Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

p
50
, increases the amount of O
2
that hemoglobin unloads
in the capillaries (Fig. 10-10). Similar increases in BPG
concentration occur in individuals suffering from disor-
ders that limit the oxygenation of the blood (hypoxia),
such as various anemias and cardiopulmonary insuffi-
ciency.
b. Fetal Hemoglobin Has a Low BPG Affinity
The effects of BPG also help supply the fetus with
oxygen. A fetus obtains its O
2
from the maternal circulation
via the placenta.This process is facilitated because fetal he-
moglobin (HbF) has a higher O
2
affinity than does mater-
nal hemoglobin (HbA; recall that HbF has the subunit
composition ␣
2
␥
2
, in which the ␥ subunit is a variant of
HbA’s  subunit; Section 7-3C). BPG occurs in about the
same concentrations in adult and fetal erythrocytes but
binds more tightly to deoxyHbA than to deoxyHbF; this ac-
counts for HbF’s greater O
2
affinity. In the next section we
shall develop the structural rationale for the effect of BPG
and for the other aspects of O
2
binding.
2 STRUCTURE AND MECHANISM
The determination of the first protein X-ray structures,
those of sperm whale myoglobin by John Kendrew in 1959
and of human deoxyhemoglobin and horse methemoglo-
bin by Max Perutz shortly thereafter, ushered in a revolu-
tion in biochemical thinking that has reshaped our under-
standing of the chemistry of life. Before the advent of
protein crystallography, macromolecular structures, if they
were considered at all, were thought of as having a rather
hazy existence of uncertain biological significance. How-
ever, as the elucidation of macromolecular structures has
continued at an ever quickening pace, it has become clear
that life is based on the interactions of complex, structurally
well-defined macromolecules.
The story of hemoglobin’s structural determination is a
tale of enormous optimism and tenacity. Perutz began this
study in 1937 at Cambridge University as a graduate student
of J. D. Bernal (who, with Dorothy Crowfoot Hodgkin, had
taken the first X-ray diffraction photographs of hydrated
protein crystals in 1934). In 1937, the X-ray crystal structure
determination of even the smallest molecule required many
months of hand computation, and the largest structure yet
determined was that of the dye phthalocyanin, which has 40
nonhydrogen atoms. Since hemoglobin has ⬃4500 nonhy-
drogen atoms, it must have seemed to Perutz’s colleagues
that he was pursuing an impossible goal. Nevertheless, the
laboratory director, Lawrence Bragg (who in 1912, with his
father William Bragg, had determined the first X-ray struc-
ture, that of NaCl), realized the tremendous biological sig-
nificance of determining a protein structure and supported
the project.
It was not until 1953 that Perutz finally hit on the method
that would permit him to solve the X-ray structure of hemo-
globin, that of isomorphous replacement. Kendrew, a col-
league of Perutz, used this technique to solve the X-ray
structure of sperm whale myoglobin, first at low resolution
in 1957, and then at high resolution in 1959. Hemoglobin’s
greater complexity delayed its low-resolution structural
determination until 1959, and it was not until 1968, over 30
years after he had begun the project, that Perutz and his as-
sociates obtained the high-resolution X-ray structure of
horse methemoglobin. Those of human and horse deoxy-
hemoglobins followed shortly thereafter.Since then, the X-
ray structures of hemoglobins from numerous different
species, from mutational variants, and with different bound
ligands have been elucidated. This, together with many of-
ten ingenious physicochemical investigations, has made he-
moglobin the most intensively studied, and perhaps the
best understood, of proteins.
In this section, we examine the molecular structures of
myoglobin and hemoglobin and consider the structural ba-
sis of hemoglobin’s oxygen-binding cooperativity, the Bohr
effect, and BPG binding.
A. Structure of Myoglobin
Myoglobin consists of eight helices (labeled A–H) that are
linked by short polypeptide segments to form an ellipsoidal
Section 10-2. Structure and Mechanism 331
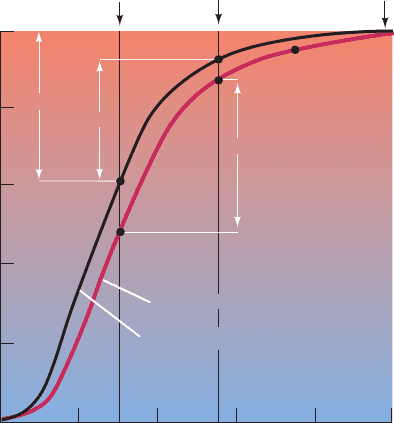
1.0
0.8
0.6
0.4
0.2
0 20 40 60 80 100
Venous
pO
2
Arterial
pO
2
at
4500 m
Arterial
pO
2
at
sea level
0.38
0.3
0.37
p
50
= 31 torr (high BPG)
p
50
= 26 torr (normal BPG)
pO
2
(torr)
Y
O
2
Figure 10-10 The O
2
-dissociation curves of blood adapted to
sea level (black curve) and to high altitude (red curve). Between
the sea level arterial and venous pO
2
values of 100 and 30 torr,
respectively, Hb normally unloads 38% of the O
2
it can maximally
carry. However, when the arterial pO
2
drops to 55 torr, as it does
at an altitude of 4500 m, this difference is reduced to 30% in
nonadapted blood. High-altitude adaptation increases the BPG
concentration in erythrocytes, which shifts the O
2
-dissociation
curve of Hb to the right.The amount of O
2
that Hb delivers to
the tissues is thereby restored to 37% of its maximum load.
JWCL281_c10_323-358.qxd 2/24/10 1:57 PM Page 331
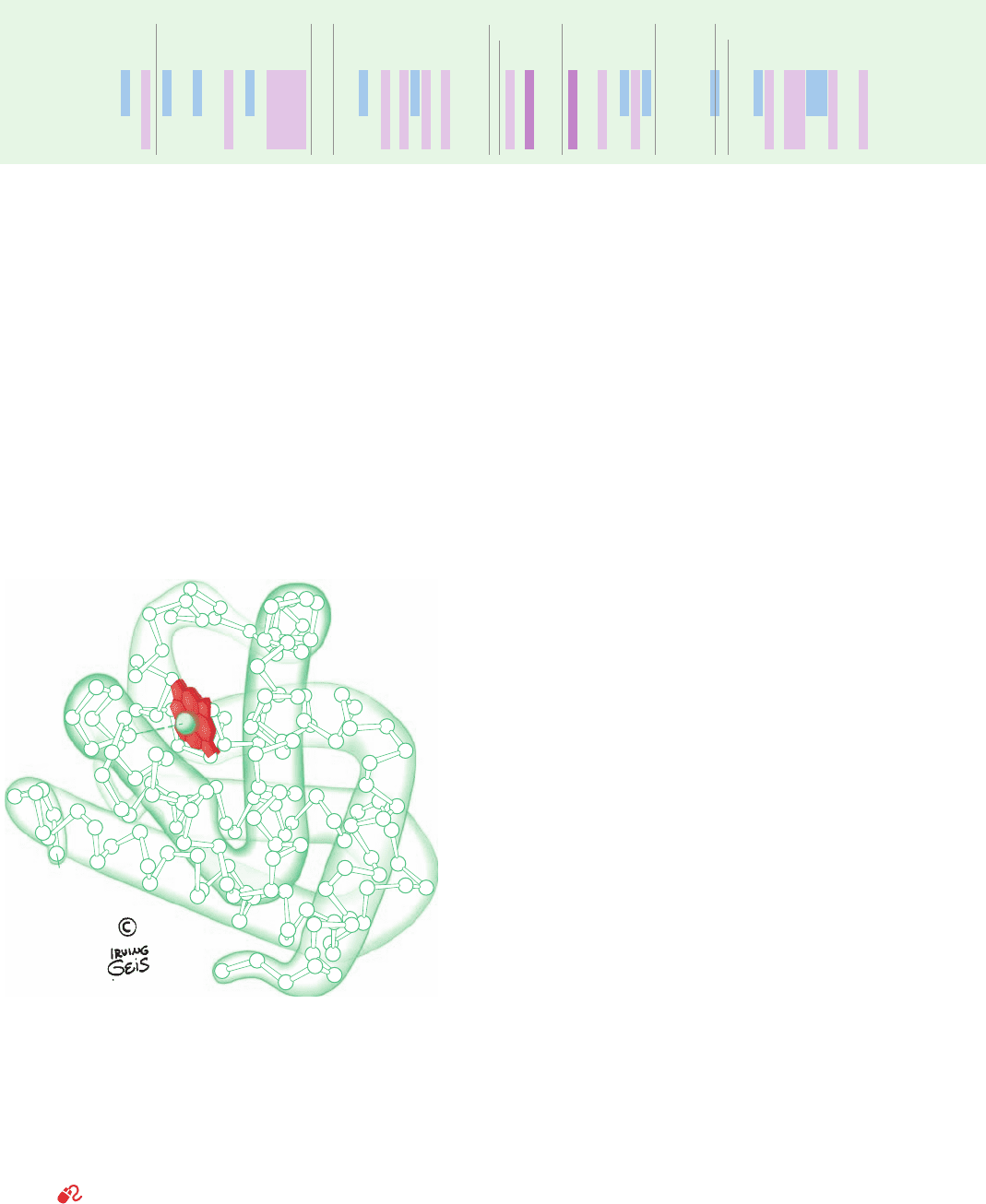
molecule of approximate dimensions 44 ⫻ 44 ⫻ 25 Å (Fig.
