Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

PurK catalyzes the ATP-dependent carboxylation of AIR
to yield N
5
-CAIR, which Class I PurE rearranges to yield
CAIR. Class I PurE is homologous to AIR carboxylase,
which is therefore also called Class II PurE. Class I PurE
alone can catalyze the AIR carboxylase reaction but since
its K
M
for HCO
3
is 110 mM, it requires an unphysiologi-
cally high (⬃100 mM) HCO
3
concentration to do so at a
significant rate. However, the action of PurK decreases the
HCO
3
concentration required for the PurE-catalyzed re-
action by 1000-fold, presumably through the ATP-driven
formation of carbonyl phosphate, as is also postulated to
occur in the carbamoyl phosphate synthetase reaction
(Section 26-2A). The observation that N
5
-CAIR is chemi-
cally unstable (it decomposes to AIR with a half-life of 15 s
at pH 7.5 and 25°C) suggests that N
5
-CAIR is channeled
between PurK and Class I PurE. In fact, in yeast and plants,
the N-terminus of Class I PurE is fused to the C-terminus
of PurK. However, in E. coli, these two enzymatic activities
occur on separate proteins for which there is no evidence
of association.
8. Acquisition of N1. Purine atom N1 is contributed by
aspartate in an amide-forming condensation reaction
yielding 5-aminoimidazole-4-(N-succinylocarboxamide)
ribotide (SAICAR) that is catalyzed by SAICAR syn-
thetase (PurC).The reaction,which is driven by the hydrol-
ysis of ATP to ADPP
i
, chemically resembles Reaction 3.
9. Elimination of fumarate. SAICAR is cleaved with
the release of fumarate, yielding 5-aminoimidazole-4-
carboxamide ribotide (AICAR) in a reaction catalyzed by
adenylosuccinate lyase (PurB). Reactions 8 and 9 chemically
resemble the reactions in the urea cycle in which citrulline
is aminated to form arginine (Sections 26-2C and 26-2D).
In both pathways, aspartate’s amino group is transferred to
an acceptor through an ATP-driven coupling reaction fol-
lowed by the elimination of the aspartate carbon skeleton
as fumarate. In plants and microorganisms, AICAR is also
formed in the biosynthesis of histidine (Section 26-5Be)
but since in that process the AICAR is derived from ATP,
it provides for no net purine biosynthesis.
10. Acquisition of C2. The final purine ring atom is ac-
quired through formylation by N
10
-formyltetrahydrofolate,
yielding 5-formaminoimidazole-4-carboxamide ribotide
(FAICAR) in a reaction catalyzed by AICAR transform-
ylase (PurH). In bacteria, this reaction and that of Reaction
4 are indirectly inhibited by sulfonamides, which, it will be
recalled, prevent the synthesis of folate by competing with
its p-aminobenzoate component (Section 26-4D).Animals,
including humans, must acquire folate through the diet,
since they are incapable of synthesizing it. They are there-
fore unaffected by sulfonamides. The antibiotic properties
of sulfonamides are therefore largely a result of their inhi-
bition of nucleic acid biosynthesis in susceptible bacteria.
11. Cyclization to form IMP. The final reaction in the
pathway, ring closure to form IMP, occurs through the elim-
ination of water as catalyzed by IMP cyclohydrolase
(PurJ). In contrast to Reaction 6, the cyclization that forms
the imidazole ring, this reaction does not entail ATP
hydrolysis.
In animals, the activities catalyzing Reactions 3, 4, and 6,
Reactions 7 and 8, and Reactions 10 and 11 occur on single
polypeptides. The intermediate products of these multi-
functional enzymes are not readily released to the medium
but are channeled to the succeeding enzymatic activities of
the pathway, thereby increasing the overall rates of these
multistep processes and protecting the intermediates from
degradation by other cellular enzymes. We have previously
seen, for example, that the formation of acetyl-CoA from
pyruvate takes place on the pyruvate dehydrogenase mul-
tienzyme complex, which contains three enzymes catalyz-
ing five consecutive reactions (Section 21-2A); that all
seven enzymatic activities catalyzing fatty acid synthesis
in animals occur on a single protein molecule (Section
25-4Ca,b); and that the multifunctional enzymes carbamoyl
phosphate synthase I (Section 26-2Aa), glutamate synthase
(Section 26-5Aa), tryptophan synthase (Section 26-5Bd),
and amidophosphoribosyltransferase (see above) pass re-
active intermediate products between their active sites via
protein tunnels. It is becoming increasingly apparent that
the association of functionally related enzymes is a wide-
spread phenomenon.
B. Synthesis of Adenine and Guanine
Ribonucleotides
IMP does not accumulate in the cell but is rapidly con-
verted to AMP and GMP. AMP, which differs from IMP
only in the replacement of its 6-keto group by an amino
The aromatization of the imidazole ring is facilitated by the
tautomeric shift of the reactant from its imine to its ena-
mine form.
7. Acquisition of C6. In higher eukaryotes, purine C6 is
introduced as HCO
3
(CO
2
) in an ATP-dependent reaction
catalyzed by AIR carboxylase that yields carboxyaminoim-
idazole ribotide (CAIR) and ADP P
i
. However, in yeast,
plants, and most prokaryotes (including E. coli), this over-
all reaction occurs in two steps that are mediated by sepa-
rate enzymatic activities: PurK and Class I PurE.
