Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

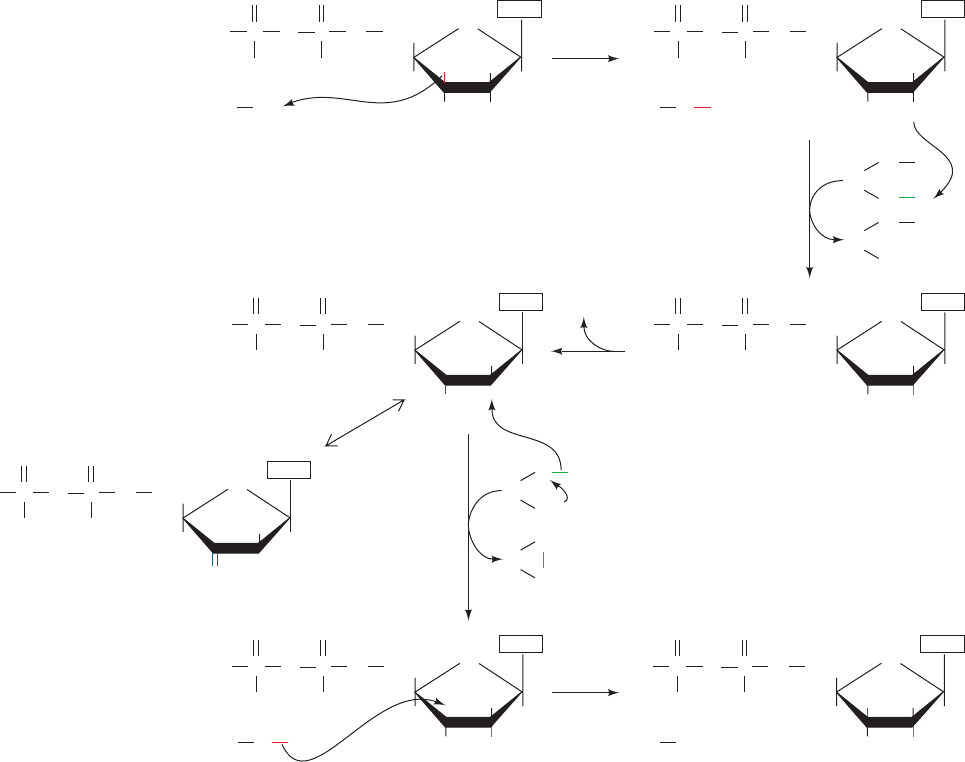
formulate the following catalytic mechanism for E. coli
RNR (Fig. 28-13):
1. RNR’s free radical (X) abstracts an H atom from
C3¿ of the substrate in the reaction’s rate-determining step.
2 and 3. Acid-catalyzed cleavage of the C2¿¬OH bond
releases H
2
O to yield a radical cation intermediate. The
radical mediates the stabilization of the C2¿ cation by the
3¿¬OH group’s unshared electron pair, thereby account-
ing for the radical’s catalytic role.
4. The radical cation intermediate is reduced by the en-
zyme’s redox-active sulfhydryl pair to yield a 3¿-deoxynu-
cleotide radical and a protein disulfide group.
5. The 3¿ radical reabstracts an H atom from the protein
to yield the product deoxynucleoside diphosphate and re-
store the enzyme to its radical state.A small fraction of the
originally abstracted H atom exchanges with solvent be-
fore it can be replaced, thus accounting for the release of
3
H on reduction of [3¿-
3
H]UDP.
The Tyr 122 radical in R2 is too far away (10 Å) from the
enzyme’s catalytic site to abstract an electron directly from
the substrate. Evidently, the protein mediates electron
transfer from this tyrosyl radical to some other group (X in
Fig. 28-13) that is in close proximity to the substrate
C3¿¬H group. Site-directed mutagenesis studies suggest
that Cys 439 of R1, in its thiyl radical form (—S), is the
most plausible candidate for X (which makes RNR the
only enzyme in which a Cys residue is known to reduce a
carbohydrate substrate). Similar studies suggest that Cys
225 and Cys 462 of R1 form the redox-active sulfhydryl
pair that directly reduces substrate. Moreover,the resulting
disulfide bond is subsequently reduced to regenerate ac-
tive enzyme via disulfide interchange with Cys 754 and Cys
759 on R1, which are apparently positioned to accept elec-
trons from external reducing agents (see below).Thus, each
R1 subunit contains at least five Cys residues that chemi-
cally participate in nucleotide reduction.
These observations are confirmed by the X-ray struc-
ture of R1 in complex with R2’s 20-residue C-terminal
Section 28-3. Formation of Deoxyribonucleotides 1121
Figure 28-13 Enzymatic mechanism of ribonucleotide
reductase. The reaction occurs via a free radical–mediated
process in which reducing equivalents are supplied by the
X
C
H
H
OHOH
O
H
Base
H
2
POP OO
–
O
–
O
–
S
S
O
O
EX•
H
C
•
H
OHOH
O
H
Base
H
2
POP OO
–
O
–
O
–
O
O
EX
H
H
E
H
H
S
S
E
H
–
C
•
H
+
OHOH
O
H
Base
H
2
POP OO
–
O
–
O
–
O
O
H
2
C
•
H
OH
O
H
Base
H
2
POP OO
–
O
–
O
–
O
O
H
+
C
•
H
OH
O
H
Base
H
2
POP OO
–
O
–
O
–
O
O
H
+
H
2
O
S
S
E
H
S
S
E
–
:
C
•
H
OH
O
H
Base
H
2
POP OO
–
O
–
O
–
O
O
EX
H
H
H
C
•
H
OH
O
H
Base
H
2
POP OO
–
O
–
O
–
O
O
E
H
H
H
1
2
3
4
5
3 2
formation of an enzyme disulfide bond. [After Stubbe, J.A., Biol.
Chem. 265, 5330 (1990).]
JWCL281_c28_1107-1142.qxd 4/22/10 9:16 AM Page 1121
polypeptide (R1 does not crystallize satisfactorily in the
absence of this polypeptide), also determined by Eklund
(Fig. 28-12d). The central domain of the three-domain R1
monomer consists of a novel 10-stranded ␣/ barrel that is
formed by the antiparallel joining of two topologically sim-
ilar half-barrels, each comprising five parallel  strands
connected by four ␣ helices. As with the similar 8-stranded
␣/ barrels that form the active sites of numerous enzymes
(Section 8-3Bh), R1’s active site Cys residues (439, 225,
462) are located in the mouth of the 10-stranded ␣/ barrel.
The two R1 Cys residues, 754 and 759, that are impli-
cated in the regeneration of the active enzyme are compo-
nents of R1’s C-terminal segment, which is not visible in
the X-ray structure of R1 and is presumably disordered.
This observation supports the hypothesis that this C-terminal
segment acts to flexibly shuttle reducing equivalents from
the enzyme surface to its active site.
b. Radical Generation in Class I RNR Requires
the Presence of O
2
One of the most remarkable aspects of Class I RNR is
its ability to stabilize its normally highly reactive TyrOⴢ
radical (its half-life is 4 days in the protein vs milliseconds
in solution). Yet quenching the radical, say, by hydrox-
yurea, inactivates the enzyme.How, then, is the radical gen-
erated in the first place? The radical may be restored in
vitro by simply treating the inactive enzyme with Fe(II)
and a reducing agent in the presence of O
2
.