10-11; see also Fig. 8-39).The helices range in length from 7
to 26 residues and incorporate 121 of myoglobin’s 153
residues (Table 10-1). They are largely ␣ helical but with
some distortions from this geometry such as a tightening of
the final turns of helices A, C, E, and G to form segments of
3
10
helix.
In a helix numbering convention peculiar to globins,
residues are designated according to their position in a he-
lix or interhelical segment. For example, residue B5 is the
fifth residue from the N-terminus of the B helix and
residue FG3 is the third residue from the N-terminus in
the nonhelical segment connecting helices F and G. The
nonhelical N- and C-terminal segments are designated NA
and HC, respectively. The usual convention of sequentially
numbering all amino acid residues from the N-terminal
residue of the polypeptide is also used, and often both con-
ventions are used together. For example, Glu EF7(83) of
human Mb is the 83rd residue from its N-terminus and the
7th residue in the nonhelical segment connecting its E and
F helices.
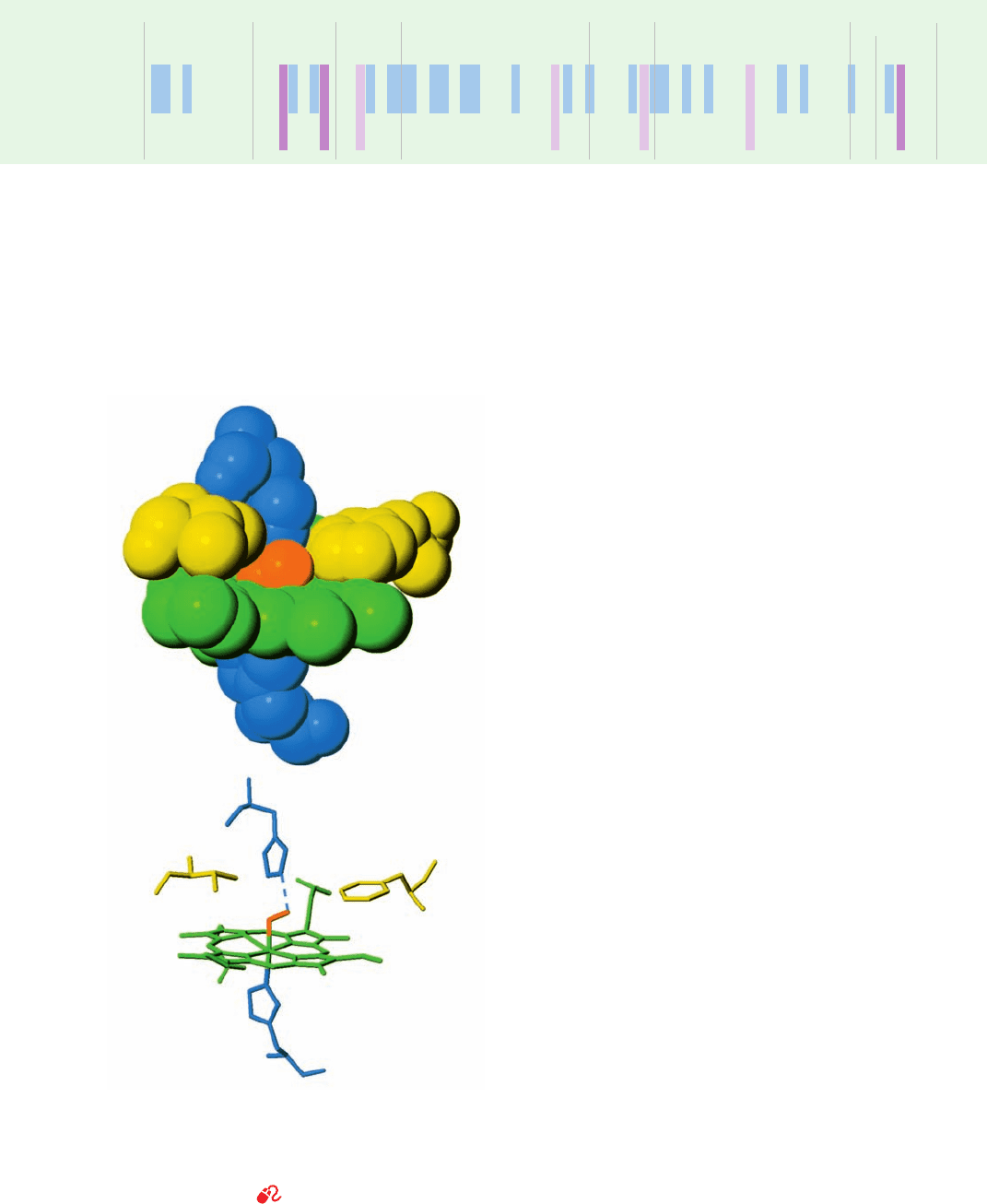
The heme is tightly wedged in a hydrophobic pocket
formed mainly by helices E and F but which includes
contacts with helices B, C, G, and H as well as the CD and
FG segments. The fifth ligand of the heme Fe(II) is His
F8, the proximal (near) histidine. In oxyMb, the Fe(II) is
positioned 0.22 Å out of the heme plane on the side of
the proximal His and is coordinated by O
2
with the bent
geometry shown in Fig. 10-12. His E7, the distal (distant)
histidine, hydrogen bonds to the O
2
. In deoxyMb, the
sixth liganding position of the Fe(II) is unoccupied be-
cause the distal His is too far away from the Fe(II) to co-
ordinate with it. Furthermore, the Fe(II) has moved to a
point 0.55 Å out of the heme plane. Other structural
changes in Mb on changing oxygenation states consist of
small motions of various chain segments and slight read-
justments of side chain conformations. By and large, how-
ever, the structures of oxy- and deoxyMb are nearly super-
imposable.