AIR carboxylase
(Class II PurE)
H
2
N R5P H
2
N
N
N
R5P
N
N
CO
2
O
C
_
O
N
H
R5P
N
N
C
AIR CAIR
N
5
-CAIR
A
TP + HCO
3
_
ADP + P
i
O
PurK
_
O
Class I PurE
Section 28-1. Synthesis of Purine Ribonucleotides 1111
JWCL281_c28_1107-1142.qxd 6/8/10 10:39 AM Page 1111
NH
2
N
N
N
N
H
O
Ribose-5-phosphate
IMP
N
N
N
N
NH
Ribose-5-phosphate
Adenylosuccinate
N
N
N
N
H
O
Ribose-5-phosphate
Xanthosine monophosphate (XMP)
CH
2
CH COO
––
OOC
O
H
Aspartate + GTP
GDP + P
i
adenylosuccinate
synthetase
NAD
+
+ H
2
O
NADH + H
+
IMP dehydrogenase
Glutamine + ATP + H
2
O
Glutamate + AMP + PP
i
N
N
N
N
Ribose-5-phosphate
AMP
N
N
N
N
H
O
Ribose-5-phosphate
GMP
H
2
N
Fumarate
adenylosuccinate
lyase
GMP
synthetase
group, is synthesized in a two-reaction pathway (Fig. 28-4,
left). In the first reaction, aspartate’s amino group is linked
to IMP in a reaction driven by the hydrolysis of GTP to
GDP P
i
to yield adenylosuccinate. In the second reac-
tion, adenylosuccinate lyase eliminates fumarate from
adenylosuccinate to form AMP.This enzyme also catalyzes
Reaction 9 of the IMP pathway (Fig. 28-2).
GMP is also synthesized from IMP in a two-reaction
pathway (Fig. 28-4, right). In the first reaction, IMP dehy-
drogenase catalyzes the NAD
-dependent oxidation of
IMP to form xanthosine monophosphate (XMP; the ri-
bonucleotide of the base xanthine). XMP is then converted
to GMP by the replacement of its 2-keto group with gluta-
mine’s amide nitrogen in a reaction driven by the hydroly-
sis of ATP to AMP PP
i
(and subsequently to 2 P
i
).
IMP dehydrogenase, a homotetramer of 514-residue
subunits, was incubated with IMP, NAD
, and the fungally
produced inhibitor mycophenolic acid (MPA).
The X-ray structure of the resulting complex, determined
by Keith Wilson, reveals that the enzyme had bound MPA
together with a reaction intermediate in which IMP atom
Mycophenolic acid (MPA)
O
OH
OH
O
OCH
3
CH
3
CH
3
1112 Chapter 28. Nucleotide Metabolism
Figure 28-4 IMP is converted to AMP or GMP in separate
two-reaction pathways. The X-ray structures for all of the
enzymes catalyzing these reactions are shown to the outside of
the corresponding reaction arrow. The X-ray structures for these
homooligomers are shown as described in the legend to Fig. 28-2.
Adenylosuccinate synthetase from E. coli, determined by
Herbert Fromm and Richard Honzatko, Iowa State University, is
a C
2
dimer in complex with IMP (green), GDP (red), and
hadacidin (magenta; a competitive inhibitor of aspartate); PDBid
1GIM.Adenylosuccinate lyase, from Thermatoga maritima,
determined by Todd Yeates, UCLA, is a D
2
tetramer; PDBid
1C3U. IMP dehydrogenase from Chinese hamsters, determined
by Keith Wilson,Vertex Pharmaceuticals, Cambridge,
Massachusetts, is a C
4
tetramer in complex with oxidized IMP
(red) and MPA (purple); PDBid 1JR1. GMP synthetase from
E. coli, determined by Janet Smith, Purdue University, is a D
2
tetramer in complex with AMP (red), pyrophosphate (blue), and
citrate (purple); PDBid 1GPM.
JWCL281_c28_1107-1142.qxd 4/22/10 9:16 AM Page 1112
C2 had become covalently linked to the Cys 331 S atom and
then dehydrogenated by NAD
to yield a thioimidate ester:
The mutagenic replacement of Cys 331 by Ala inactivates
the enzyme.These observations strongly support a catalytic
mechanism in which the Cys 331 thiol group nucleophili-
cally attacks IMP’s C2 atom, followed by hydride transfer
to NAD
to yield the above covalently bound intermedi-
ate, which is subsequently hydrolyzed to yield XMP. The
MPA binds to the enzyme with its bicyclic ring stacked on
the purine ring (as would be expected for NAD
’s nicoti-
namide ring) and with its phenolic hydroxyl group in the
proposed hydrolytic water site. This blocks the hydrolysis
of the thioimidate ester, thereby inactivating the enzyme.
IMP dehydrogenase activity is essential to the immune
response (Section 35-2) because it is required by the im-
mune system cells known as B and T lymphocytes to gener-
ate the guanosine nucleotides they need to proliferate.
Moreover, certain cancer cells have increased IMP dehy-
drogenase activity. Hence,IMP dehydrogenase is a target for
both immunosuppressive therapy and cancer chemotherapy.