This is a four-electron reduction of O
2
in which the reduc-
ing agent that supplies the electron represented by e
–
may
be ascorbate or even excess Fe
2⫹
.
c. The Inability of Oxidized RNR to Bind Substrate
Serves an Important Protective Function
Comparison of the X-ray structures of reduced R1 (in
which the redox-active Cys 225 and Cys 462 residues are in
their SH forms) with that of oxidized R1 (in which Cys 225
and Cys 462 are disulfide linked) reveals that Cys 462 in re-
duced R1 has rotated away from its position in oxidized R1
to become buried in a hydrophobic pocket, whereas Cys 225
moves into the region formerly occupied by Cys 462.The dis-
tance between the formerly disulfide-linked S atoms thereby
increases from 2.0 to 5.7 Å.These movements are accompa-
nied by small shifts of the surrounding polypeptide chain.
Oxidized RNR does not bind substrate because its R1 Cys
OO
2
H
Tyr 122-R2
O•
++++2Fe
2+
+ Fe
3+
+ H
2
OFe
3+
O
2
_
H
+
e
_
Tyr 122-R2
225 would prevent the binding of substrate through steric in-
terference of its S atom with the substrate NDP’s O2¿ atom.
The inability of oxidized RNR to bind substrate has
functional significance. In the absence of substrate, the en-
zyme’s free radical is stored in the interior of the R2 pro-
tein, close to its dinuclear iron center. When substrate is
bound, the radical is presumably transferred to it via a se-
ries of protein side chains in both R2 and R1. If the sub-
strate is unable to properly react after accepting this free
radical, as would be the case if RNR were in its oxidized
state, this could result in the destruction of the substrate
and/or the enzyme. Indeed, the mutation of the redox-
active Cys 225 to Ser results in an enzyme that permits the
formation of the substrate radical (Fig. 28-13); however,
since the mutant enzyme is incapable of reducing it,the sub-
strate radical instead decomposes followed by the release of
its base and phosphate moieties. More importantly, a tran-
sient peptide radical forms, which cleaves and inactivates
the R1 polypeptide chain while consuming the radical and
thereby inactivating R2. Thus, an important role of the en-
zyme is to control the release of the radical’s powerful oxi-
dizing capability. It does so in part by preventing the bind-
ing of substrate while the enzyme is in its oxidized form.
d. Ribonucleotide Reductase Is Regulated by
Effector-Induced Oligomerization
The synthesis of the four dNTPs in the amounts re-
quired for DNA synthesis is accomplished through feed-
back control. The maintenance of the proper intracellular
ratios of dNTPs is essential for normal growth. Indeed, a
deficiency of any dNTP is lethal, whereas an excess is muta-
genic because the probability that a given dNTP will be
erroneously incorporated into a growing DNA strand
increases with its concentration relative to those of the other
dNTPs.
The activities of both E. coli and mammalian Class I
RNRs are allosterically responsive to the concentrations of
various (d)NTPs. Thus, as Reichard has shown, ATP in-
duces the reduction of CDP and UDP; dTTP induces the
reduction of GDP and inhibits the reduction of CDP and
UDP; dGTP induces the reduction of ADP and, in mam-
mals but not E. coli, inhibits the reduction of CDP and
UDP; and dATP inhibits the reduction of all NDPs.
Barry Cooperman has shown that the catalytic activity
of mouse RNR varies with its state of oligomerization (that
is, RNR is a morpheein; Section 26-4Ac), which in turn is
governed by the binding of nucleotide effectors to three in-
dependent allosteric sites on R1: (1) the specificity site,
which binds ATP, dATP, dGTP, and dTTP; (2) the activity
site, which binds ATP and dATP; and (3) the hexameriza-
tion site, which binds only ATP. On the basis of molecular
mass, ligand binding, and activity studies on mouse RNR,
Cooperman formulated a model that quantitatively ac-
counts for the allosteric regulation of Class I RNR. It has
the following features (Fig. 28-14a):
1. The binding of ATP, dATP, dGTP, or dTTP to the
specificity site induces the catalytically inactive R1
monomers to form a catalytically active dimer, R1
2
.
1122 Chapter 28. Nucleotide Metabolism
JWCL281_c28_1107-1142.qxd 8/9/10 9:46 AM Page 1122
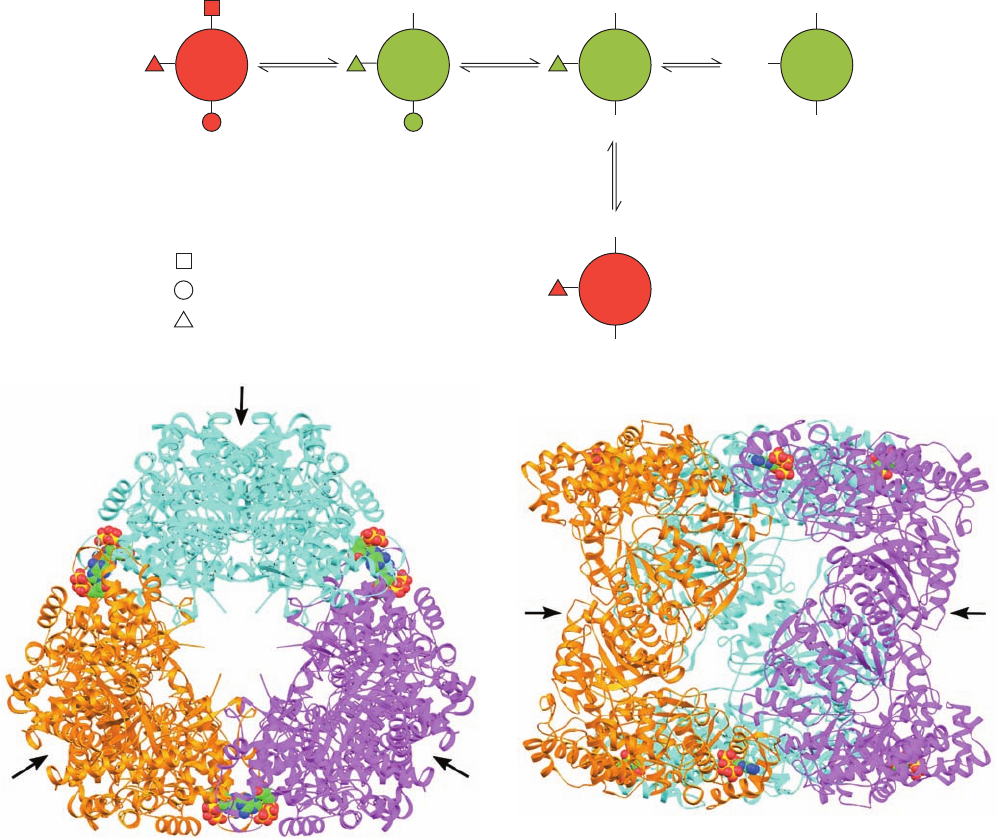
2. The binding of dATP or ATP to the activity site
causes the dimers to form catalytically active tetramers,
R1
4a
, that slowly but reversibly change conformation to a
catalytically inactive state, R1
4b
.
3. The binding of ATP to the hexamerization site in-
duces the tetramers to further aggregate to form catalyti-
cally active hexamers, R1
6
, RNR’s major active form.
The concentration of ATP in a cell is such that, in vivo,
R1 is almost entirely in its tetrameric or hexameric forms.
As a consequence, ATP couples the overall rate of DNA
synthesis to the cell’s energy state.