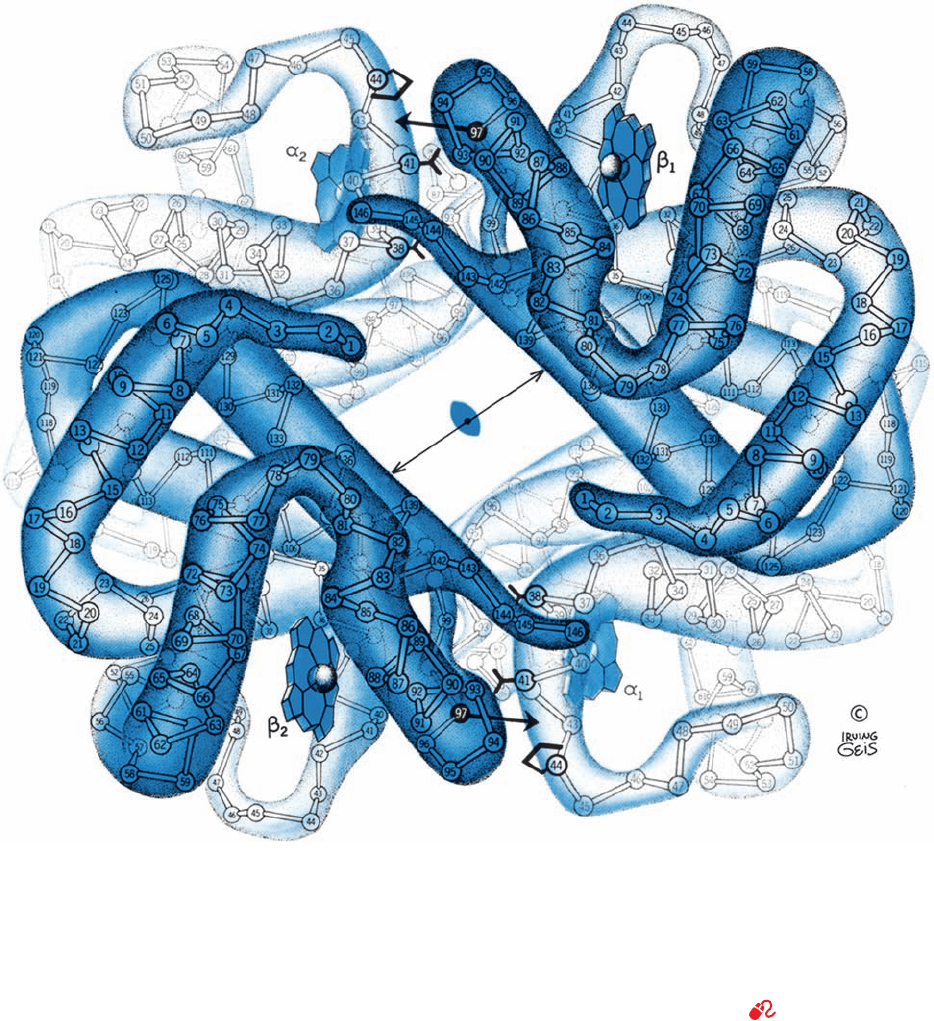
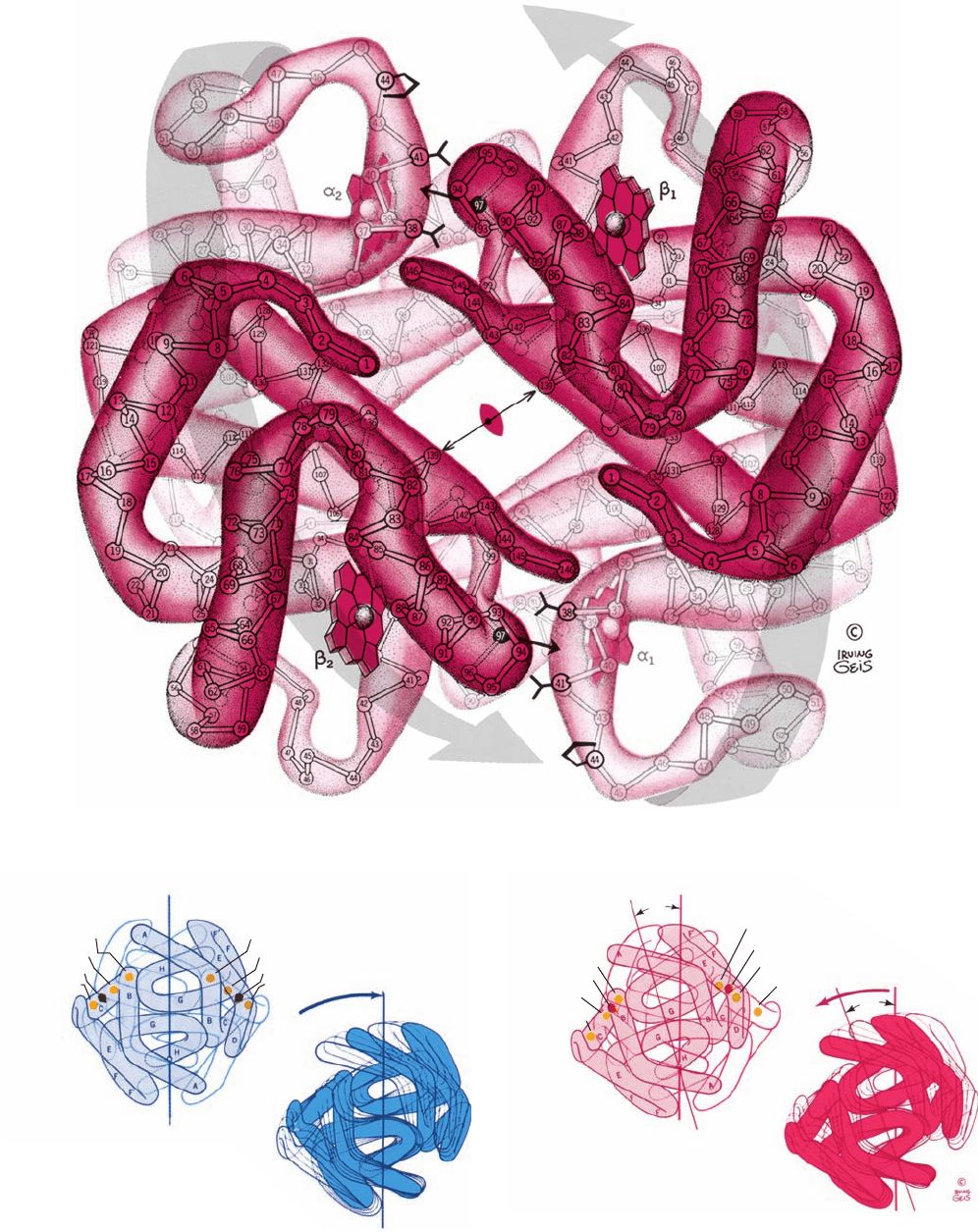
B. Structure of Hemoglobin
The hemoglobin tetramer is a spheroidal molecule of di-
mensions 64 ⫻ 55 ⫻ 50 Å. Its two ␣ protomers are sym-
metrically related by a twofold rotation (Fig. 10-13; see also
Fig. 8-64). The tertiary structures of the ␣ and  subunits are
remarkably similar, both to each other and to that of Mb
(Figs. 10-11 and 10-13), even though only 18% of the corre-
sponding residues are identical among these three
polypeptides (Table 10-1) and there is no D helix in hemo-
globin’s ␣ subunit. Indeed, the a and b subunits in the
tetramer are related by pseudo- (inexact) 2-fold rotations so
that the subunits occupy the vertices of a tetrahedron
(pseudo-D
2
symmetry; Section 8-5B).
The polypeptide chains of Hb are arranged such that
there are extensive interactions between unlike subunits. The
␣
1
–
1
interface (and its ␣
2
–
2
symmetry equivalent) in-
volves 35 residues, whereas the ␣
1
–
2
(and ␣
2
–
1
) interface
involves 19 residues. These associations are predominantly
332 Chapter 10. Hemoglobin: Protein Function in Microcosm
Table 10-1 The Amino Acid Sequences of the ␣ and  Chains of Human Hemoglobin and of Human Myoglobin
a,b
Helix Boundaries A1 A16 B1 B16 C7 D1 D7
C1 E1
1 5 10 15 20 25 30 35 40 45 50 55 60 65
Hb ␣..................V– LSPADKTNVKAA WGKVGAHAGEY GAEA LERMFLSFPTTKTYFPHF– DL SH–––––G SAQVKGHGKK VADALT
1 5 10 15 20 25 30 35 40 45 50 55 60 65 70
Hb ..................VHLTPEEKSAVTALWGKV––NVDE V GGE A LGR L LVVY PWT QR FFESF GD L STPDAVMG NPK VKA HGKK V LGA FS
1 5 10 15 20 25 30 35 40 45 50 55 60 65 70
Mb ....................G–LSDGEWQLVLNVWGKVEADI PGHGQEVLIRL FK GHPET LEKFDKFKHLKSEDEMKA SEDLKKHGAT VLTA LG
Figure 10-11 Structure of sperm whale myoglobin. Its 153 C
␣
positions are numbered from the N-terminus and its eight helices
are sequentially labeled A through H.The last half of the EF
corner is now regarded as a turn of helix and is therefore
designated the F¿ helix.The heme group is shown in red. Also see
Fig. 8-39. [Illustration, Irving Geis. Image from the Irving Geis
Collection, Howard Hughes Medical Institute. Reprinted with
permission.] Based on an X-ray structure by John Kendrew,
MRC Laboratory of Molecular Biology, Cambridge, U.K. PDBid
1MBN.]