Indeed, MPA is in clinical use to prevent the rejection of
transplanted kidneys.
a. Nucleoside Diphosphates and Triphosphates Are
Synthesized by the Phosphorylation of Nucleoside
Monophosphates
In order to participate in nucleic acid synthesis, nucleo-
side monophosphates must first be converted to the corre-
sponding nucleoside triphosphates. In the first of the two
sequential phosphorylation reactions that do so, nucleoside
diphosphates are synthesized from the corresponding
nucleoside monophosphates by base-specific nucleoside
monophosphate kinases. For example, adenylate kinase
(Section 17-4Fd) catalyzes the phosphorylation of AMP
to ADP:
Similarly, GDP is produced by a guanine-specific enzyme:
These nucleoside monophosphate kinases do not discrimi-
nate between ribose and deoxyribose in the substrate.
Nucleoside diphosphates are converted to the corre-
sponding triphosphates by nucleoside diphosphate kinase;
for instance,
Although this reaction is written with ATP as the phospho-
ryl donor and GDP as the acceptor, nucleoside diphos-
phate kinase is nonspecific as to the bases on either of its
ATP GDP Δ ADP GTP
GMP ATP Δ GDP ADP
AMP ATP Δ 2ADP
N
N
N
HN
O
Ribose-5-P
Enzyme–product thioimidate ester
S
Enzyme
substrates and as to whether their sugar residues are ribose
or deoxyribose. The reaction occurs via a Ping Pong mecha-
nism in which the substrate NTP phosphorylates an enzyme
His residue, which in turn, phosphorylates the substrate NDP.
The phosphoglycerate mutase reaction of glycolysis also has
a phospho-His intermediate (Section 17-2H).The nucleoside
diphosphate kinase reaction, as might be expected from the
nearly identical structures of its substrates and products,
normally operates close to equilibrium (G ⬇ 0). ADP is,
of course, also converted to ATP by a variety of energy-
releasing reactions such as those of glycolysis and oxidative
phosphorylation. Indeed, it is these reactions that ultimately
drive the foregoing kinase reactions.
C. Regulation of Purine Nucleotide Biosynthesis
The pathways involved in nucleic acid metabolism are
tightly regulated, as is evidenced, for example, by the in-
creased rates of nucleotide synthesis during cell prolifera-
tion. In fact, the pathways synthesizing IMP,ATP, and GTP
are individually regulated in most cells so as not only to
control the total amounts of purine nucleotides produced
but also to coordinate the relative amounts of ATP and
GTP. This control network is diagrammed in Fig. 28-5.
The IMP pathway is regulated at its first two reactions:
those catalyzing the synthesis of PRPP and 5-phosphoribo-
Section 28-1. Synthesis of Purine Ribonucleotides 1113
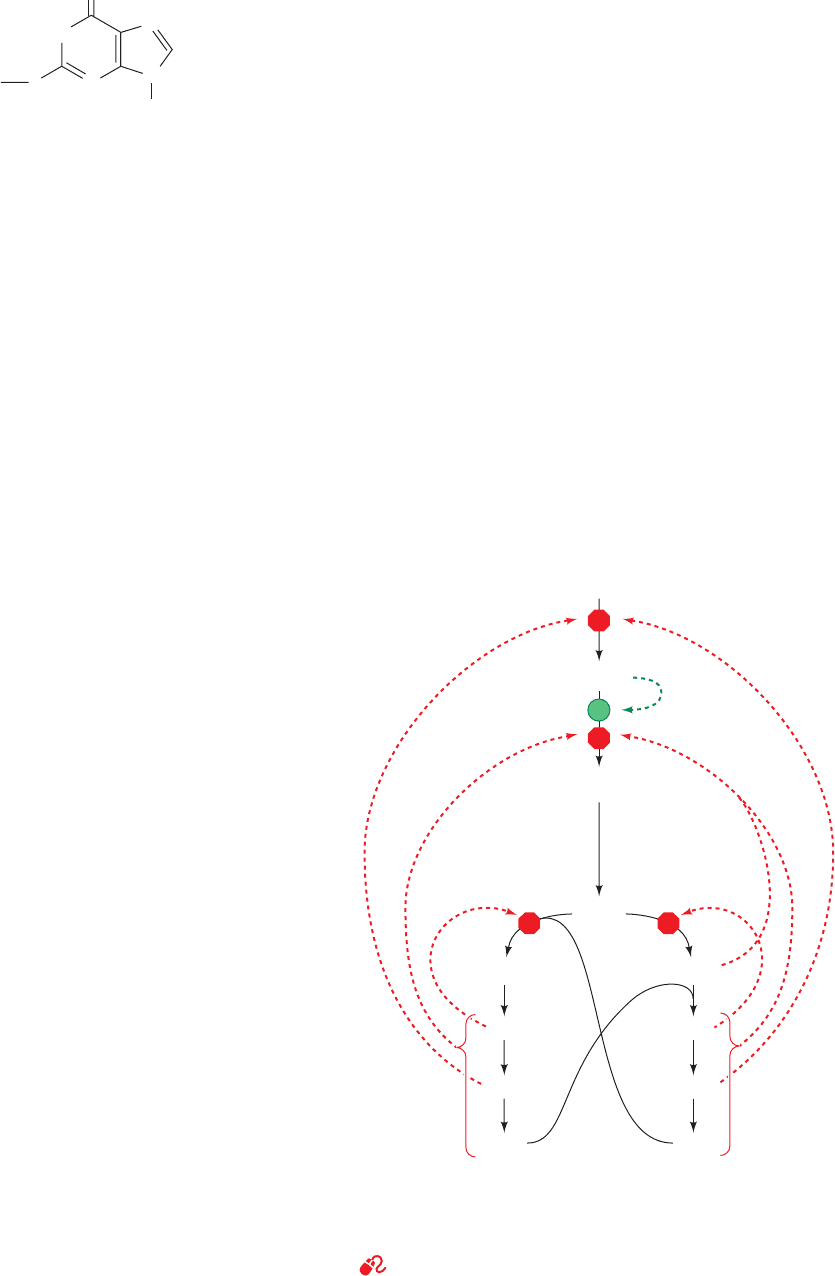
Figure 28-5 Control network for the purine biosynthesis
pathway. Red octagons and green dots indicate control points.
