subunits, which re-
sults in cyanosis. Mutations affecting subunit interfaces may sta-
bilize either the R state or the T state, which, respectively, in-
crease and decrease hemoglobin’s O
2
affinity. Sickle-cell
anemia is caused by the homozygous Hb mutant Glu 6 S Val,
which promotes the gelation of the resulting deoxyHbS to form
rigid 14-strand fibers that deform erythrocytes. Under gelation
conditions,fiber growth occurs via a two-stage nucleation mech-
anism, resulting in a delay time that varies with the 30th to 50th
power of the initial HbS concentration. Agents that increase
this delay time to longer than the transit times of erythrocytes
through the capillaries should therefore prevent sickle-cell
crises and thus relieve the symptoms of sickle-cell anemia.
4 Allosteric Regulation The Adair equation rationalizes
the O
2
-binding cooperativity of Hb by assigning a separate
dissociation constant to each O
2
bound. Positive cooperativity
results if these constants decrease sequentially. However, the
Adair equation offers no physical insight as to why this occurs.
The symmetry model proposes that symmetrical oligomers
can exist in one of two conformational states, R and T, that dif-
fer in ligand-binding affinity. Ligand binding to the high-
affinity state forces the oligomer to assume this conformation,
which facilitates the binding of additional ligand. This ho-
motropic model is extended to heterotropic effects by postu-
lating that activator and substrate can bind only to the R state
and inhibitor can bind only to the T state. The binding of acti-
vator forces the oligomer into the R state, which facilitates the
binding of substrate and additional activator. The binding of
inhibitor, however, forces the oligomer into the T state, which
prevents substrate and activator binding. The sequential
model postulates that an induced fit between ligand and sub-
strate confers conformational strain on the protein that alters
its affinity for binding other ligands without requiring the
oligomer to maintain its symmetry. The Perutz mechanism for
O
2
binding to Hb is structurally largely consistent with the
symmetry model but exhibits some elements of the sequential
model. However, Hb’s ligand-binding cooperativity is in full
accord with the symmetry model.
CHAPTER SUMMARY
General
Bunn, F.H. and Forget, B.G., Hemoglobin: Molecular, Genetic and
Clinical Aspects, Saunders (1986). [A valuable compendium on
normal and abnormal hemoglobins.]
Dickerson, R.E. and Geis, I., Hemoglobin, Benjamin/Cummings
(1983). [A beautifully written and lavishly illustrated treatise
on the structure, function, and evolution of hemoglobin.]
REFERENCES
JWCL281_c10_323-358.qxd 2/24/10 1:58 PM Page 355
356 Chapter 10. Hemoglobin: Protein Function in Microcosm
Ferry, G., Max Perutz and the Secret of Life, Cold Spring Harbor
Laboratory Press (2007). [A definitive biography.]
Hendgen-Cotta, U.B., et al., Nitrite reductase activity of myoglo-
bin regulates respiration and cellular viability in myocardial
ischemia–reperfusion injury, Proc. Natl. Acad. Sci. 105,
10256–10261 (2008).
Judson, H.F., The Eighth Day of Creation (expanded edition),
Chapters 9 and 10, Cold Spring Harbor Laboratory Press
(1996). [Includes a fascinating historical account of how our
present perception of hemoglobin structure and function came
about.]
Lukin, J.A. and Ho, C.,The structure–function relationship of he-
moglobin in solution at atomic resolution, Chem. Rev. 104,
1219–1230 (2004).
Royer, W.E., Jr., Zhu, H., Gorr, T.A., Flores, J.F., and Knapp, J.E.,
Allosteric hemoglobin assembly: diversity and similarity, J.
Biol. Chem. 280, 27477–27480 (2005). [Reviews the different
types of hemoglobins.]