See Kinemage Exercise 6-1
34
31
51
55
56
58
57
50
49
48
152
151
153
137
139
138
140
143
142
141
144
146
145
148
150
149
133
135
10 9
114
115
120
119
121
122
124
126
130
108
107
32
36
43
47
46
45
105
101
100
1
82
2
3
8
6
13
7
10
12
11
14
16
15
17
20
24
23
19
18
22
59
61
60
62
63
66
69
70
72
71
68
67
64
73
74
76
75
77
80
79
78
81
83
86
89
90
93
94
95
98
97
96
92
91
87
88
85
84
5
4
21
39
40
41
42
44
123
FG
CD
D
AB
NA
HC
H
GH
A
EF
F
⬘
F
G
B
E
C
JWCL281_c10_323-358.qxd 8/10/10 9:40 AM Page 332
hydrophobic in character, although numerous hydrogen
bonds and several ion pairs are also involved (Section
10-2C). In contrast, contacts between like subunits,
␣
1
–␣
2
and 
1
–
2
, are few and largely polar in character.
This is because like subunits face each other across an
⬃20-Å-diameter solvent-filled channel that parallels
the 50-Å length of the exact 2-fold axis (Figs. 8-64
and 10-13).
a. Oxy- and Deoxyhemoglobins Have Different
Quaternary Structures
Oxygenation causes such extensive quaternary struc-
tural changes to Hb that oxy- and deoxyHb have different
crystalline forms; indeed, crystals of deoxyHb shatter on
exposure to O
2
. The crystal structures of hemoglobin’s oxy
and deoxy forms therefore had to be determined inde-
pendently. The quaternary structural change preserves he-
moglobin’s exact 2-fold symmetry and takes place entirely
across its a
1
–b
2
(and a
2
–b
1
) interface. The ␣
1
–
1
(and ␣
2
–
2
)
contact is unchanged, presumably as a result of its more ex-
tensive close associations. This contact provides a conven-
ient frame of reference from which the oxy and deoxy con-
formations may be compared. Viewed in this way,
oxygenation rotates the ␣
1

1
dimer ⬃15° with respect to
the ␣
2

2
dimer (Fig. 10-14), so that some atoms at the ␣
1
–
2
interface shift by as much as 6 Å relative to each other
(compare Figs. 10-13a and 10-13b).
The quaternary conformation of deoxyHb is named the
T state (T for “tense”). That of oxyHb, which is essentially
independent of the ligand used to induce it (e.g., O
2
, met,
CO, CN
⫺
, and NO hemoglobins all have the same quater-
nary structure), is called the R state (R for “relaxed”). Sim-
ilarly, the tertiary conformational states for the deoxy and
liganded subunits are designated as the t and r states,
respectively.The structural differences between the quater-
nary and tertiary conformations are described in the fol-
lowing subsection in terms of hemoglobin’s O
2
-binding
mechanism.
Section 10-2. Structure and Mechanism 333
a
The residues have been aligned in structurally analogous positions.The blue boxes shade the residues that are identical in both Hb chains, the purple
boxes shade the residues that are identical in both Hb chains and in Mb, and the dark purple boxes shade residues that are invariant in all vertebrate Hb
and Mb chains (Thr C4, Phe CD1, Leu F4, His F8, and Tyr HC2).
b
The first and last residues in helices A–H are indicated, whereas the residues between helices constitute the intervening “segments.”The refined Hb
structure reveals that much of what is designated the EF segment is really helical in both chains: It encompasses residues EF4–F2 and is designated the
F¿ helix.
Source: Dickerson, R.E. and Geis, I., Hemoglobin, pp. 68–69, Benjamin/Cummings (1983).
NAV AHVDDM PNALSA LSD L HA
D GLAHL DNLKGTFA TLSELHC
G I LKKKGHHEA E I K P L AQSHA
Hb ␣...
Hb ...
Mb.......
HKLRVDPV NFKLLSHCLLVT L
D KLHV DPE NFRLLGNVLVCVL
TKH K IPVKYLEFISECIIQVL
AAHL PAE F T PAV HASLDK FL A S
AHHFGKEF T PPVQAAYQKVV AG
QSKHPGDFG ADA QGAMNK ALEL
VSTVLTSKY R
V ANA L AHKYH
F RKDM A S N YKELGFQG
E19 F1 F9G1 G19 H1 H19
H21
H26
70 75 80 85 90 95 100 105 110 115 120 125 130 135 140
75 80 85 90 95 100 105 110 115 120 125 130 135 140 145
75 80 85 90 95 100 105 110 115 120 125 130 135 140 145 150
Figure 10-12 The heme complex in oxyMb. In the upper
drawing, atoms are represented as spheres of van der Waals radii.
The lower drawing shows the corresponding skeletal model with
a dashed line representing the hydrogen bond between the distal
His and the bound O
2
. [After Phillips, S.E.V., J. Mol. Biol. 142,
544 (1980). PDBid 1MBO.]
See Kinemage Exercise 6-1
His E7
Phe CD1
O
2
O
2
Val E11
His F8
Fe
JWCL281_c10_323-358.qxd 2/24/10 1:57 PM Page 333
C. Mechanism of Oxygen-Binding Cooperativity
The positive cooperativity of O
2
binding to Hb arises from
the effect of the ligand-binding state of one heme on the
ligand-binding affinity of another. Yet the distances of 25 to
37 Å between the hemes in an Hb molecule are too large
for these heme–heme interactions to be electronic in char-
acter. Rather, they are mechanically transmitted by the pro-
tein. The elucidation of how this occurs has motivated much
of the structural research on Hb for the past four decades.
X-ray crystal structure analysis has provided “snapshots”
of the R and T states of Hb in various states of ligation but
does not indicate how the protein changes states. It is difficult
to determine the sequence of events that result in such trans-
formations because to do so requires an understanding of
the inner workings of proteins that is presently lacking. It is
as if you were asked to explain the mechanism of a compli-
cated mechanical clock from its out-of-focus photographs
when you had only a hazy notion of how gears, levers, and
springs might function. Nevertheless, largely on the basis of
the X-ray structures of Hb, Perutz formulated the following
mechanism of Hb oxygenation, the Perutz mechanism.
334 Chapter 10. Hemoglobin: Protein Function in Microcosm
Figure 10-13 The X-ray structures of (a) deoxyHb and
(b) oxyHb as viewed down their exact 2-fold axes. The C
␣
atoms,
numbered from each N-terminus, and the heme groups are
shown.The Hb tetramer contains a solvent-filled central channel
paralleling its 2-fold axis, whose flanking  chains draw closer
together on oxygenation (compare the lengths of the double-
headed arrows). In the deoxy state, His FG4(97) (small single-
headed arrow) fits between Thr C6(41)␣ and Pro CD2(44)␣
(lower right and upper left).The relative movements of the two
␣ protomers on oxygenation (large gray arrows in Part b) shift
His FG4(97) to a new position between Thr C3(38)␣ and Thr
C6(41)␣. See Fig. 8-64 for a similarly viewed space-filling model
of deoxyHb. [Illustration, Irving Geis. Image from the Irving
Geis Collection, Howard Hughes Medical Institute. Reprinted
with permission.] Based on X-ray structures by Max Perutz,
MRC Laboratory of Molecular Biology, Cambridge, U.K.