Feedback inhibition is indicated by dashed red arrows and
feedforward activation is represented by dashed green arrows.
See the Animated Figures
Ribose-5-phosphate
Inhibition
PRPP
5-Phosphoribosylamine
IMP
Adenylosuccinate
AMP
ADP
ATP
GMP
GDP
GTP
XMP
Activation
JWCL281_c28_1107-1142.qxd 4/22/10 9:16 AM Page 1113
sylamine. We have already seen that ribose phosphate py-
rophosphokinase, the enzyme catalyzing Reaction 1 of the
IMP pathway, is inhibited by both ADP and GDP (Section
28-1A). Amidophosphoribosyltransferase, the enzyme cat-
alyzing the first committed step of the IMP pathway (Reac-
tion 2), is likewise subject to feedback inhibition. In this
case, however, the enzyme binds ATP, ADP, and AMP at
one inhibitory site and GTP, GDP, and GMP at another.
The rate of IMP production is consequently independently
but synergistically controlled by the levels of adenine nu-
cleotides and guanine nucleotides. Moreover, amidophos-
phoribosyltransferase is allosterically stimulated by PRPP
(feedforward activation).
A second level of regulation occurs immediately below
the branch point leading from IMP to AMP and GMP (Fig.
28-4). AMP and GMP are each competitive inhibitors of
IMP in their own synthesis, so that excessive buildup of
these products is impeded. In addition, the synthesis rates
of adenine and guanine nucleotides are coordinated be-
cause GTP powers the synthesis of AMP from IMP,
whereas ATP powers the synthesis of GMP from IMP. This
reciprocity serves to balance the production of AMP and
GMP (which are required in roughly equal amounts in
nucleic acid biosynthesis): The rate of synthesis of GMP
increases with [ATP], whereas that of AMP increases with
[GTP].
D. Salvage of Purines
Most cells have an active turnover of many of their nucleic
acids (particularly some types of RNA) which, through
degradative processes described in Section 28-4A, result in
the release of adenine, guanine, and hypoxanthine. These
free purines are reconverted to their corresponding nu-
cleotides through salvage pathways. In contrast to the
de novo purine nucleotide synthesis pathway, which is vir-
tually identical in all cells, salvage pathways are diverse in
character and distribution. In mammals, purines are, for the
most part, salvaged by two different enzymes. Adenine
phosphoribosyltransferase (APRT) mediates AMP forma-
tion through the transfer of adenine to PRPP with the
release of PP
i
:
Hypoxanthine–guanine phosphoribosyltransferase (HGPRT)
catalyzes the analogous reaction for both hypoxanthine
and guanine:
a. Lesch–Nyhan Syndrome Results from
HGPRT Deficiency
The symptoms of Lesch–Nyhan syndrome, which is
caused by a severe HGPRT deficiency, indicate that purine
salvage reactions have functions other than conservation
of the energy required for de novo purine biosynthesis.This
sex-linked congenital defect (affects almost only males) re-
sults in excessive uric acid production (uric acid is a purine
Guanine ⫹ PRPP Δ GMP ⫹ PP
i
Hypoxanthine ⫹ PRPP Δ IMP ⫹ PP
i
Adenine ⫹ PRPP Δ AMP ⫹ PP
i
degradation product; Section 28-4A) and neurological ab-
normalities such as spasticity, mental retardation, and
highly aggressive and destructive behavior, including a
bizarre compulsion toward self-mutilation. For example,
many children with Lesch–Nyhan syndrome have such an
irresistible urge to bite their lips and fingers that they must
be restrained. If the restraints are removed, communicative
patients will plead that the restraints be replaced even as
they attempt to injure themselves.
The excessive uric acid production in patients with
Lesch–Nyhan syndrome is readily explained. The lack of
HGPRT activity leads to an accumulation of the PRPP that
would normally be used in the salvage of hypoxanthine and
guanine.The excess PRPP activates amidophosphoribosyl-
transferase (which catalyzes Reaction 2 of the IMP biosyn-
thesis pathway; Fig. 28-2), thereby greatly increasing the
rate of synthesis of purine nucleotides and consequently
that of their degradation product, uric acid.Yet the physio-
logical basis of the associated neurological abnormalities
remains obscure.That a defect in a single enzyme can cause
such profound but well-defined behavioral changes never-
theless has important psychiatric implications.
2 SYNTHESIS OF PYRIMIDINE
RIBONUCLEOTIDES
The biosynthesis of pyrimidines is a simpler process than
that of purines. Isotopic labeling experiments have shown
that atoms N1, C4, C5, and C6 of the pyrimidine ring are all
derived from aspartic acid, C2 arises from HCO
3
⫺
, and N3
is contributed by glutamine (Fig. 28-6). In this section we
discuss the pathways for pyrimidine ribonucleotide biosyn-
thesis and how these processes are regulated.