The specificity and activity sites have been located in
X-ray structures of E. coli R1 (Fig. 28-12d);the hexameriza-
tion site has not yet been identified.The R1 hexamer had, in
fact, been previously observed in the X-ray structures of R1
(Fig. 28-14b,c), but the interactions between its contacting
dimers are so tenuous that it was assumed that they are
merely artifacts of crystallization with no physiological sig-
nificance.Yet, since the activity site is located at this contact
Section 28-3. Formation of Deoxyribonucleotides 1123
Figure 28-14 Ribonucleotide reductase regulation. (a) A
model for the allosteric regulation of Class I RNR via its
oligomerization. States shown in green have high activity and
those shown in red have little or no activity. R2 has been omitted
for simplicity. [After Kashlan, O.B., Scott, C.P., Lear, J.D., and
Cooperman, B.S., Biochemistry 41, 461 (2002).] (b) The X-ray
structure of the R1 hexamer, which has D
3
symmetry, in complex
with AMPPNP as viewed along its 3-fold axis. Each of its three
dimers are differently colored (the X-ray structure of a dimer is
ATP
dATP
dGTP
dTTP
(d)NTP
dATP
ATP
ATP
ATP
R1 R1
2
d(NTP)
R1
4b
(d)NTP
R1
6
(d)NTP
(d)ATP
(d)ATP
(d)ATP
R1
4a
Specificity site
Activity site
Hexamerization site
(slow)
shown in Fig. 28-12d). The AMPPNP, which binds to the
enzyme’s activity sites, is drawn in space-filling form with
C green, N blue, O red, and P gold.The black arrows point along
the R1 dimers’ 2-fold axes and indicate the probable docking
sites for the binding of R2 dimers. (c) The R1 AMPPNP
hexamer as viewed along the vertical 2-fold axis in Part b. [Parts
b and c based on an X-ray structure by Hans Eklund, Swedish
University of Agricultural Sciences, Uppsala, Sweden. PDBid
3R1R.]
(a)
(b)
(c)
JWCL281_c28_1107-1142.qxd 4/22/10 9:16 AM Page 1123
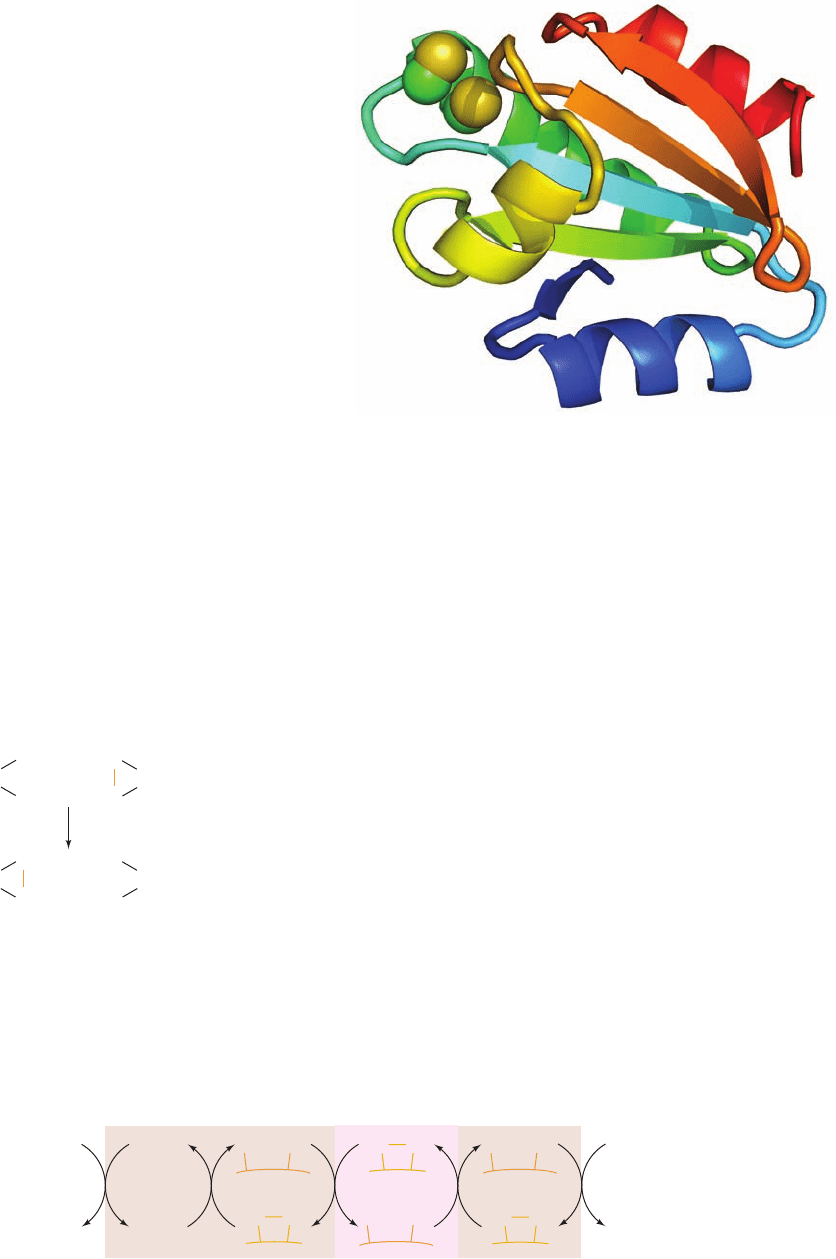
site, it now seems likely that its binding of (d)ATP induces
R1 oligomerization through local conformational changes.
The foregoing model has, for simplicity, neglected the
presence of R2 subunits although, of course, R1 and R2
must be present in equimolar amounts in the active en-
zyme. Presumably, the R1 and R2 dimers bind to one an-
other such that their 2-fold axes coincide.The lack of space
on the inside of the R1 hexamer dictates that the R2 dimers
must contact the R1 dimers from outside the hexamer (Fig.
28-14b).
dCTP is not an effector of RNR. This is presumably be-
cause the intracellular balance between dCTP and dTTP is
not controlled by RNR but, rather,is maintained by deoxy-
cytidine deaminase, which converts dCTP to dUMP, the
precursor of dTTP. This enzyme is activated by dCTP and
inhibited by dTTP.
e. Thioredoxin and Glutaredoxin Are Class I
Ribonucleotide Reductase’s Physiological
Reducing Agents
The final step in the RNR catalytic cycle is the reduction of
the enzyme’s newly formed disulfide bond to reform its re-
dox-active sulfhydryl pair. Dithiols such as that of
2-mercaptoethanol (Section 7-1B) can serve as the reducing
agent for this process in vitro through a disulfide interchange
reaction. One of the enzyme’s physiological reducing agents,
however, is thioredoxin (Trx), a ubiquitous monomeric 105-
residue protein that has a pair of closely proximal redox-
active Cys residues, Cys 32 and Cys 35 (we have previously
encountered thioredoxin in our study of the light-induced
activation of the Calvin cycle; Section 24-3B). Thioredoxin
reduces oxidized RNR via disulfide interchange.