PDBids (a) 2DHB and (b) 2MHB.]
See Kinemage Exercises
6-2 and 6-3
(a)
JWCL281_c10_323-358.qxd 8/10/10 9:40 AM Page 334
Section 10-2. Structure and Mechanism 335
DeoxyHb
C3(38)
␣
2
C6(41)␣
2
FG4(97)
1
CD2(44)␣
2
C3(38)␣
2
C3(38)␣
1
C6(41)␣
1
FG4(97)
2
CD2(44)␣
1
␣
2
␣
1

2

1
(a)
OxyHb
(b)


1
15⬚
C3(38)␣
2
FG4(97)
1
C6(41)␣
2
CD2(44)␣
2
C3(38)␣
1
FG4(97)
2
C6(41)␣
1
CD2(44)␣
1
OxyHb
(b)
15⬚
␣
2
␣
1

2

1
15⬚
Figure 10-14 The major structural differences between the
quaternary conformations of (a) deoxyHb and (b) oxyHb. On
oxygenation, the ␣
1

1
(shaded) and ␣
2

2
(outline) dimers move,
as indicated on the right, as rigid units such that there is an ⬃15°
off-center rotation of one protomer relative to the other that
preserves the molecule’s exact 2-fold symmetry. Note how the
Figure 10-13 (continued)
position of His FG4 (pentagons) changes with respect to Thr
C3␣,Thr C6␣, and Pro CD2␣ (yellow dots) at the ␣
1
–
2
and
␣
2
–
1
interfaces.The view is from the right side relative to that in
Fig. 10-13. [Illustration, Irving Geis. Image from the Irving
Geis Collection, Howard Hughes Medical Institute. Reprinted
with permission.]
(b)
JWCL281_c10_323-358.qxd 8/10/10 9:40 AM Page 335
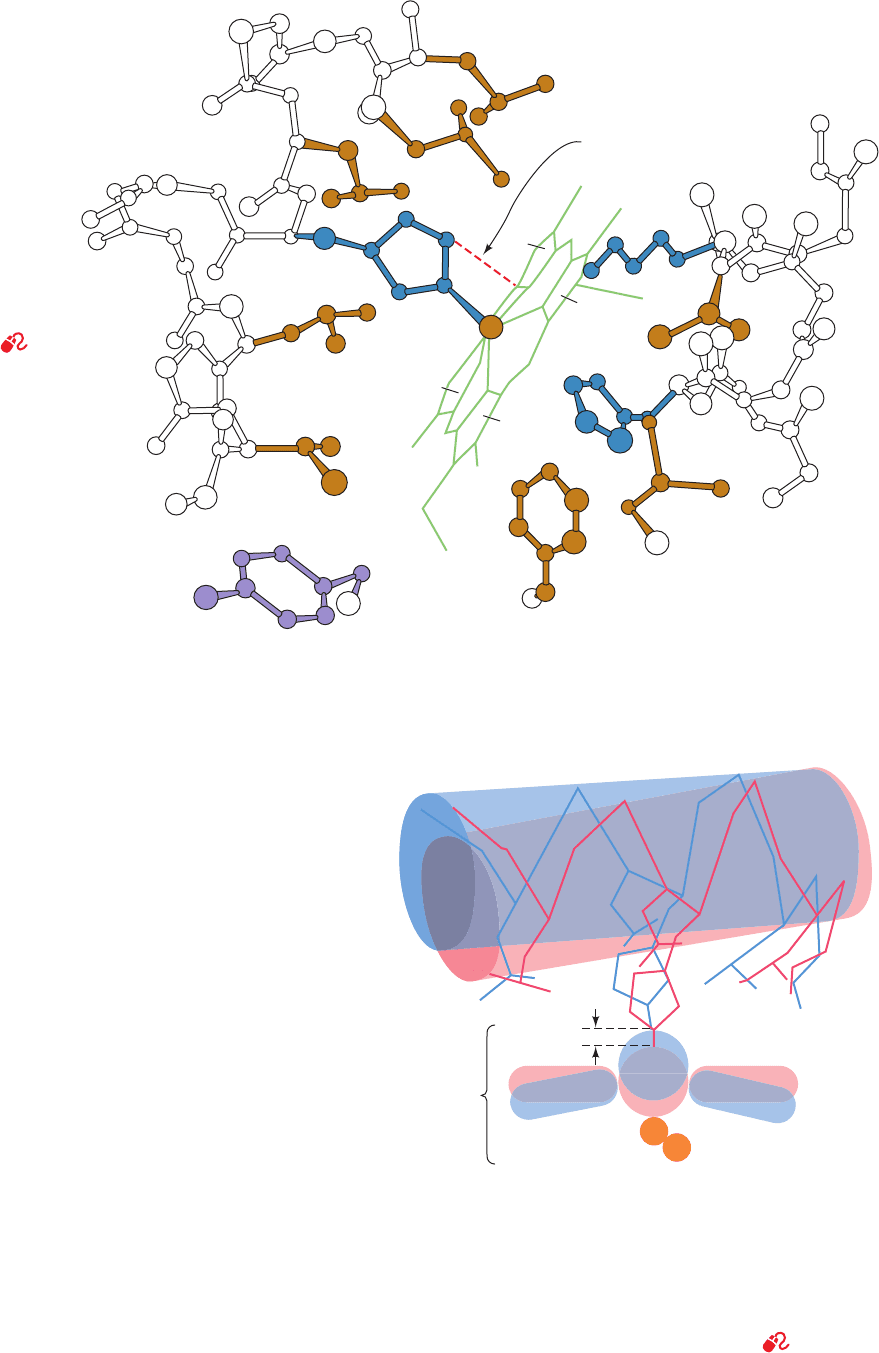
a. The Movement of Fe(II) Into the Heme Plane
Triggers the T S R Conformational Shift
In the t state, the Fe(II) is situated ⬃0.6 Å out of the
heme plane on the side of the proximal His because of a
pyramidal doming of the porphyrin skeleton and because
the Fe¬N
porphyrin
bonds are too long to allow the Fe to lie
in the porphyrin plane (Figs. 10-15 and 10-16). The change
in the heme’s electronic state on binding O
2
, however,
causes the doming to subside and the Fe¬N
porphyrin
bonds
to contract by ⬃0.1 Å. Consequently, on changing from the
t to the r state, the Fe(II) moves to the center of the heme
plane (Fig. 10-16) where O
2
can coordinate it without steric
interference from the porphyrin.The Fe’s movement drags
the proximal His along with it, which tilts the attached
F helix and translates it ⬃1 Å across the heme plane
(Fig. 10-16). This lateral translation occurs because, in the
t state, the imidazole ring of the proximal His is oriented
such that its direct movement of ⬃0.6 Å toward the heme
plane would cause it to collide with the heme (Figs. 10-15
and 10-16); however, the F helix shift reorients the imida-
zole ring, thereby permitting the Fe(II) to move into the
heme plane. In addition, in the t state of the  but not the ␣
subunits, Val E11 partially occludes the O
2
-binding pocket
so that it must be moved aside before O
2
binding can occur.
b. The ␣
1
–
2
and ␣
2
–
1
Contacts Have Two
Stable Positions
As we saw above, the difference between hemoglobin’s
R and T conformations occurs mainly in the ␣
1
–
2
(and the
336 Chapter 10. Hemoglobin: Protein Function in Microcosm
Figure 10-16 Triggering mechanism for the T S R transition
in Hb. In the T form (blue), the Fe is ⬃0.6 Å above the mean
plane of the domed porphyrin ring. On assuming the R form
(red), the Fe moves into the plane of the now undomed
porphyrin, where it can readily bind O
2
, and, in doing so, pulls
the proximal His F8 and its attached F helix with it.The Fe¬O
2
bond is thereby strengthened because of the relaxation of the
steric interference between the O
2
and the heme. See
Kinemage Exercise 6-4 and the Animated Figures
Leu F4 (83)
His
F8 (87)
Leu H19
(136)
Leu F7
Leu FG3
(91)
Val FG5 (93)
Tyr C7 (42)
(86)
Phe CD4
(46)
His E7
(58)
Leu G8
(101)
Val E11
(62)
Lys E10
(61)
Close
contact
A
B
C
D
Porphyrin Porphyrin
Fe
2
+
Heme
Helix F
Leu F7
Leu F4 Leu FG3
Val FG5
His F8
0.6 A
O
2
°
Figure 10-15 The heme group and its
environment in the unliganded ␣ chain
of human Hb. Only selected side chains
are shown and the heme D propionate
group is omitted for clarity. The F helix
runs along the left side of the
drawing.The close contact
between the proximal His
and the heme group that
inhibits oxygenation
of t-state hemes is
indicated by a
dashed red line.