A. Synthesis of UMP
The major breakthrough in the determination of the path-
way for the de novo biosynthesis of pyrimidine ribonu-
cleotides was the observation that mutants of the bread
mold Neurospora crassa, which are unable to synthesize
pyrimidines and therefore require both cytosine and uracil
in their growth medium, grow normally when supplied in-
stead with the pyrimidine orotic acid (uracil-6-carboxylic
acid).
This observation led to the elucidation of the following six-
reaction pathway for the biosynthesis of UMP (Fig. 28-7).
Note that, in contrast to the case for purine nucleotides, the
pyrimidine ring is coupled to the ribose-5-phosphate moi-
ety after the ring has been synthesized.
Orotic acid (uracil-6-carboxylic acid)
COOH
N
N
O
O
H
H
1114 Chapter 28. Nucleotide Metabolism
JWCL281_c28_1107-1142.qxd 8/9/10 9:46 AM Page 1114
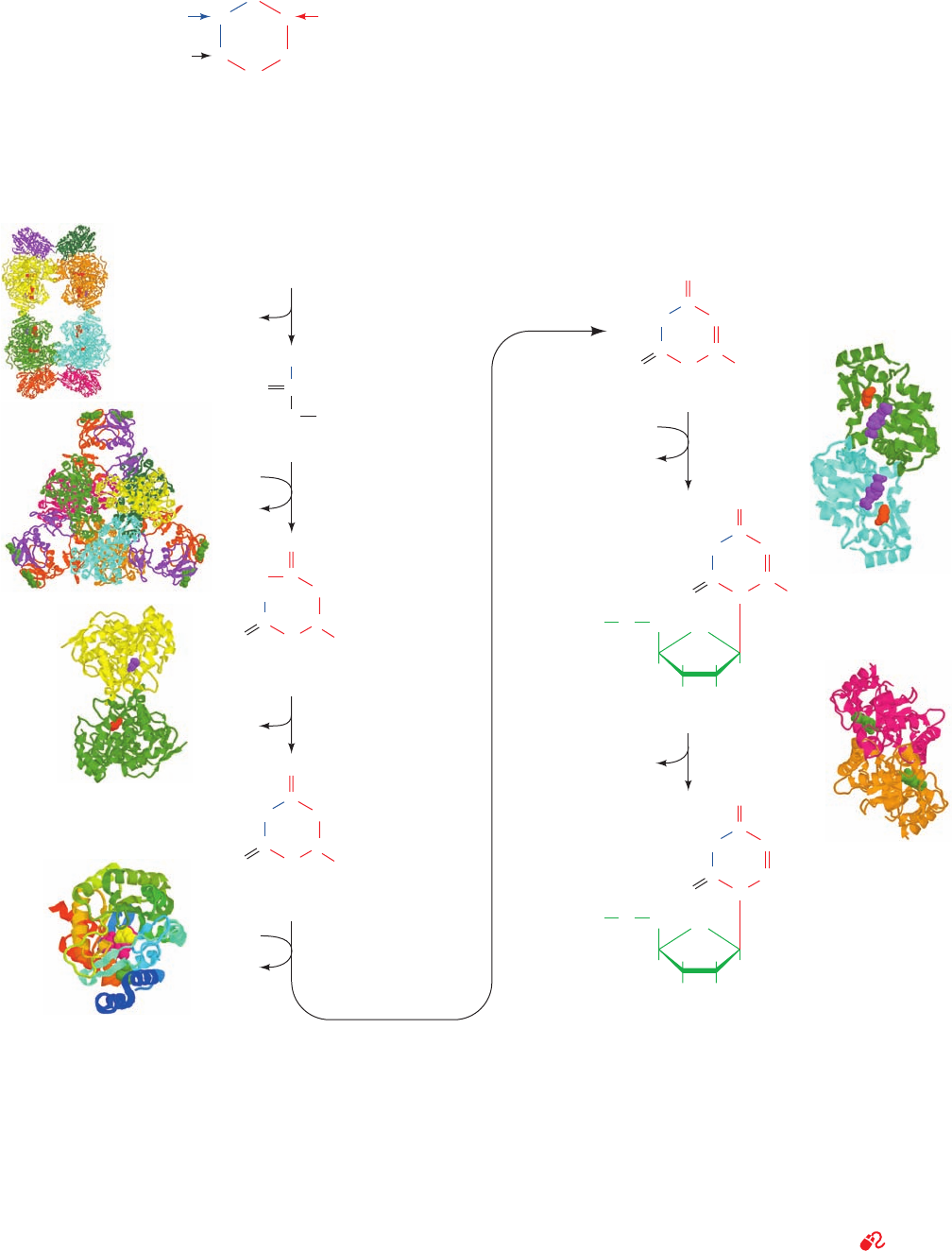
1. Synthesis of carbamoyl phosphate. The first reaction
of pyrimidine biosynthesis is the synthesis of carbamoyl
phosphate from HCO
3
and the amide nitrogen of glutamine
by the cytosolic enzyme carbamoyl phosphate synthetase II
(CPS II). This reaction is unusual in that it does not use
biotin and consumes two molecules of ATP: One provides
a phosphate group and the other energizes the reaction.
We have previously discussed the synthesis of carbamoyl
phosphate in connection with the formation of arginine
(Section 26-2A).The carbamoyl phosphate that is used to
Section 28-2. Synthesis of Pyrimidine Ribonucleotides 1115
Figure 28-6 The biosynthetic origins of pyrimidine ring atoms.