Vinogradov, S.N. and Moens, L., Diversity of globin function: enzy-
matic, transport, storage, and sensing, J. Biol. Chem. 283,
8773–8777 (2008). [Discusses globin-mediated reactions with
NO as well as neuroglobin and cytoglobin.]
Structures of Myoglobin, Hemoglobin, and
Model Compounds
Brunori, M., Nitric oxide moves myoglobin to center stage,Trends
Biochem. Sci. 26, 209–210 (2001).
Fermi, G., Perutz, M.F., Shaanan, B., and Fourme, R., The crystal
structure of human deoxyhaemoglobin at 1.74 Å, J. Mol. Biol.
175, 159–174 (1984).
Jameson, G.B., Molinaro, F.S., Ibers, J.A., Collman, J.P., Brauman,
J.I., Rose, E., and Suslick, K.S., Models for the active site of
oxygen-binding hemoproteins. Dioxygen binding properties
and the structures of (2-methylimidazole)-meso-tetra(␣,␣,␣,␣-
o-pivalamidophenyl)porphinato iron(II)-ethanol and its dioxy-
gen adduct, J. Am. Chem. Soc. 102, 3224–3237 (1980). [The
picket-fence complex.]
Liddington, R., Derewenda, Z., Dodson, G., and Harris, D., Struc-
ture of the liganded T state of haemoglobin identifies the ori-
gin of cooperative oxygen binding,Nature 331, 725–728 (1988).
Ordway, G.A. and Garry, D.J., Myoglobin: an essential hemopro-
tein in striated muscle, J. Exp. Biol. 207, 341–3446 (2004).
Phillips, S.E.V., Structure and refinement of oxymyoglobin at 1.6
Å resolution, J. Mol. Biol. 142, 531–554 (1980).
Shaanan, B., Structure of human oxyhaemoglobin at 2.1 Å resolu-
tion, J. Mol. Biol. 171, 31–59 (1983).
Takano, T., Structure of myoglobin refined at 2.0 Å resolution,
J. Mol. Biol. 110, 537–568, 569–584 (1977).
Mechanism of Hemoglobin Oxygen Binding
Baldwin, J. and Chothia, C., Haemoglobin: The structural changes
related to ligand binding and its allosteric mechanism, J. Mol.
Biol. 129, 175–220 (1979). [The exposition of a detailed mech-
anism of O
2
binding to Hb based on the structures of oxyHb
and deoxyHb.]
Barrick, D., Ho, N.T., Simplaceanu,V., Dahlquist, F.W., and Ho, C.,
A test of the role of the proximal histidines in the Perutz
model for cooperativity in haemoglobin, Nature Struct. Biol. 4,
78–83 (1997). [Describes the experiments in which the proxi-
mal His is detached from the F helix.]
Gelin, B.R., Lee, A.W.-N., and Karplus, M., Haemoglobin tertiary
structural change on ligand binding, J. Mol. Biol. 171, 489–559
(1983).[A theoretical study of the dynamics of O
2
binding to Hb.]
Perutz, M.F., Stereochemistry of cooperative effects in haemoglo-
bin, Nature 228, 726–734 (1970). [The landmark paper in which
the Perutz mechanism was first proposed. Although many of
its details have since been modified, the basic model remains
intact.]
Perutz, M.F., Regulation of oxygen affinity of hemoglobin, Annu.
Rev. Biochem. 48, 327–386 (1979). [An examination of the
Perutz mechanism in light of structural and spectroscopic data.]
Perutz, M.F., Mechanisms of cooperativity and allosteric regula-
tion in proteins, Q. Rev. Biophys. 22, 139–236 (1989). [Contains
a detailed structural description of allosterism in hemoglobin.]
Perutz, M.F., Wilkinson, A.J., Paoli, M., and Dodson, G.G., The
stereochemical mechanism of the cooperative effects in hemo-
globin revisited, Annu. Rev. Biophys. Biomol. Struct. 27, 1–34
(1998).