The X-ray structure of reduced E. coli Trx (Fig. 28-15)
reveals that the side chain of the redox-active Cys 32 is
exposed on the protein’s surface, where it is available for
oxidation. Oxidized thioredoxin is, in turn, reduced by
Thioredoxin
(reduced)
Ribonucleotide
reductase
(oxidized)
+
+
S
S
S
S
Ribonucleotide
reductase
(reduced)
Thioredoxin
(oxidized)
HS
SH
SH
HS
NADPH in a reaction mediated by the flavoprotein thiore-
doxin reductase. NADPH therefore serves as the terminal
reducing agent in the RNR-mediated reduction of NDPs
to dNDPs (Fig. 28-16).
The existence of a viable E. coli mutant devoid of thiore-
doxin indicates that this protein is not the only substance
capable of reducing oxidized RNR in vivo. This observation
led to the discovery of glutaredoxin, a disulfide-containing,
monomeric, 85-residue protein that can also reduce RNR
(mutants devoid of both thioredoxin and glutaredoxin are
nonviable). Oxidized glutaredoxin is reduced, via disulfide
interchange, by the Cys-containing tripeptide glutathione
which, in turn, is reduced by NADPH as catalyzed by
glutathione reductase (GR; Section 21-2Ba). The relative
1124 Chapter 28. Nucleotide Metabolism
Figure 28-15 X-ray structure of human thioredoxin in its
reduced (sulfhydryl) state. The 105-residue polypeptide chain is
drawn in ribbon form colored in rainbow order from its N-terminus
(blue) to its C-terminus (red).The side chains of the redox-active
residues, Cys 32 and Cys 35, are shown in space-filling form with
C green and S yellow. This structure closely resembles those of
the homologous a and a¿ domains of protein disulfide isomerase
(PDI, Fig. 9-16). [Based on an X-ray structure by William
Montfort, University of Arizona. PDBid 1ERT.]
Figure 28-16 Electron-transfer pathway for nucleoside
diphosphate (NDP) reduction. NADPH provides the reducing
NADPH
NADP
+
FAD
FADH
2
SH SH
S
S
NDP
dNDP
SH SH
SH SH
S
S
S
S
Thioredoxin reductase Thioredoxin Ribonucleotide
reductase
equivalents for this process through the intermediacy of
thioredoxin reductase, thioredoxin, and ribonucleotide reductase.
JWCL281_c28_1107-1142.qxd 4/22/10 9:16 AM Page 1124
importance of thioredoxin and glutaredoxin in the reduc-
tion of RNRs remains to be established.
f. Thioredoxin Reductase Alternates Its
Conformation with Its Redox State
Thioredoxin reductase (TrxR), a homodimer of 316-
residue subunits, is a homolog of GR that catalyzes a simi-
lar reaction: the reduction of a substrate disulfide bond by
NADPH as mediated by an FAD prosthetic group and a
redox active sulfhydryl pair (Cys 135 and Cys 138). How-
ever, the X-ray structure of the C138S mutant of E. coli
TrxR in complex with NADP
(Fig. 28-17a), determined by
Charles Williams and John Kuriyan, reveals that TrxR and
GR differ in their active site arrangements such that their
redox-active sulfhydryl pairs are on opposite sides of the
flavin rings in the two enzymes. Nevertheless,TrxR’s redox-
active sulfhydryl pair appears properly positioned to re-
duce the flavin ring. However, the NADP
’s nicotinamide
ring is 17 Å from the flavin ring and the redox-active
sulfhydryl pair is buried such that it could not react with
the enzyme’s Trx substrate. How then does TrxR manage to
transfer an electron pair from its bound NADPH via its
flavin ring and redox-active sulfhydryl pair to Trx?
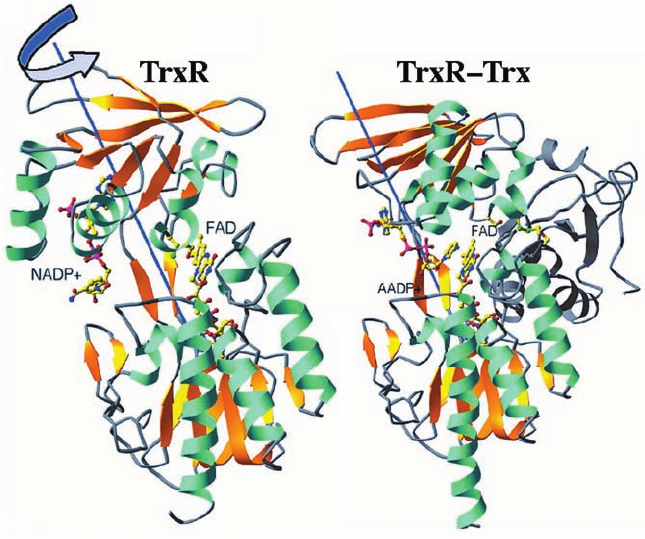
This question was answered by Williams and Martha
Ludwig through their X-ray structure determination of the
C135S mutant of TrxR, whose Cys 138 is disulfide-linked to
Cys 32 of the C35S mutant of E. coli Trx (probably the
physiologically relevant disulfide bond) and which is in
complex with the NADP
analog 3-aminopyridine adenine
dinucleotide phosphate (AADP
). In this complex (Fig.
28-17b),TrxR’s NADP
-binding domain has rotated by 67°
Section 28-3. Formation of Deoxyribonucleotides 1125
Figure 28-17 X-ray structures of E. coli
thioredoxin reductase (TrxR). (a) The C138S
mutant TrxR in complex with NADP
.The
protein is shown in ribbon form colored
according to its secondary structure. The
NADP
, the FAD, and the side chains of
Cys 135 and Ser 138 are drawn in ball-and-
stick form with C yellow, N blue, O red, S
green, and P magenta. (b) The C135S
mutant TrxR in complex with AADP
and
covalently linked to the C35S mutant of Trx
via a disulfide bond between TrxR Cys 138
and Trx Cys 32. The TrxR is represented as in
Part a, the Trx ribbon is blue-gray, and its
Cys 32 and Ser 35 side chains are drawn in
ball-and-stick form. Comparison of these
two structures reveals that TrxR’s
NADP
-binding domain (residues 120–243)
undergoes a 67° rotation about the axis
drawn in blue relative to the rest of the
protein, which is shown in the same
orientation in both structures. [Courtesy of
Martha Ludwig, University of Michigan.
PDBids (a) 1TDF and (b) 1F6M.]
(a)
(b)
relative to the rest of the protein compared to its position
in TrxR alone (Fig. 28-17a). This positions the AADP
’s
pyridine ring to react with the flavin ring and positions
TrxR’s redox-active sulfhydryl pair to undergo a disulfide
interchange reaction with that of Trx. Moreover, in this
latter conformation, the NADP
-binding domain appears
to provide the recognition site for the substrate Trx.
Evidently, TrxR alternates its conformation with each suc-
cessive step in the process of transferring an electron pair
from NADPH to the flavin to its redox-active sulfhydryl
pair to its bound Trx substrate. This added mechanistic
complication relative to that of GR, which does not un-
dergo a significant conformational change in reducing
glutathione disulfide (Section 21-2Ba), has apparently
evolved to permit TrxR to reduce its protein substrate: Trx
would be too large for its redox-active sulfhydryl pair to
properly approach the active site sulfhydryl pair in a GR-
like enzyme.
g. The Three Classes of Ribonucleotide
Reductases Are Evolutionarily Related
We have seen that the active forms of Class I RNRs are
R1
2
R2
2
,R1
4
R2
4
, and R1
6
R2
6
oligomers that have mecha-
nistically essential tyrosyl radicals that are stabilized by
oxo-bridged binuclear Fe(III) complexes, have NDPs for
substrates, and obtain their reducing equivalents from
thioredoxin and glutaredoxin. In contrast, Class II RNRs,
which are monomers or
2
dimers, utilize a 5¿-deoxyadeno-
sylcobalamin cofactor (coenzyme B
12
; Section 25-2Eb) for
radical generation,have NDPs for substrates, and are reduced
by thioredoxin and glutaredoxin; whereas Class III RNRs,
JWCL281_c28_1107-1142.qxd 4/22/10 9:16 AM Page 1125
which are
2
dimers that interact with a radical-generating
protein
2
that contains a [4Fe–4S] cluster and requires
S-adenosylmethionine (SAM; Section 26-3Ea) and NADPH
for activity, have NTPs for substrates, and their reducing
equivalents are provided by the oxidation of formate to CO
2
.