[After Gelin, B.R.,
Lee, A.W.N., and Karplus, M.,
J. Mol. Biol. 171, 542 (1983).
PDBid 2HHB.]
See
Kinemage Exercise 6-4
JWCL281_c10_323-358.qxd 2/24/10 1:58 PM Page 336
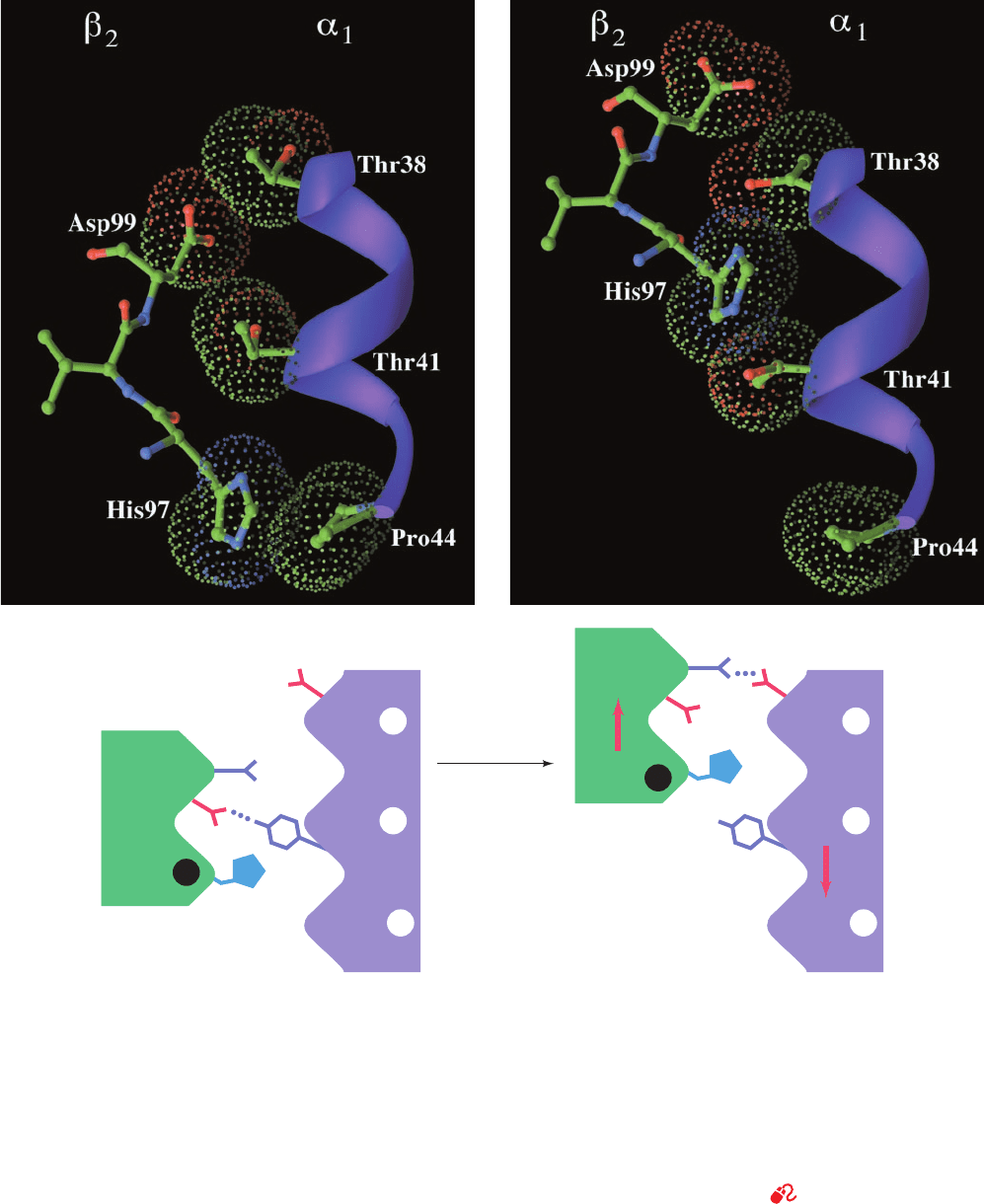
symmetrically related ␣
2
–
1
) interface, which consists of
the C helix and FG segment of ␣
1
, respectively, contacting
the FG segment and C helix of 
2
. The quaternary change
results in a 6-Å relative shift at the ␣
1
C–
2
FG interface
(Fig. 10-14). In the T state, His FG4(97) is in contact with
Thr C6(41)␣ (Figs. 10-13a and 10-17a), whereas in the R
state it is in contact with Thr C3(38)␣, one turn back along
the C helix (Figs. 10-13b and 10-17b). In both conforma-
tions, the “knobs” on one subunit mesh nicely with the
“grooves”on the other (Fig.10-17).An intermediate position,
Section 10-2. Structure and Mechanism 337
Figure 10-17 The ␣
1
C–
2
FG interface of Hb in (a) the T state
and (b) the R state. The upper drawings show the C helix in
ribbon form (purple) and its contacting portion of the FG region
in ball-and-stick form colored according to atom type (C green,
N blue, and O red).The dots outline the contacting van der
Waals surfaces and are also colored according to atom type.The
lower drawings are the corresponding schematic diagrams of the
␣
1
C–
2
FG contact. On a T S R transformation, this contact
snaps from one position to the other with no stable intermediate
(a) T State (deoxy)
oxygenation
(b) R State (oxy)
Asp G1 (99)
Tyr C7
(42)
Asn G4 (102)
Asp G1 (94)
β
2
α
1
β
2
His FG4
α
1
_
_
–
–
97
Thr C3 38
Thr C6 41
Pro CD2 44
Thr C3 38
Thr C6 41
Pro CD2 44
His FG4
97
(note how, in both conformations, the knobs formed by the side
chains of His 97 and Asp 99 fit between the grooves on the C
helix formed by the side chains of Thr 38␣, Thr 41␣, and Pro
44␣).The subunits are joined by different hydrogen bonds in the
two quarternary states. Figures 10-13 and 10-14 provide
additional structural views of these interactions. [Based on X-ray
structures by Giulio Fermi, Max Perutz, and Boaz Shaanan,
MRC Laboratory of Molecular Biology, Cambridge, U.K.
PDBids (a) 2HHB and (b) 1HHO.]
See Kinemage Exercise 6-5
JWCL281_c10_323-358.qxd 6/3/10 10:27 AM Page 337
however, would be severely strained because it would
bring His FG4(97) and Thr C6(41)␣ too close together
(knobs on knobs). Hence these contacts, which are joined by
different but equivalent sets of hydrogen bonds in the two
states (Fig. 10-17), act as a binary switch that permits only
two stable positions of the subunits relative to each other. In
contrast, the quaternary change causes only a 1-Å shift at
the ␣
1
FG–
2
C contact, so its side chains maintain the same
associations throughout the change. These side chains
therefore act as flexible joints or hinges about which the a
1
and b
2
subunits pivot during the quaternary change.
c. The T State Is Stabilized by a Network of Salt
Bridges That Must Break to Form the R State
The R state is stabilized by ligand binding. But in the
absence of ligand, why is the T state more stable than the
R state? In the electron density maps of R-state Hb, the C-
terminal residues of each subunit (Arg 141␣ and His 146)
appear as a blur, which suggests that these residues are free
to wave about in solution.Maps of the T form,however,show
these residues firmly anchored in place via several intersub-
unit and intrasubunit salt bridges, which evidently help stabi-
lize the T state (Fig. 10-18). The structural changes accompa-
nying the T S R transition tear away these salt bridges in a
process driven by the Fe¬O
2
bonds’ energy of formation.
d. Hemoglobin’s O
2
-Binding Cooperativity Derives
from the T S R Conformational Shift
The hemoglobin molecule resembles a finely tooled
mechanism that has very little slop. The binding of O
2
re-
quires a series of tightly coordinated movements:
1. The Fe(II) of any subunit cannot move into its
heme plane without the reorientation of its proximal His
so as to prevent this residue from bumping into the por-
phyrin ring.