Bohr Effect and BPG Binding
Arnone, A., X-ray studies of the interaction of CO
2
with human
deoxyhaemoglobin, Nature 247, 143–145 (1974).
Benesch, R.E. and Benesch, R., The mechanism of interaction of
red cell organic phosphates with hemoglobin, Adv. Prot. Chem.
28, 211–237 (1974).
Kilmartin, J.V. and Rossi-Bernardi, L.,Interactions of hemoglobin
with hydrogen ion, carbon dioxide and organic phosphates,
Physiol. Rev. 53, 836–890 (1973).
Lenfant, C., Torrance, J., English, E., Finch, C.A., Reynafarje, C.,
Ramos, J., and Faura, J., Effect of altitude on oxygen binding by
hemoglobin and on organic phosphate levels, J. Clin. Invest. 47,
2652–2656 (1968).
Perutz, M.F., Kilmartin, J.V., Nishikura, K., Fogg, J.H., and Butler,
P.J.G., Identification of residues contributing to the Bohr effect
of human haemoglobin, J. Mol. Biol. 138, 649–670 (1980).
Richard, V., Dodson, G.G., and Mauguen, Y., Human deoxy-
haemoglobin-2,3-diphosphoglycerate complex low-salt struc-
ture at 2.5 Å resolution, J. Mol. Biol. 233, 270–274 (1993).
Sun, D.P., Zou, M., Ho, N.T., and Ho, C., Contribution of surface
histidyl residues in the ␣-chain of the Bohr effect of human
normal adult hemoglobin: Roles of global electrostatic effects,
Biochemistry 36, 6663–6673 (1997).
Abnormal Hemoglobins
Baudin-Chich, V., Pagnier, J., Marden, M., Bohn, B., Lacaze, N.,
Kister, J., Schaad, O., Edelstein, S.J., and Poyart, C., Enhanced
polymerization of recombinant human deoxyhemoglobin 6
Glu S Ile, Proc. Natl. Acad. Sci. 87, 1845–1849 (1990).
Bunn, F.H., Pathogenesis and treatment of sickle cell disease, New
Engl. J. Med. 337, 762–769 (1997).
Bunn, F.H., Human hemoglobins: sickle hemoglobin and other
mutants, in Stamatoyannopoulos, G., Majerus, P.W., Perlmut-
ter, R.M., and Varmus, H. (Eds.), The Molecular Basis of Blood
Diseases (3rd ed.), Chapter 7, Elsevier (2001).
Eaton,W.A. and Hofrichter,J., Sickle cell hemoglobin polymeriza-
tion, Adv. Prot. Chem. 40, 63–279 (1990). [An exhaustive re-
view of HbS polymerization.]
Eaton, W.A. and Hofrichter, J., The biophysics of sickle cell hy-
droxyurea therapy, Science 268, 1142–1143 (1995).
Harrington, D.J.,Adachi, K.,and Royer,W.E., Jr.,The high resolu-
tion crystal structure of deoxyhemoglobin S, J. Mol. Biol. 272,
398–407 (1997).
Nagel, R.L., Haemoglobinopathies due to structural mutations, in
Provan, D. and Gribben, J. (Eds.), Molecular Haematology, pp.
121–133, Blackwell Science (2000).
Perutz, M., Protein Structure. New Approaches to Disease and
Therapy, Chapter 6, Freeman (1992).
Perutz, M.F. and Lehmann, H., Molecular pathology of human
JWCL281_c10_323-358.qxd 2/24/10 1:58 PM Page 356
Problems 357
haemoglobin, Nature 219, 902–909 (1968). [A ground-breaking
study correlating the clinical symptoms and inferred structural
alterations of numerous mutant hemoglobins.]
Steinberg, M.H., Management of sickle cell disease, New Engl.
J. Med. 340, 1021–1030 (1999).
Strasser, B.J., Sickle-cell anemia, a molecular disease, Science 286,
1488–1490 (1999). [A short history of Pauling’s characteriza-
tion of sickle-cell anemia.]