Since all known cellular life synthesizes its deoxy-
ribonucleotides from ribonucleotides, the rise of an RNR
must have preceded the evolutionary transition from the
RNA world (Section 1-5Ca) to DNA-based life-forms. Did
the three classes of RNRs arise independently or are they
evolutionarily related? Despite the seemingly large differ-
ences between these different classes of RNRs, the reac-
tions they catalyze are surprisingly similar. All replace the
2¿ OH group of ribose with H via a free radical mechanism
involving a thiyl radical with the reducing equivalents pro-
vided by a Cys sulfhydryl group (Fig. 28-13; the second Cys
residue of the redox-active sulfhydryl pair in Class I and II
RNRs is replaced by formate in Class III RNRs).They dif-
fer mainly in the way they generate the free radical. [In
Class II RNRs, the radical is generated by the homolytic
cleavage of its 5¿-deoxyadenosylcobalamin cofactor’s
C¬Co(III) bond (Section 25-2Ec). In Class III RNRs, it is
generated by the NADPH-supplied and [4Fe–4S] cluster–
mediated one-electron reductive cleavage of SAM by the
2
protein to yield methionine and the 5¿-deoxyadenosyl
radical (the same radical generated by the homolytic cleav-
age of 5¿-deoxyadenosylcobalamin), which then abstracts
the H atom from a C
¬H group of a specific Gly on the
subunit to yield 5¿-deoxyadenosine and a stable but O
2
-
sensitive glycyl radical.] Moreover, the X-ray structures of
both Class II and Class III RNRs reveal that their active
sites are formed by 10-stranded / barrels that have the
same connectivity as and are closely superimposable on
that of Class I RNRs. It therefore appears that all three
classes of RNRs are evolutionarily related. Reichard has
proposed that, since life arose under anaerobic conditions
and that formate, one of simplest organic reductants, was
probably widely available on primitive Earth (Section
1-5B), the primordial RNR was a Class III–like enzyme.
The rise of photosynthetic organisms that generated O
2
then promoted the evolution of Class II RNRs, which can
function under both anaerobic and aerobic conditions.
Class I RNRs, which require the presence of O
2
for activa-
tion, evolved last, presumably from a Class II RNR.
h. dNTPs Are Produced by Phosphorylation
of dNDPs
In pathways involving Class I and Class II RNRs, the
final step in the production of dNTPs is the phosphorylation
of the corresponding dNDPs:
This reaction is catalyzed by nucleoside diphosphate
kinase, the same enzyme that phosphorylates NDPs (Sec-
tion 28-1Ba).As before, the reaction is written with ATP as
the phosphoryl donor, although any NTP or dNTP can
function in this capacity. In pathways involving Class III
RNRs, the production of NTPs from NDPs precedes the
reduction of NTPs to dNTPs.
dNDP ATP Δ dNTP ADP
B. Origin of Thymine
a. dUTP Diphosphohydrolase
The dTMP component of DNA is synthesized,as we dis-
cuss below, by methylation of dUMP. The dUMP is gener-
ated through the hydrolysis of dUTP by dUTP diphospho-
hydrolase (dUTPase; also called dUTP pyrophosphatase):
The reason for this apparently energetically wasteful
process (dTMP, once formed, is rephosphorylated to dTTP)
is that cells must minimize their concentration of dUTP in
order to prevent incorporation of uracil into their DNA.
This is because, as we discuss in Section 30-5Bd, DNA poly-
merase does not discriminate between dUTP and dTTP.
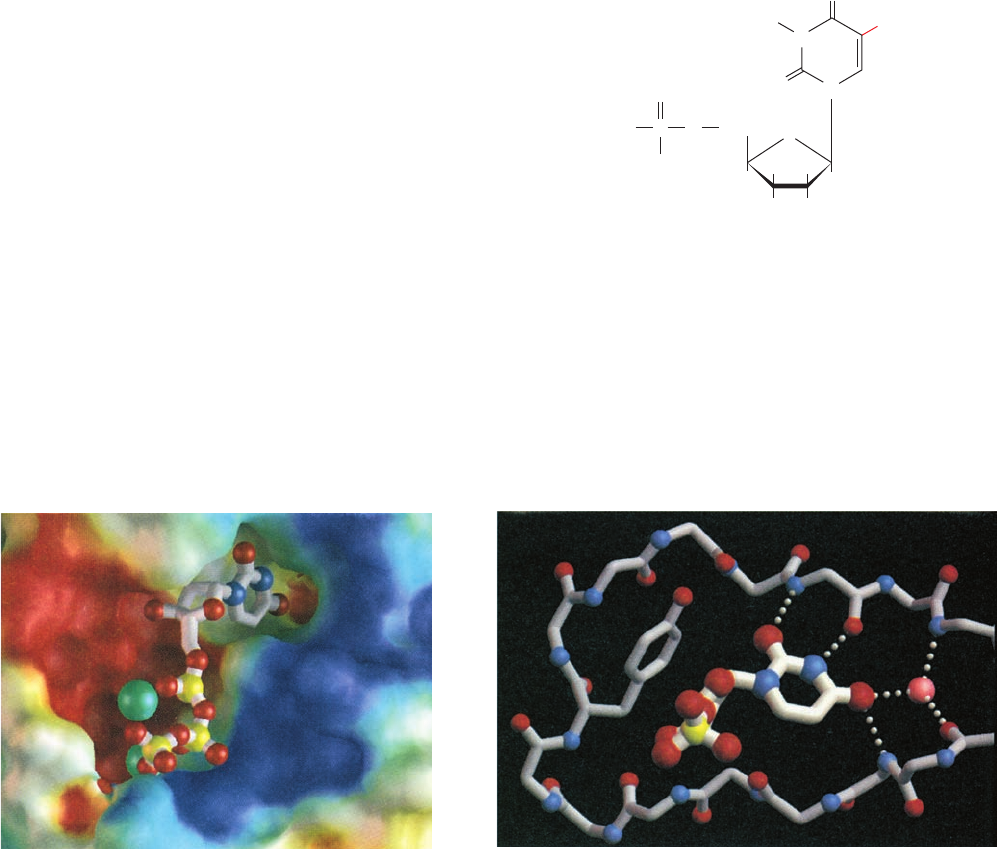
The X-ray structure of human dUTPase, determined by
John Tainer, reveals the basis for this enzyme’s exquisite
specificity for dUTP. This homotrimer of 141-residue subunits
binds dUTP in a snug-fitting cavity that sterically excludes
thymine’s C5 methyl group via the side chains of conserved
residues (Fig. 28-18a). It differentiates uracil from cytosine via
a set of hydrogen bonds from the protein backbone that
mimic adenine’s base-pairing interaction (Fig. 28-18b), and it
differentiates dUTP from UTP by the steric exclusion of
ribose’s 2¿ OH group by the side chain of a conserved Tyr.