2. The proximal His is so tightly packed by its surround-
ing groups that it cannot reorient unless this movement is
accompanied by the previously described translation of the
F helix across the heme plane.
3. The F helix translation is only possible in concert
with the quaternary shift that steps the ␣
1
C–
2
FG contact
one turn along the ␣
1
C helix.
4. The inflexibility of the ␣
1
–
1
and ␣
2
–
2
interfaces re-
quires that this shift simultaneously occur at both the ␣
1
–
2
and the ␣
2
–
1
interfaces.
Consequently, no one subunit or dimer can greatly
change its conformation independently of the others. In-
deed, the two stable positions of the a
1
C–b
2
FG contact limit
the Hb molecule to only two quaternary forms, R and T.
We are now in a position to structurally rationalize hemo-
globin’s O
2
-binding cooperativity. Any deoxyHb subunit
binding O
2
is constrained to remain in the t state by the T
conformation of the tetramer. However, the t state has re-
duced O
2
affinity, most probably because its Fe¬O
2
bond is
stretched beyond its normal length by the steric repulsions be-
tween the heme and the O
2
and in the b subunits, by the need
to move Val E11 out of the O
2
-binding site. As more O
2
is
bound to the Hb tetramer,this strain,which derives from the
Fe¬O
2
bond energy, accumulates in the liganded subunits
until it is of sufficient strength to snap the molecule into the
R conformation. All the subunits are thereby converted to the
r state whether or not they are liganded. Unliganded subunits
in the r state have an increased O
2
affinity because they are al-
ready in the O
2
-binding conformation. This accounts for the
high O
2
affinity of nearly saturated Hb.
338 Chapter 10. Hemoglobin: Protein Function in Microcosm
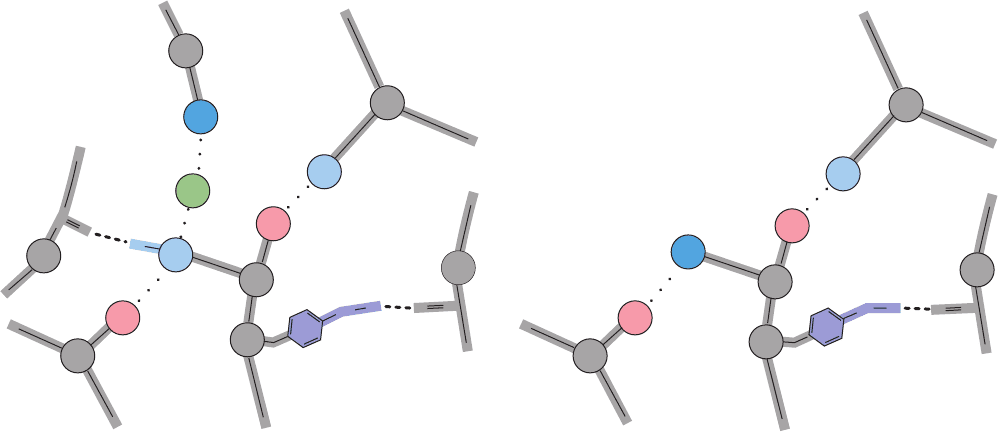
Figure 10-18 Networks of salt bridges and hydrogen bonds in
deoxyHb. These bonds, which involve the last two residues of (a)
the ␣ chains and (b) the  chains, are all ruptured in the T S R
transition.The two groups that participate in the Bohr effect by
Val
Val
Lys
Val
β
2
α
2
α
1
α
2
α
1
α
2
Asp
Tyr
Arg
N-terminal
+
127
Cl
-
34
126
_
+
_
141
140
+
93
C
O
H
O
H
O
C
C-terminal
1
(a) α Chains
Val
Lys
β
2
β
2
α
1
Asp
Tyr
His
40
94
_
_
146
145
+
98
O
H
O
C
C-terminal
β
2
+
(b) β Chains
becoming partially deprotonated in the R state are indicated by
white plus signs. [Illustration, Irving Geis. Image from the Irving
Geis Collection, Howard Hughes Medical Institute. Reprinted
with permission.]
JWCL281_c10_323-358.qxd 8/10/10 9:41 AM Page 338
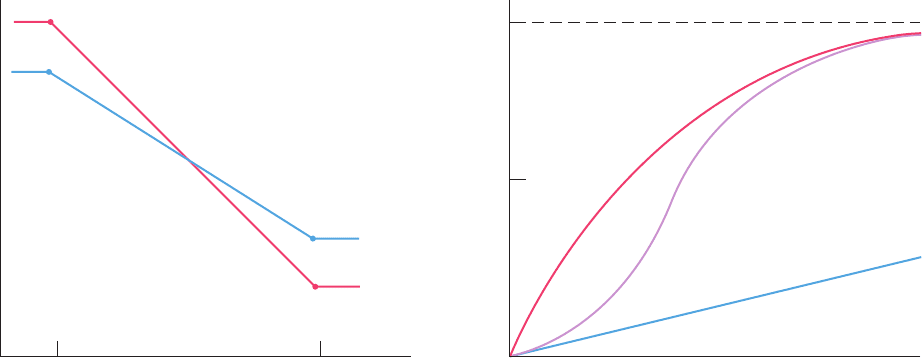
e. Hemoglobin’s Sigmoidal O
2
-Binding Curve Is a
Composite of Its Hyperbolic R- and T-State Curves
The relative stabilities of the T and R states, as indicated
by their free energies, vary with fractional saturation
(Fig. 10-19a). In the absence of ligand, the T state is more
stable than the R state, and vice versa when all ligand-
binding sites are occupied.The formation of Fe¬O
2
bonds
causes the free energy of both the T and the R states to de-
crease (become more stable) with oxygenation, although
the rate of this decrease is smaller for the T state as a result
of the strain that liganding imposes on t-state subunits. The
R 12 T transformation is, of course, an equilibrium process,
so that Hb molecules, at intermediate levels of fractional
saturation (1, 2, or 3 bound O
2
molecules), continually in-
terconvert between the R and the T states.
The O
2
-binding curve of Hb can be understood as a com-
posite of those of its R and T states (Fig. 10-19b). For pure
states, such as R or T, these curves are hyperbolic because lig-
and binding at one protomer is unaffected by the state of
other protomers in the absence of a quaternary structural
change.At low pO
2
’s,Hb follows the low-affinity T-state curve
and at high pO
2
’s, it follows the high-affinity R-state curve.At
intermediate pO
2
’s, Hb exhibits an O
2
affinity that changes
from T-like to R-like as pO
2
increases. The switchover results
in the sigmoidal shape of hemoglobin’s O
2
-binding curve.