Watowich, S.J., Gross, L.J., and Josephs, R., Intermolecular con-
tacts within sickle hemoglobin fibers, J.Mol. Biol. 209, 821–828
(1989).
Weatherall, D.J., Clegg, J.B., Higgs, D.R., and Wood, W.G., The he-
moglobinopathies, in Valle, D. (Ed.), The Online Metabolic &
Molecular Bases of Inherited Disease http://ommbid.com/. [A
detailed review of abnormal hemoglobins.]
Allosteric Regulation
Ackers, G.A. and Holt, J.M., Asymmetric cooperativity in a sym-
metric tetramer: human hemoglobin, J. Biol. Chem. 281,
11441–11443 (2006); and Ackers, G.A., Deciphering the molec-
ular code of hemoglobin allostery, Adv. Prot. Chem. 51,
185–253 (1998).
Eaton, W.A., Henry, E.R., Hofrichter, J., Bettati, S., Viappiani, C.,
and Mozzarelli, A., Evolution of allosteric models for hemo-
globin, IUBMB Life 59, 586–589 (2007). [A critical review.]
Fersht, A., Structure and Mechanism in Protein Science, Chapter
10, Freeman (1999).
Koshland, D.E., Jr., Némethy, G., and Filmer, D., Comparison of
experimental binding data and theoretical models in proteins
containing subunits, Biochemistry 5, 365–385 (1966). [The
formulation of the sequential model of allosteric regulation.]
Monod, J., Wyman, J., and Changeux, J.P., On the nature of al-
losteric transitions: A plausible model, J. Mol. Biol. 12, 88–118
(1965). [The exposition of the symmetry model of allosteric
regulation.]
Yonetani, T. and Laberge, M., Protein dynamics explain the al-
losteric behaviours of hemoglobin, Biochim. Biophys. Acta
1784, 1146–1158 (2008).
1. The urge to breathe in humans results from a high blood
CO
2
content; there are no direct physiological sensors of blood
pO
2
. Skindivers often hyperventilate (breathe rapidly and deeply
for several minutes) just before making a protracted dive in the be-
lief that they will thereby increase the O
2
content of their blood.
This belief results from the fact that hyperventilation represses the
breathing urge by expelling significant quantities of CO
2
from the
blood. In light of what you know about the properties of hemoglo-
bin, is hyperventilation a useful procedure? Is it safe? Explain.
2. Explain why the Hill constant, n, can never be larger than
the number of ligand-binding sites on the protein.
*3. In the Bohr effect, protonation of the N-terminal amino
groups of hemoglobin’s ␣ chains is responsible for ⬃30% of the
0.6 mol of H
⫹
that combine with Hb on the release of 1 mol of O
2
at pH 7.4. Assuming that this group has pK ⫽ 7.0 in oxyHb, what
is its pK in deoxyHb?
4. As one of the favorites to win the La Paz, Bolivia,marathon,
you have trained there for the several weeks it requires to become
adapted to its 3700-m altitude. A manufacturer of running equip-
ment who sponsors an opponent has invited you for the weekend
to a prerace party at a beach house near Lima, Peru, with the as-
surance that you will be flown back to La Paz at least a day before
the race. Is this a token of his respect for you or an underhanded
attempt to handicap you in the race? Explain (see Fig. 10-9).
5. In active muscles, the pO
2
may be 10 torr at the cell surface
and 1 torr at the mitochondia (the organelles where oxidative me-
tabolism occurs). How might myoglobin (p
50
⫽ 2.8 torr) facilitate
the diffusion of O
2
through these cells? Active muscles consume
O
2
much faster than do other tissues. Could myoglobin also be an
effective O
2
-transport protein in other tissues? Explain.
6. Erythrocytes that have been stored for over a week in stan-
dard acid–citrate–dextrose medium become depleted in BPG.