b. Thymidylate Synthase
dTMP is synthesized from dUMP by thymidylate
synthase (TS) with N
5
,N
10
-methylenetetrahydrofolate
(N
5
,N
10
-methylene-THF) as the methyl donor:
(THF cofactors are discussed in Section 26-4D). Note that
the transferred methylene group (in which the carbon has
the oxidation state of formaldehyde) is reduced to a methyl
group (which has the oxidation state of methanol) at the
R =
C
COO
_
O
_
;
CH
2
H
2
N
H
2
C
H
2
N
H
N
N
N
H
N
R
CH
3
N
H
H
N
CH
2
CH
2
CH
2
CH
2
CH
2
H
N
C
dTMP
dUMP N
5
,N
10
-Methylenetetrahydrofolate
Dihydrofolate
H
N
H
N
O
()
n
n =
16
R
H
NH
H
N
N
N
dRib P
N
H
O
H
N
H
dRib P
O
+
+
8
7
6
5
9
10
5
6
O
O
O
C
O
O
dUTP H
2
O Δ dUMP PP
i
1126 Chapter 28. Nucleotide Metabolism
JWCL281_c28_1107-1142.qxd 7/21/10 7:09 PM Page 1126
expense of the oxidation of the THF cofactor to dihydro-
folate (DHF).
The catalytic mechanism of TS, a highly conserved ho-
modimeric protein (with 264-residue subunits in E. coli),
has been extensively investigated. On incubation of the en-
zyme with N
5
,N
10
-methylene-[6-
3
H]THF and dUMP, the
3
H is quantitatively transferred to the methyl group of
the product dTMP. When [5-
3
H]dUMP is the substrate,
however, the
3
H is released into the aqueous solvent. Such
information, together with the knowledge that uracil C6,
which occupies the  position of an ␣,-unsaturated ke-
tone, is susceptible to nucleophilic attack, led Daniel Santi
to propose the following mechanistic scheme for the TS
reaction (Fig. 28-19):
1. An enzyme nucleophile, identified as the thiolate
group of Cys 146, attacks C6 of dUMP to form a covalent
adduct.
2. C5 of the resulting enolate ion attacks the CH
2
group
of the iminium cation in equilibrium with N
5
,N
10
-methylene-
THF to form an enzyme–dUMP–THF ternary covalent
complex.
3. An enzyme base abstracts the acidic proton at the C5
position of the enzyme-bound dUMP, forming an exocyclic
methylene group and eliminating the THF cofactor. The
abstracted proton subsequently exchanges with solvent.
4. The redox change occurs via the migration of the
N6¬H atom of THF as a hydride ion to the exocyclic
methylene group, converting it to a methyl group (thus
accounting for the above described transfer of
3
H) and
yielding DHF. This reduction promotes displacement of
the Cys thiolate group from the intermediate so as to
release product, dTMP, and reform active enzyme.
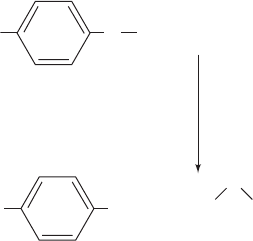
c. 5-Fluorodeoxyuridylate Is a Potent
Antitumor Agent
The above mechanism is supported by the observation
that 5-fluorodeoxyuridylate (FdUMP)
is an irreversible inhibitor of TS.This substance, like dUMP,
binds to the enzyme (an F atom has approximately the
same radius as an H atom) and undergoes the first two steps
of the normal enzymatic reaction. In Step 3, however, the
enzyme cannot abstract the F atom as F
⫹
(recall that F is the
most electronegative element), so that the enzyme is all but
permanently immobilized as the enzyme–FdUMP–THF
N
H
N
O
F
O
5-Fluorodeoxyuridylate (FdUMP)
O
H
2
C
HH
HOH
H
H
O
O
P
–
O
O
–
Section 28-3. Formation of Deoxyribonucleotides 1127
Figure 28-18 X-ray structure of human dUTPase. (a) The
molecular surface at the substrate binding site showing how the
enzyme differentiates uracil from thymine. Bound dUTP is
drawn in stick form with its N, O, and P atoms represented by
blue, red, and yellow spheres. Mg
2⫹
ions that were modeled into
the structure are represented by green spheres.The protein’s
molecular surface is colored according to its electrostatic
potential with positive, negative, and near neutral regions blue,
red, and white, respectively. Note how the snug fit of the uracil
ring into its binding site would sterically exclude thymine’s C5
methyl group. (b) The substrate binding site indicating how the
enzyme differentiates uracil from cytosine and 2¿-deoxyribose
from ribose. dUMP bound at the active site is drawn as in Part a.
The protein, mainly the backbone of a  hairpin motif, is
similarly drawn but with thinner gray bonds. Hydrogen bonds are
shown as dotted white lines, and a tightly bound, conserved water
molecule is represented by a pink sphere. The pattern of
hydrogen bonding donors and acceptors on the protein would
prevent cytosine from binding in the active site pocket.The
conserved Tyr side chain sterically excludes ribose’s 2’ OH group.
[Courtesy of John Tainer,The Scripps Research Institute, La
Jolla, California.]
(a)
(b)
JWCL281_c28_1107-1142.qxd 8/9/10 9:46 AM Page 1127
ternary covalent complex analogous to that after Step 2 in
Fig. 28-19. Indeed, X-ray structural analysis by William
Montfort revealed that crystals of E. coli TS that had been
soaked in a solution containing FdUMP and N
5
,N
10
-
methylene-THF contain precisely this complex (Fig. 28-20).
Enzymatic inhibitors such as FdUMP, which inactivate an
enzyme only after undergoing part or all of its normal
catalytic reaction, are called mechanism-based inhibitors
(alternatively, suicide substrates because they cause the en-
zyme to “commit suicide”). Mechanism-based inhibitors, be-
ing targeted for particular enzymes, are among the most pow-
erful, specific, and therefore useful enzyme inactivators.
The strategic position of thymidylate synthase in DNA
biosynthesis has led to the clinical use of FdUMP as an an-
titumor agent. Rapidly proliferating cells, such as cancer
cells, require a steady supply of dTMP in order to survive
and are therefore killed by treatment with FdUMP. In con-
trast, most normal mammalian cells, which grow slowly if at
all, have a lesser requirement for dTMP, so that they are
1128 Chapter 28. Nucleotide Metabolism
Figure 28-20 The X-ray structure of the E. coli thymidylate
synthase in covalent complex with FdUMP and THF. The active
site region of one subunit of this dimeric enzyme is shown in
ribbon form with its polypeptide chain colored in rainbow order
from its N-terminus (blue) to its C-terminus (red).The FdUMP
and N
5
,N
10
-methylene-THF are drawn in stick form with FdUMP
C cyan, N
5
,N
10
-methylene-THF C green, N blue, O red,
F purple, and P orange. The C5 and C6 atoms of FdUMP form
covalent bonds with the CH
2
group substituent to N5 of THF
and the S atom of Cys 146, whose side chain is drawn in stick
form with C green and S yellow. [Based on an X-ray structure by
William Montfort, University of Arizona, PDBid 1TSN.]