Figure 28-7 Metabolic pathway for the de novo synthesis of
UMP. The pathway consists of six enzymatically catalyzed
reactions. Note that, in contrast to the case for purine
biosynthesis (Fig. 28-2), the pyrimidine ring is formed before its
attachment to a ribose ring.The X-ray structures for the enzymes
are drawn as described in the legend to Fig. 28-2. Enzyme 1, from
E. coli, determined by Hazel Holden, University of Wisconsin, is
an
4
4
heterooctamer with D
2
symmetry, whose large subunits
each bind two ADPNPs (red) and one ornithine (purple); PDBid
1D3H. Enzyme 2, from E. coli, determined by William Lipscomb,
Harvard University, is a c
6
r
6
heterododecamer with D
3
symmetry,
whose regulatory (r) subunits each bind a CTP (green); PDBid
N
C
C
C
C
N
1
3
2
4
5
6
Glutamine amide Aspartate
HCO
–
3
5AT1. Enzyme 3 from E. coli, determined by Hazel Holden,
University of Wisconsin, is a C
2
dimer that binds carbamoyl
aspartate (purple) in one subunit and orotate (red) in the other;
PDBid 1J79. Enzyme 4, from humans, determined by Jon Clardy,
Cornell University, is a monomer that binds orotate (yellow),
FMN (magenta), and A77 1726 (green); PDBid 1D3H. Enzyme 5,
from Salmonella typhimurium, determined by James Sacchettini,
Albert Einstein College of Medicine, is a C
2
dimer that binds
orotate (orange) and PRPP (purple); PDBid 1OPR. Enzyme 6,
from B. subtilis, determined by Steven Ealick, Cornell University,
is a C
2
dimer that binds UMP (green); PDBid 1DBT. See the
Animated Figures
CO
PO
3
2
–
P
i
2 ATP +
HCO
3
–
+
Glutamine
2 ADP +
Glutamate
carbamoyl phosphate
synthetase II
O
NH
2
Carbamoyl phosphate
Aspartate
O
C
CH
2
CH
N
H
C
O
NH
HO
2
COO
–
Carbamoyl aspartate
dihydroorotase
H
2
O
O
C
CH
2
CH
N
H
C
O
HN
COO
–
Dihydroorotate
dihydroorotate
dehydrogenase
Quinone
Reduced
quinone
PP
i
PRPP
O
C
CH
C
N
H
C
O
HN
Orotate
orotate
phosphoribosyl
transferase
O
C
CH
C
N
C
O
HN
COO
–
COO
–
Orotidine-5-monophosphate (OMP)
CH
2
HH
HH
OH OH
O
O
O
3
2
–
P
β
OMP decarboxylase
CO
2
O
C
CH
C
N
C
O
HN
Uridine monophosphate (UMP)
CH
2
HH
HH
OH OH
O
O
O
3
2
–
P
H
1
2
3
4
4
6
5
+
P
i
aspartate
transcarbamoylase
(ATCase)
+
H
2
O
JWCL281_c28_1107-1142.qxd 6/8/10 10:39 AM Page 1115
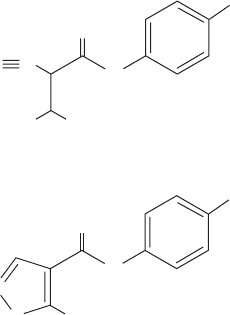
synthesize arginine via the urea cycle is synthesized by a
separate mitochondrial enzyme, carbamoyl phosphate syn-
thetase I (CPS I), which uses ammonia as its nitrogen
source. Prokaryotes only have one carbamoyl phosphate
synthetase, which supplies both pyrimidine and arginine
biosynthesis and utilizes glutamine. This latter enzyme, as
we have seen, contains three different active sites that are
connected by a remarkable 96-Å-long tunnel through
which intermediate products diffuse (Fig. 26-9).
The pyrimidine biosynthetic pathway is a target for
antiparasitic drugs. For example, the parasitic protozoan
Toxoplasma gondii, which infects most mammals, causes
toxoplasmosis, a disease whose complications include
blindness, neurological dysfunction, and death in immuno-
compromised individuals (e.g., those with AIDS). Most
parasites have evolved to take advantage of nutrients sup-
plied by their hosts. However, T. gondii is unable to meet its
needs exclusively through nucleotide salvage pathways and
retains the ability to synthesize uracil de novo. Drugs that
target the parasite’s carbamoyl phosphate synthetase II
(an enzyme whose structure and kinetics distinguish it
from its mammalian counterpart) could therefore prevent
T. gondii growth. Moreover, there is evidence that T. gondii
strains that have been engineered to lack carbamoyl phos-
phate synthetase II are avirulent and could be useful as
vaccines in humans and livestock.
2. Synthesis of carbamoyl aspartate. Condensation of
carbamoyl phosphate with aspartate to form carbamoyl
aspartate is catalyzed by aspartate transcarbamylase
(ATCase). This reaction, the pathway’s flux-generating
step, occurs without need of ATP because carbamoyl phos-
phate is intrinsically activated. The structure and regula-
tion of E. coli ATCase is discussed in Section 13-4.
3. Ring closure to form dihydroorotate. The third reac-
tion of the pathway was elucidated by Arthur Kornberg
following his observation that microorganisms made to uti-
lize orotic acid as a carbon source first reduce it to dihy-
droorotate. The reaction forming the pyrimidine ring
yields dihydroorotate in an intramolecular condensation
catalyzed by the zinc metalloenzyme dihydroorotase.