Discuss the merits of using fresh versus week-old blood in blood
transfusions.
7. The following fractional saturation data have been meas-
ured for a certain blood sample:
PROBLEMS
pO
2
pO
2
20 0.14 60 0.59
30 0.26 70 0.66
40 0.39 80 0.72
50 0.50 90 0.76
Y
O
2
Y
O
2
What are the Hill constant and the p
50
of this blood sample? Are
they normal?
8. An anemic individual, whose blood has only half the normal
Hb content, may appear to be in good health.Yet a normal individ-
ual is incapacitated by exposure to sufficient carbon monoxide to
occupy half of his/her heme sites ( pCO of 1 torr for ⬃1 h; CO
binds to Hb with 200-fold greater affinity than does O
2
). Explain.
*9. The X-ray structure of Hb Rainier (Tyr 145 S Cys) indi-
cates that the mutant Cys residue forms a disulfide bond with Cys
93 of the same subunit. This holds the  subunit’s C-terminal
residue in a quite different orientation than it assumes in HbA.
How would the following quantities for Hb Rainier compare with
those of HbA? Explain. (a) The oxygen affinity, (b) the Bohr ef-
fect, (c) the Hill constant, and (d) the BPG affinity.
10. The crocodile, which can remain under water without
breathing for up to 1 h, drowns its air-breathing prey and then
dines at its leisure. An adaptation that aids the crocodile in doing
so is that it can utilize virtually 100% of the O
2
in its blood,
whereas humans, for example, can extract only ⬃65% of the O
2
in
their blood. Crocodile Hb does not bind BPG. However, crocodile
deoxyHb preferentially binds . How does this help the
crocodile obtain its dinner?
11. The gelation time of an equimolar mixture of HbA and HbS
is less than that of a solution of only HbS in the same concentration
HCO
⫺
3
JWCL281_c10_323-358.qxd 2/24/10 1:58 PM Page 357

358 Chapter 10. Hemoglobin: Protein Function in Microcosm
15. Derive the equilibrium constant for the reaction R
2
34 T
2
for a symmetry model n-mer in terms of the parameters L, c,
and ␣.
*16. Derive the equation for the fraction of protein molecules
in the R state, , for the homotropic symmetry model in terms of
the parameters n, L, c, and ␣. Plot this function versus ␣ for n ⫽ 4,
L ⫽ 1000, and c ⫽ 0 and discuss its physical significance.
17. In the symmetry model of allosterism, why must an in-
hibitor (which causes a negative heterotropic effect with the sub-
strate) undergo a positive homotropic effect?
18. At low concentrations, the hemoglobin tetramer re-
versibly dissociates into two ␣
1

1
dimers.What is the Hill constant
for O
2
binding to these dimers? Explain.
19. Describe the nature of the allosteric changes (homotropic
or heterotropic, positive or negative) that take place in the
GroEL/ES system during the various stages of its catalytic cycle
(Fig. 9-25).
R
that it has in the mixture. What does this observation imply about
the participation of HbA in the gelation of HbS?
12. The severely anemic condition of homozygotes for HbS
results in an elevated BPG content in their erythrocytes. Discuss
whether or not this is a beneficial effect.
13. As organizer of an expedition that plans to climb several
very high mountains, it is your responsibility to choose its mem-
bers. Each of the applicants for one of the positions on the team is
a heterozygote for one of the following variant hemoglobins:
(1) HbS, (2) Hb Hyde Park [His F8(92) S Tyr], (3) Hb
Riverdale–Bronx [Gly B6(24) S Arg], (4) Hb Memphis [Glu
B4(23)␣ S Gln], and (5) Hb Cowtown [His HC3(146) S Leu].
Assuming that all of these candidates are equal in ability at low al-
titudes, which one would you choose for the position? Explain
your reasoning.