E
HN
N
S
H
N
O
–
N
O
H
dRib P
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
N
H
N
H
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
H
O
H
2
C
H
2
C
H
2
C
CH
2
N
N
N
5
, N
10
-Methylene-THF
H
N
O
N
O
H
dRib P
dTMP
CH
3
R
10
N
N
H
N
H
H
O
N
R
HN
+
H
H
N
O
N
O
H
dRib P
dUMP
5
6
H
E
B:
S
–
E
B:
..
S
–
..
S
H
N
O
N
O
H
dRib P
N
N
H
N
H
H
O
CH
2
CH
2
N
H
E
B:
HN
S
N
O
N
O
H
dRib P
N
N
H
N
H
H
O
N
–
H
E
CH
2
HB
+ H
+
+
B
:
+
1
2
3
4
HN
N
N
H
N
H
O
CH
2
N
R
DHF
R
5
6
R
H
+
Figure 28-19 Catalytic mechanism of thymidylate synthase. The methyl group is supplied by
N
5
,N
10
-methylene-THF, which is concomitantly oxidized to dihydrofolate.
JWCL281_c28_1107-1142.qxd 6/8/10 10:39 AM Page 1128
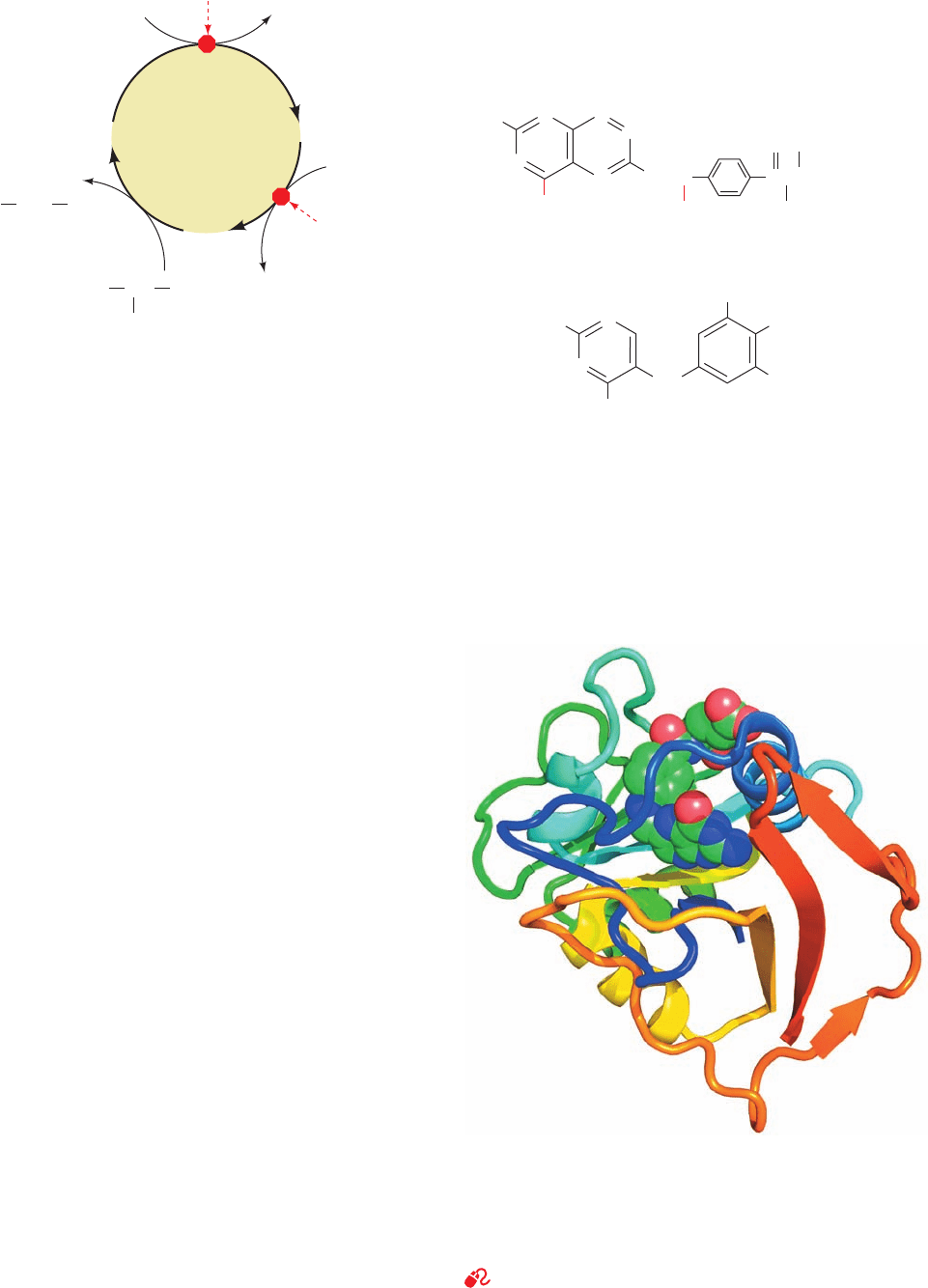
relatively insensitive to FdUMP (some exceptions are the
bone marrow cells that comprise the blood-forming tissues
and much of the immune system, the intestinal mucosa, and
hair follicles). 5-Fluorouracil and 5-fluorodeoxyuridine are
also effective antitumor agents since they are converted to
FdUMP through salvage reactions.
d. N
5
,N
10
-Methylene-THF Is Regenerated
in Two Reactions
The thymidylate synthase reaction is biochemically unique
in that it oxidizes THF to DHF; no other enzymatic reaction
employing a THF cofactor alters this coenzyme’s net oxida-
tion state. The DHF product of the thymidylate synthase re-
action is recycled to the enzyme’s N
5
,N
10
-methylene-THF
cofactor through two sequential reactions (Fig. 28-21):
1. DHF is reduced to THF by NADPH as catalyzed by
dihydrofolate reductase (DHFR; Section 26-4D).Although,
in most organisms, DHFR is a monomeric monofunctional
enzyme, in protozoa and at least some plants, DHFR and TS
occur on the same polypeptide chain to form a bifunctional
enzyme that has been shown to channel DHF from its TS to
its DHFR active sites.
2. Serine hydroxymethyltransferase (Section 26-3Bb)
transfers the hydroxymethyl group of serine to THF, yield-
ing N
5
,N
10
-methylene-THF and glycine.
e. Antifolates Are Anticancer Agents
Inhibition of DHFR quickly results in all of a cell’s lim-
ited supply of THF being converted to DHF by the thymidy-
late synthase reaction. Inhibition of DHFR therefore not
only prevents dTMP synthesis (Fig. 28-21), but also blocks
all other THF-dependent biological reactions such as the
synthesis of purines (Section 28-1A),methionine (Section 26-
5Ba), and, indirectly, histidine (Section 26-5Be). DHFR (Fig.
28-22) therefore offers an attractive target for chemotherapy.