D. Testing the Perutz Mechanism
The Perutz mechanism is a description of the dynamic be-
havior of Hb that is largely based on the static structures
of its R and T end states. Accordingly, without the direct
demonstration that Hb actually follows the postulated
pathway in changing conformational states, the Perutz
mechanism must be taken as being at least partially con-
jectural. Unfortunately, the physical methods that can fol-
low dynamic changes in proteins are, as yet, incapable of
providing detailed descriptions of these changes. Never-
theless, certain aspects of the Perutz mechanism are sup-
ported by static measurements, as is described below and
in Section 10-3.
a. C-Terminal Salt Bridges Are Required
to Maintain the T State
The proposed function of the C-terminal salt bridges in
stabilizing the T state has been corroborated by chemically
modifying human Hb. Removal of the C-terminal Arg 141␣
(by treating isolated ␣ chains with carboxypeptidase B fol-
lowed by reconstitution) drastically reduces the cooperativ-
ity of O
2
binding (Hill constant of 1.7, reduced from its nor-
mal value of 2.8). Cooperativity is abolished by the further
removal of the other C-terminal residue, His 146 (Hill con-
stant of ⬃1.0). Apparently, in the absence of its C-terminal
salt bridges, the T form of Hb is unstable. Indeed, human
deoxy-Hb, with its C-terminal residues removed, crystal-
lizes in a form very similar to that of normal human oxyHb.
b. Fe¬O
2
Bond Tension Has Been
Spectroscopically Demonstrated
If movement of the Fe into the heme plane on oxygena-
tion is mechanically coupled via the proximal His to the
T S R transformation, then conversely, forcing oxyHb into
the T form must exert a tension on the Fe, through the prox-
imal His, that tends to pull the Fe out of the heme plane. Pe-
rutz demonstrated the existence of this tension as follows.
Section 10-2. Structure and Mechanism 339
Figure 10-19 Free energy and saturation curves for O
2
binding
to hemoglobin. (a) The variation of the free energies of
hemoglobin’s T and R states with their fractional saturation,
In the absence of O
2
the T state is more stable, and when
saturated with O
2
the R state is more stable. The free energy of
both states is reduced with increasing oxygenation as a
consequence of O
2
liganding.The Fe(II)¬O
2
bonding is more
Y
O
2
.
(a) (b)
R State
T State
R State
Hb
T State
Free
energy,
10
R
0
T
0
R
4
T
4
0.5
1.0
G
pO
2
Y
O
2
Y
O
2
exergonic in the R state than it is in the T state, however, so that
the relative stabilities of these two states reverse order at
intermediate levels of oxygenation. (b) The sigmoid O
2
-binding
curve of Hb (purple) is a composite of its hyperbolic R-state
(red) and T-state (blue) binding curves: It is more T-like at lower
pO
2
values and more R-like at higher pO
2
values.
JWCL281_c10_323-358.qxd 2/24/10 1:58 PM Page 339
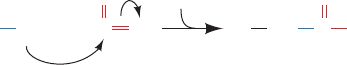
IHP’s six phosphate groups cause it to bind to deoxyHb with
much greater affinity than does BPG (the structural basis of
BPG binding to Hb is discussed in Section 10-2F); the pres-
ence of IHP therefore tends to force Hb into the T state.
Conversely, nitric oxide (NO) binds to Hb far more strongly
than does O
2
and thereby tends to force Hb into the R state.
Spectroscopic analysis indicates the consequences of simul-
taneously binding both NO and IHP to Hb:
1. The NO, as expected, pulls the Fe into the plane of
the heme.
2. The IHP forces the Hb molecule into the T state,
which through the “gears and levers” coupling the 4° and 3°
conformational changes, pulls the proximal His in the
opposite direction, away from the Fe.
The bond between the proximal His and the Fe lacks the
strength to withstand these two opposing “irresistible”
forces; it simply breaks. The spectroscopic observation of
this phenomenon therefore confirms the existence of the
heme–protein tension predicted by the Perutz mechanism.
c. Detaching the Proximal His from the F Helix
Eliminates Most Cooperativity
In a further experimental investigation of the origin of
cooperativity in hemoglobin, Chien Ho mutagenically
changed the proximal His residue to Gly on only the ␣ sub-
units, on only the  subunits, and on both the ␣ and  sub-
units. The missing imidazole ring of the proximal His was
then replaced by imidazole (which a variety of evidence in-
dicates ligands the heme Fe as does the proximal His). This,
in effect, detaches the proximal His from the protein,
thereby cutting the covalent bond that, according to the Pe-
rutz model, links the ligand-induced movement of the Fe
into the heme plane to the accompanying movement of he-
lix F. In all three cases, this proximal detachment, in agree-
ment with the Perutz model, significantly increases hemo-
globin’s ligand-binding affinity,reduces its cooperativity, and
prevents its T S R quaternary switch. However, these mu-
tant hemoglobins exhibit a small amount of residual cooper-
ativity, suggesting that the heme groups also communicate
via pathways that do not require covalent coupling between
the F helix and the proximal His. These pathways may in-
volve movements of protein groups in contact with the heme
(see Figs. 10-12 and 10-15) in response to the subsidence of
heme doming on ligand binding. They may also involve
movements of the distal His residues of the ␣ and  subunits,
and/or the movement of Val E11 of the  subunits, all of
whose side chains must move aside when ligand binds to Hb.
E. Origin of the Bohr Effect
The Bohr effect, hemoglobin’s release of H
⫹
on binding O
2
,
is also observed when Hb binds other ligands. It arises from
pK changes of several groups caused by changes in their
local environments that accompany hemoglobin’s T S R
transition. The groups involved include the N-terminal
amino groups of the ␣ subunits and the C-terminal His of
the  subunits. These have been identified through chemi-
cal and structural studies, and their quantitative contribu-
tions to the Bohr effect have been estimated.
Reaction of the ␣ subunits of Hb with cyanate results in
the specific carbamoylation of the N-terminal amino
groups (Fig. 10-20). When such carbamoylated ␣ subunits
are mixed with normal  subunits, the resulting reconsti-
tuted Hb lacks 20 to 30% of the normal Bohr effect. The
reason for this is seen on comparing the X-ray structure
of deoxyHb with that of carbamoylated deoxyHb. In
deoxyHb, a Cl
⫺
ion binds between the N-terminal amino
group of Val 1␣
2
and the guanidino group of Arg 141␣
1
(the
C-terminal residue; Fig. 10-18a). This Cl
⫺
is absent in car-
bamoylated deoxyHb. It is also absent in normal R-state
Hb because its C-terminal residues are not held in place by
salt bridges (which partially accounts for the preferential
binding of Cl
⫺
to deoxyHb; Section 10-1C). N-Terminal
amino groups of polypeptides normally have pK’s near 8.0.
On deoxyHb ␣ subunits, however, the N-terminal amino
group is electrostatically influenced by its closely associ-
ated Cl
⫺
to increase its positive charge by binding protons
more tightly, that is, to increase its pK. Since at the pH of
blood (7.4) N-terminal amino groups are normally only
partially charged, this pK shift causes them to bind signifi-
cantly more protons in the T state than in the R state.
The Hb  chain also contributes to the Bohr effect. Re-
moval of its C-terminal residue, His 146, reduces the Bohr
effect by 40%. In normal deoxyHb, the imidazole ring of
His 146 associates with the carboxylate of Asp 94 on the
same subunit (Fig. 10-18b) to form a salt bridge that is ab-
sent in the R state. Proton NMR measurements indicate
that formation of this salt bridge increases the pK of the
imidazole group from 7.1 to 8.0. This effect more than ac-
counts for His 146’s share of the Bohr effect.
We have not yet accounted for about 30 to 40% of the
Bohr effect. It is largely due to small contributions from the
numerous surface-exposed His residues whose environ-
ments are altered on hemoglobin’s T S R transition [since
His is the only residue with an intrinsic pK (6.04) in the
physiological range, small changes in its pK will signifi-
cantly alter the number of protons it binds]. Indeed, NMR
measurements by Ho indicate that the T S R transition in-
duces small shifts in the pK’s of these various His residues,
although, interestingly, some of these shifts are in the direc-
tion that diminishes the magnitude of the Bohr effect.
340 Chapter 10. Hemoglobin: Protein Function in Microcosm
Figure 10-20 Reaction of cyanate with the unprotonated
(nucleophilic) forms of primary amino groups. At physiological
pH’s, N-terminal amino groups, which have pK’s near 8.0, readily
react with cyanate. Lys ε-amino groups (pK ⬇ 10.8), however, are
fully protonated under these conditions and are therefore
unreactive.
C
O
R NH
2
+ N
–
R NH
C
O
NH
2
Terminal
amino
group
Cyanate Carbamoylated
terminal amino
group
..
H
+
JWCL281_c10_323-358.qxd 2/24/10 1:58 PM Page 340