14. Show that the Adair equation for a tetramer reduces to
the Hill equation for k
1
⬇ k
2
⬇ k
3
W k
4
and to a hyperbolic rela-
tionship for k
1
⫽ k
2
⫽ k
3
⫽ k
4
.
JWCL281_c10_323-358.qxd 2/24/10 1:58 PM Page 358

359
CHAPTER 11
Sugars and
Polysaccharides
1 Monosaccharides
A. Classification
B. Configurations and Conformations
C. Sugar Derivatives
2 Polysaccharides
A. Carbohydrate Analysis
B. Disaccharides
C. Structural Polysaccharides: Cellulose and Chitin
D. Storage Polysaccharides: Starch and Glycogen
E. Glycosaminoglycans
3 Glycoproteins
A. Proteoglycans
B. Bacterial Cell Walls
C. Glycoprotein Structure and Function
D. Glycomics
weight. Polysaccharides such as starch in plants and glyco-
gen in animals serve as important nutritional reservoirs.
The elucidation of the structures and functions of carbo-
hydrates has lagged well behind those of proteins and nu-
cleic acids. This can be attributed to several factors. Carbo-
hydrate compounds are often heterogeneous, both in size
and in composition, which greatly complicates their physi-
cal and chemical characterization. They are not subject to
the types of genetic analysis that have been invaluable in
the study of proteins and nucleic acids because saccharide
sequences are not genetically specified but are built up
through the sequential actions of specific enzymes (Section
23-3B). Furthermore, it has been difficult to establish as-
says for the biological activities of polysaccharides because
of their largely passive roles. Nevertheless, it is abundantly
clear that carbohydrates are essential elements in many, if
not most, biological processes.
In this chapter, we explore the structures, chemistry,
and, to a limited extent, the functions of carbohydrates,
alone and in association with proteins. Glycolipid struc-
tures are considered in Section 12-1D. The biosynthesis of
complex carbohydrates is discussed in Section 23-3.
1 MONOSACCHARIDES
Monosaccharides or simple sugars are aldehyde or ketone
derivatives of straight-chain polyhydroxy alcohols contain-
ing at least three carbon atoms. Such substances, for exam-
ple,
D-glucose and D-ribulose, cannot be hydrolyzed to
form simpler saccharides.
In this section, the structures of the monosaccharides and
some of their biologically important derivatives are dis-
cussed.
H
H
O
HO HC
C
C
OH
HCOH
HC
CH
2
OH
OH
1
2
3
4
6
5
D-Glucose
OC
CH
2
OH
HCOH
HC
CH
2
OH
OH
1
2
3
5
4
D-Ribulose
Carbohydrates or saccharides (Greek: sakcharon, sugar)
are essential components of all living organisms and are, in
fact, the most abundant class of biological molecules. The
name carbohydrate, which literally means “carbon hy-
drate,” stems from their chemical composition, which is
roughly (C ⴢ H
2
O)
n
, where n ⱖ 3. The basic units of carbo-
hydrates are known as monosaccharides. Many of these
compounds are synthesized from simpler substances in a
process named gluconeogenesis (Section 23-1). Others
(and ultimately nearly all biological molecules) are the
products of photosynthesis (Section 24-3), the light-powered
combination of CO
2
and H
2
O through which plants and
certain bacteria form “carbon hydrates.” The metabolic
breakdown of monosaccharides (Chapters 17 and 21)
provides much of the energy used to power biological
processes. Monosaccharides are also principal components
of nucleic acids (Section 5-1A), as well as important ele-
ments of complex lipids (Section 12-1D).