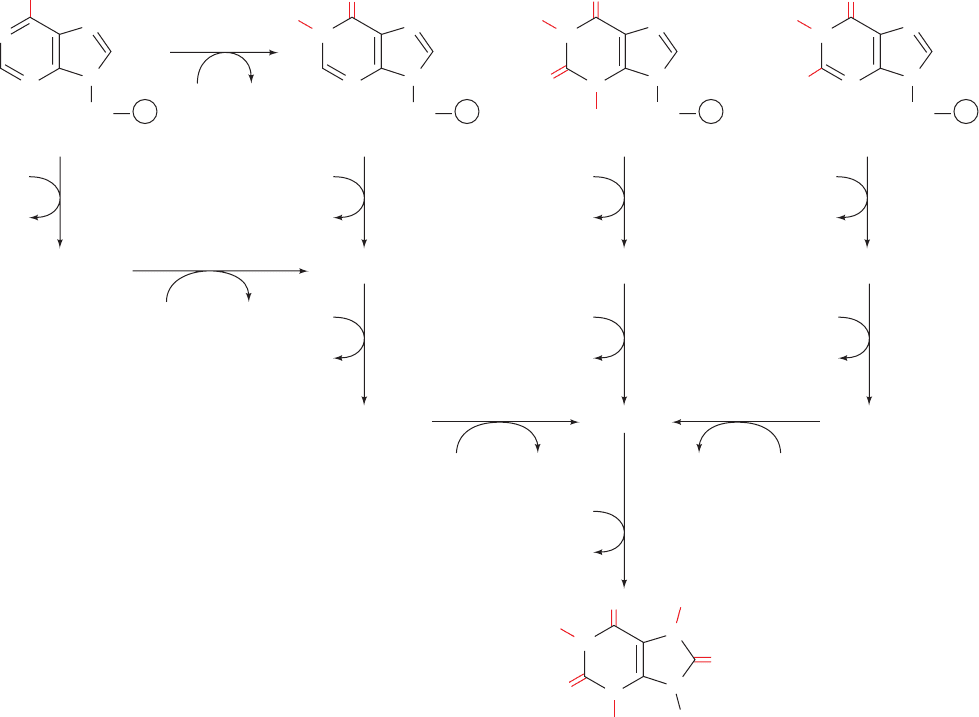
Methotrexate (amethopterin), aminopterin, and tri-
methoprim
are DHF analogs that competitively although all but irre-
versibly bind to DHFR with an ⬃1000-fold greater affinity
than does DHF. These antifolates (substances that interfere
with the action of folate cofactors) are effective anticancer
agents, particularly against childhood leukemias. In fact, a
successful chemotherapeutic strategy is to treat a cancer
OCH
N
CH
2
COOCH
2
–
H
2
N
N
N
CH
CH
2
N
Trimethoprim
COO
–
N
HR
NCH
NH
2
R = H
R = CH
Aminopterin
Methotrexate (amethopterin)
3
N
H
2
N
N
NH
2
CH
2
3
OCH
3
OCH
3
O
C
Section 28-3. Formation of Deoxyribonucleotides 1129
Figure 28-21 Regeneration of N
5
,N
10
-methylenetetrahydro-
folate. The DHF product of the thymidylate synthase reaction is
converted back to N
5
,N
10
-methylene-THF by the sequential
actions of (1) dihydrofolate reductase and (2) serine
hydroxymethyltransferase. Thymidylate synthase is inhibited
by FdUMP, whereas dihydrofolate reductase is inhibited by the
antifolates methotrexate, aminopterin, and trimethoprim.
Figure 28-22 X-ray structure of human dihydrofolate reduc-
tase in complex with folic acid. The polypeptide is colored in
rainbow order from its N-terminus (blue) to its C-terminus (red).
The folic acid is drawn in space-filling form with C green, N blue,
and O red. [Based on an X-ray structure by Joseph Kraut,
University of California at San Diego. PDBid 1DHF.]
See Interactive Exercise 29.
N
5
,N
10
-Methylene-THF
NADPH + H
+
NADP
+
Serine
dTMP
serine
hydroxymethyl
transferase
dihydrofolate
reductase
FdUMP
Methotrexate
Aminopterin
Trimethoprim
thymidylate
synthase
DHF
THF
2
1
dUMP
CH COO
–
CH
2
OH
NH
3
+
Glycine
CH
2
COO
–
NH
3
+
JWCL281_c28_1107-1142.qxd 10/19/10 9:58 AM Page 1129
N
O
N
H
H
2
N
N
Rib
N
P
N
O
O
N
H
H
N
Rib
N
P
N
O
N
H
N
Rib
N
P
N
NH
2
N
N
Rib
N
P
H
2
O
P
i
AMP
Adenosine
nucleotidase
H
2
O
P
i
IMP
Inosine
nucleotidase
H
2
O
P
i
XMP
N
O
O
O
N
H
H
H
N
H
N
Uric acid
Xanthosine
nucleotidase
H
2
O
P
i
GMP
Guanosine
Hypoxanthine Xanthine Guanine
nucleotidase
H
2
O NH
4
AMP
deaminase
Ribose-1-P
P
i
purine
nucleoside
phosphorylase
(PNP)
Ribose-1-P
P
i
purine
nucleoside
phosphorylase
(PNP)
Ribose-1-P
P
i
purine
nucleoside
phosphorylase
(PNP)
H
2
O
2
H
2
O
2
O
2
+ H
2
O
O
2
+ H
2
O
xanthine
oxidase
+
H
2
O
adenosine
deaminase
xanthine
oxidase
H
2
O
guanine
deaminase
NH
4
+
NH
4
+
victim with a lethal dose of methotrexate and some hours
later “rescue” the patient (but hopefully not the cancer) by
administering massive doses of 5-formyl-THF and/or thymi-
dine.A low dose of methotrexate is also effective in the treat-
ment of rheumatoid arthritis, inhibiting immune system activ-
ity and thus decreasing inflammation. Trimethoprim, which
was discovered by George Hitchings and Gertrude Elion,
binds much more tightly to bacterial DHFRs than to those of
mammals and is therefore a clinically useful antibiotic.
4 NUCLEOTIDE DEGRADATION
Most foodstuffs, being of cellular origin, contain nucleic
acids. Dietary nucleic acids survive the acid medium of the
stomach; they are degraded to their component nu-
cleotides, mainly in the duodenum, by pancreatic nucleases
and intestinal phosphodiesterases. These ionic compounds,
which cannot pass through cell membranes, are then hy-
drolyzed to nucleosides by a variety of group-specific nu-
cleotidases and nonspecific phosphatases. Nucleosides may
be directly absorbed by the intestinal mucosa or first
undergo further degradation to free bases and ribose or
ribose-1-phosphate through the action of nucleosidases
and nucleoside phosphorylases:
Radioactive labeling experiments have demonstrated
that only a small fraction of the bases of ingested nucleic
acids are incorporated into tissue nucleic acids. Evidently,
the de novo pathways of nucleotide biosynthesis largely sat-
isfy an organism’s need for nucleotides. Consequently, in-
gested bases, for the most part, are degraded and excreted.
Cellular nucleic acids are also subject to degradation as part
of the continual turnover of nearly all cellular components.
In this section we outline these catabolic pathways and dis-
cuss the consequences of several of their inherited defects.
A. Catabolism of Purines
The major pathways of purine nucleotide and deoxynu-
cleotide catabolism in animals are diagrammed in
Fig. 28-23. Other organisms may have somewhat different
Nucleoside P
i
¬¬¬¬
¡
nucleoside
phosphorylase
base ribose-1-P
Nucleoside H
2
O
¬¬¬¬
¡
nucleosidase
base ribose
1130 Chapter 28. Nucleotide Metabolism
Figure 28-23 Major pathways of purine catabolism in animals.
The various purine nucleotides and deoxynucleotides are all
degraded to uric acid.
JWCL281_c28_1107-1142.qxd 4/22/10 9:16 AM Page 1130