4. Oxidation of dihydroorotate. Dihydroorotate is irre-
versibly oxidized to orotate by dihydroorotate dehydroge-
nase (DHODH). The eukaryotic enzyme, which contains
FMN, is an integral membrane protein that is located on
the outer surface of the inner mitochondrial membrane,
where ubiquinone supplies its oxidizing power. The other
five enzymes of pyrimidine nucleotide biosynthesis are
cytosolic in animal cells. Many bacterial dihydroorotate de-
hydrogenases are NAD
-linked flavoproteins that contain
FMN, FAD, and a [2Fe–2S] cluster. These enzymes nor-
mally function degradatively, that is, in the direction oro-
tate S dihydroorotate, thereby permitting these bacteria
to metabolize orotate and accounting for Kornberg’s ob-
servation. The reaction mediated by eukaryotic DHODH
involves two redox steps, as is indicated in Fig. 28-8. The
X-ray structure of human DHODH in complex with oro-
tate, determined by Jon Clardy, reveals that the pyrimidine
ring of orotate is stacked over the FMN’s flavin ring with
the orotate C6 and FMN N5 separated by 3.6 Å, a distance
that is compatible with direct hydride transfer between
these two centers. A tunnel leads from the opposite side of
the flavin ring to a hydrophobic region on the enzyme sur-
face. The enzyme presumably binds to the mitochondrial
membrane surface via this hydrophobic patch, thereby per-
mitting ubiquinone, which readily diffuses within the mito-
chondrial membrane, to approach and reoxidize the en-
zyme’s bound FMNH
2
. In the X-ray structure, this tunnel
contains a tightly bound molecule named A77 1726, which
is the primary metabolite of leflunomide (trade name
Arava),
a compound that is in clinical use for the treatment of
rheumatoid arthritis. A77 1726 attenuates this autoimmune
disease by blocking pyrimidine biosynthesis in T lympho-
cytes, thereby reducing their inappropriate proliferation.
However,A77 1726 does not inhibit bacterial DHODHs.
5. Acquisition of the ribose phosphate moiety. Orotate
reacts with PRPP to yield orotidine-5-monophosphate
(OMP) in a reaction catalyzed by orotate phosphoribosyl-
transferase and driven by hydrolysis of the eliminated PP
i
.
This reaction fixes the anomeric form of pyrimidine nu-
cleotides in the configuration. Orotate phosphoribosyl-
transferase also acts to salvage other pyrimidine bases,
such as uracil and cytosine, by converting them to their cor-
responding nucleotides. Although the various phosphori-
bosyltransferases, including HGPRT, exhibit little se-
quence similarity, their X-ray structures indicate that they
contain a common structural core that resembles the dinu-
cleotide binding fold (Section 8-3Bi) but lacks one of its
strands.
6. Decarboxylation to form UMP. The final reaction of
the pathway is the decarboxylation of OMP by OMP de-
carboxylase (ODCase) to form UMP. ODCase enhances
the rate (k
cat
/K
M
) of OMP decarboxylation by a factor of
2 10
23
over that of the uncatalyzed reaction, making it the
most catalytically proficient enzyme known. Yet ODCase
has no cofactors to help stabilize the reaction’s putative