Oligosaccharides consist of a few covalently linked
monosaccharide units. They are often associated with pro-
teins (glycoproteins) and lipids (glycolipids) in which they
have both structural and regulatory functions (glycopro-
teins and glycolipids are collectively called glycoconju-
gates). Polysaccharides consist of many covalently linked
monosaccharide units and have molecular masses ranging
well into the millions of daltons. They have indispensable
structural functions in all types of organisms but are most
conspicuous in plants because cellulose, their principal
structural material, comprises up to 80% of their dry
JWCL281_c11_359-385.qxd 6/3/10 10:35 AM Page 359
360 Chapter 11. Sugars and Polysaccharides
A. Classification
Monosaccharides are classified according to the chemical
nature of their carbonyl group and the number of their C
atoms. If the carbonyl group is an aldehyde, as in glucose,
the sugar is an aldose. If the carbonyl group is a ketone, as
in ribulose, the sugar is a ketose. The smallest monosaccha-
rides, those with three carbon atoms, are trioses. Those
with four, five, six, seven, etc., C atoms are, respectively, tet-
roses, pentoses, hexoses, heptoses, etc. These terms may be
combined so that, for example, glucose is an aldohexose,
whereas ribulose is a ketopentose.
Examination of
D-glucose’s molecular formula indicates
that all but two of its six C atoms¬C1 and C6¬are chiral
centers, so that
D-glucose is one of 2
4
⫽ 16 stereoisomers
that comprise all possible aldohexoses. In general, n-carbon
aldoses have 2
n⫺2
stereoisomers. The stereochemistry and
names of the
D-aldoses are presented in Fig. 11-1. Emil
Fischer elucidated these configurations for the aldohexoses in
1896.According to the Fischer convention (Section 4-2B),
D
sugars have the same absolute configuration at the asymmet-
ric center farthest removed from their carbonyl group as does
D-glyceraldehyde. The L sugars, in accordance with this con-
vention, are mirror images of their
D counterparts, as is
shown below in Fischer projection for glucose.
H
H
O
HO HC
C
C
OH
HCOH
HC
CH
2
OH
OH
H
HO
O
HOHC
C
C
H
HO C H
HO C
CH
2
OH
H
D-Glucose
L-Glucose
Figure 11-1 The stereochemical relationships, shown in
Fischer projection, among the
D-aldoses with three to six carbon
atoms. The arrows indicate stereochemical relationships (not
biosynthetic reactions).The configuration about C2 (red)
CHO
HCOH
CH
2
OH
D-Glyceraldehyde
HCOH
CH
2
OH
CH
O
HCOH
HCOH
CH
2
OH
CH
O
HOCH
HCOH
CH
2
OH
HCOH
HCOH
CH
O
HCOH
D-Allose
HCOH
CH
2
OH
HCOH
HCOH
CH
O
HOCH
D-Altrose
HCOH
CH
2
OH
HCOH
HCOH
CH
O
HOCH
D-Glucose
(Glc)
HCOH
CH
2
OH
CHO
HOCH
D-Talose
HCOH
CH
2
OH
HCOH
CHO
HOCH
D-Mannose
(Man)
HOCH
HCOH
CH
2
OH
HCOH
HCOH
CH
O
HOCH
D-Gulose
HCOH
CH
2
OH
HCOH
CH
O
HOCH
D-Idose
HOCH
HCOH
CH
2
OH
HCOH
CH
O
HOCH
D-Galactose
(Gal)
HOCH
HOCH
HOCH
D-Lyxose (Lyx)D-Ribose (Rib)
D-Arabinose (Ara)
D-Xylose (Xyl)
HCOH
CH
2
OH
HOCH
CH
O
HOCH
HCOH
CH
2
OH
HOCH
CH
O
HCOH
HCOH
CH
2
OH
HCOH
CH
O
HOCH
HCOH
CH
2
OH
HCOH
CH
O
HCOH
D-ThreoseD-Erythrose
Aldotriose
Aldotetroses
Aldopentoses
Aldohexoses
distinguishes the members of each pair.The L- counterparts of
these 15 sugars are their mirror images.The biologically most
common aldoses are boxed.
JWCL281_c11_359-385.qxd 6/3/10 10:35 AM Page 360