carbanion intermediate. How is it able to do so? The X-ray
structure, by Steven Ealick, of ODCase from B. subtilis in
complex with UMP indicates that a bound OMP’s C6
A77 1726
O
O
CN
N
O
CH
3
CH
3
CF
3
HO
N
H
N
H
CF
3
Leflunomide
1116 Chapter 28. Nucleotide Metabolism
JWCL281_c28_1107-1142.qxd 4/22/10 9:16 AM Page 1116
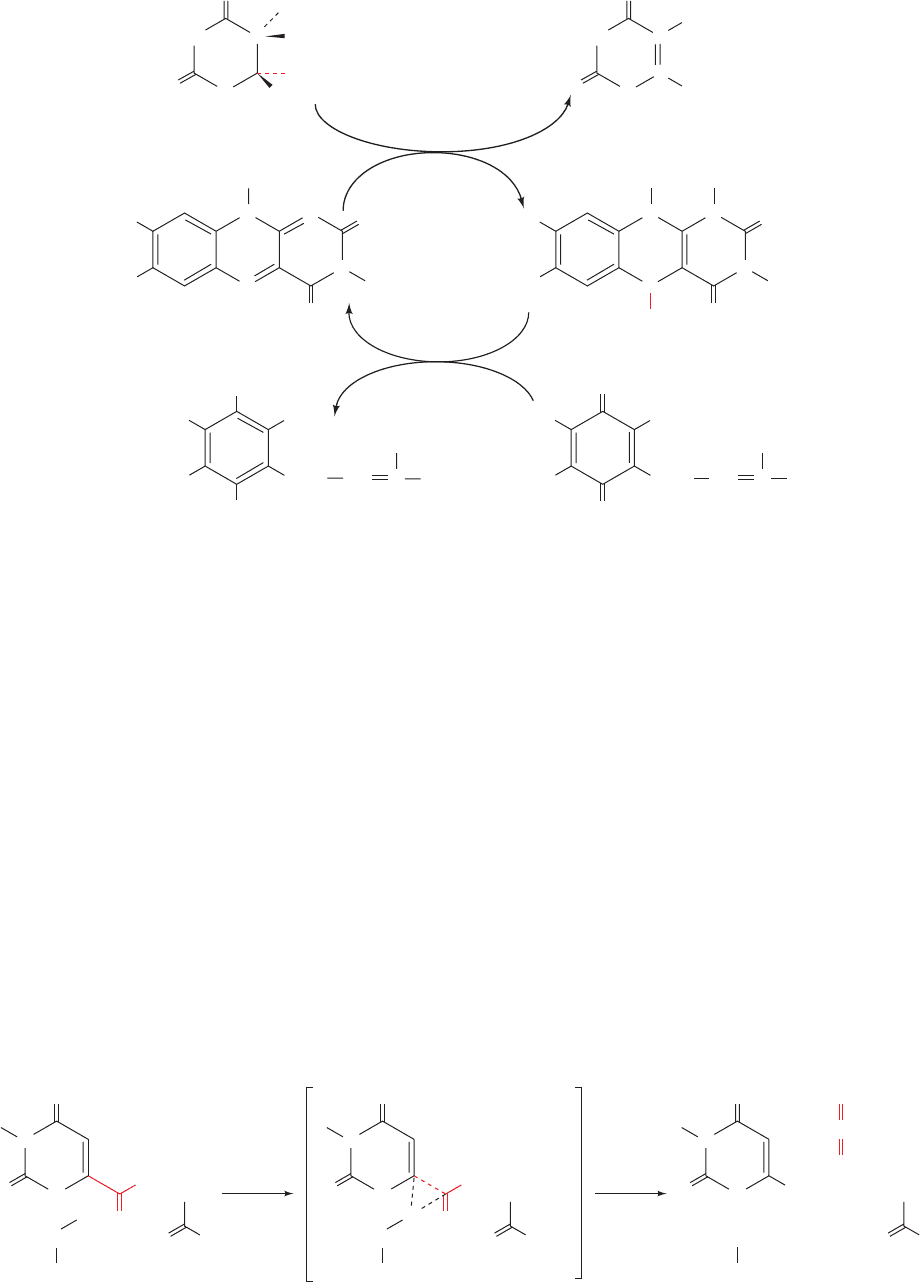
carboxyl group that is coplanar with its pyrimidine ring
would be in close proximity to the side chains of both Asp
60 and Lys 62. Ealick has therefore proposed a mechanism
(Fig. 28-9) in which the electrostatic interactions between
the closely spaced carboxyl groups of OMP and Asp 60
destabilize OMP’s ground state. This destabilization would
be reduced in the transition state by the shift of OMP’s neg-
ative charge from its carboxyl group toward C6, where it
would be stabilized by the adjacent positively charged side
chain of Lys 62. This side chain is also proposed to proton-
ate the fragmenting C¬C bond when it becomes suffi-
ciently basic to accept the proton, thus avoiding the
formation of a high-energy carbanion intermediate. The
unfavorable electrostatic interaction between OMP and
Asp 60 occurs because the enzyme tightly binds OMP
through extensive interactions with its other functional
groups. Indeed, the removal of OMP’s phosphate group,
which is quite distant from the C6 carboxyl group, decreases
the catalytic reaction’s k
cat
/K
M
by a factor of 7 10
7
, thus
providing a striking example of how binding energy can be
applied to catalysis (preferential transition state binding).
In bacteria, the six enzyme activities mediating UMP
biosynthesis occur on independent proteins (Fig. 28-7). In
animals, however, as Mary Ellen Jones demonstrated, the
first three enzymatic activities of the pathway, carbamoyl
Section 28-2. Synthesis of Pyrimidine Ribonucleotides 1117
Figure 28-8 Reactions catalyzed by eukaryotic dihydroorotate
dehydrogenase. The reaction is initiated by the enzyme-mediated
abstraction of a proton from C5 of dihydroorotate followed by
the direct hydride transfer from C6 of dihydroorotate to N5 of
FMN to yield orotate and FMNH
, which may then be
Figure 28-9 Proposed catalytic mechanism for OMP decarboxylase. [After Appleby, T.C.,
Kinsland, C., Begley, T.P., and Ealick, S.E., Proc. Natl. Acad. Sci. 97, 2005 (2000).]
O
O
O
O
H
CH
H
N
H
HN
Dihydroorotate
FMN
Coenzyme QH
2
COO
_
H
5
5
6
O
O
C
C
H
N
H
HN
Orotate
COO
_
H
3
C
H
3
C
H
3
CO
H
3
CO CH
3
(CH
2
CH
3
CH
2
)
10
H
CH C
N
N
N
N
R
O
O
H
FMNH
2
H
3
C
H
3
C
N
N
H
N
N
R
H
OH
OH
Coenzyme Q
H
3
CO
H
3
CO CH
3
(CH
2
CH
3
CH
2
)
10
H
CH C
O
O
O
–
–
O
O
O
H
H
2
N+
Lys 62
O
N
R
H
N
O
Asp 60
–
O
O
C
O
O
NH
2
Lys 62
O H
N
R
H
N
O
Asp 60
O
–
O
O
O
‡
H
H
2
N␦+
Lys 62
O
N
R
H
N
O
Asp 60
␦–
␦–
protonated to yield FMNH
2
.The FMNH
2
(or FMNH
) then
reacts with coenzyme Q acquired from the inner mitochondrial
membrane to regenerate the enzyme in its FMN form and yield
coenzyme QH
2
, which then re-enters the inner mitochondrial
membrane.
JWCL281_c28_1107-1142.qxd 6/8/10 10:39 AM Page 1117
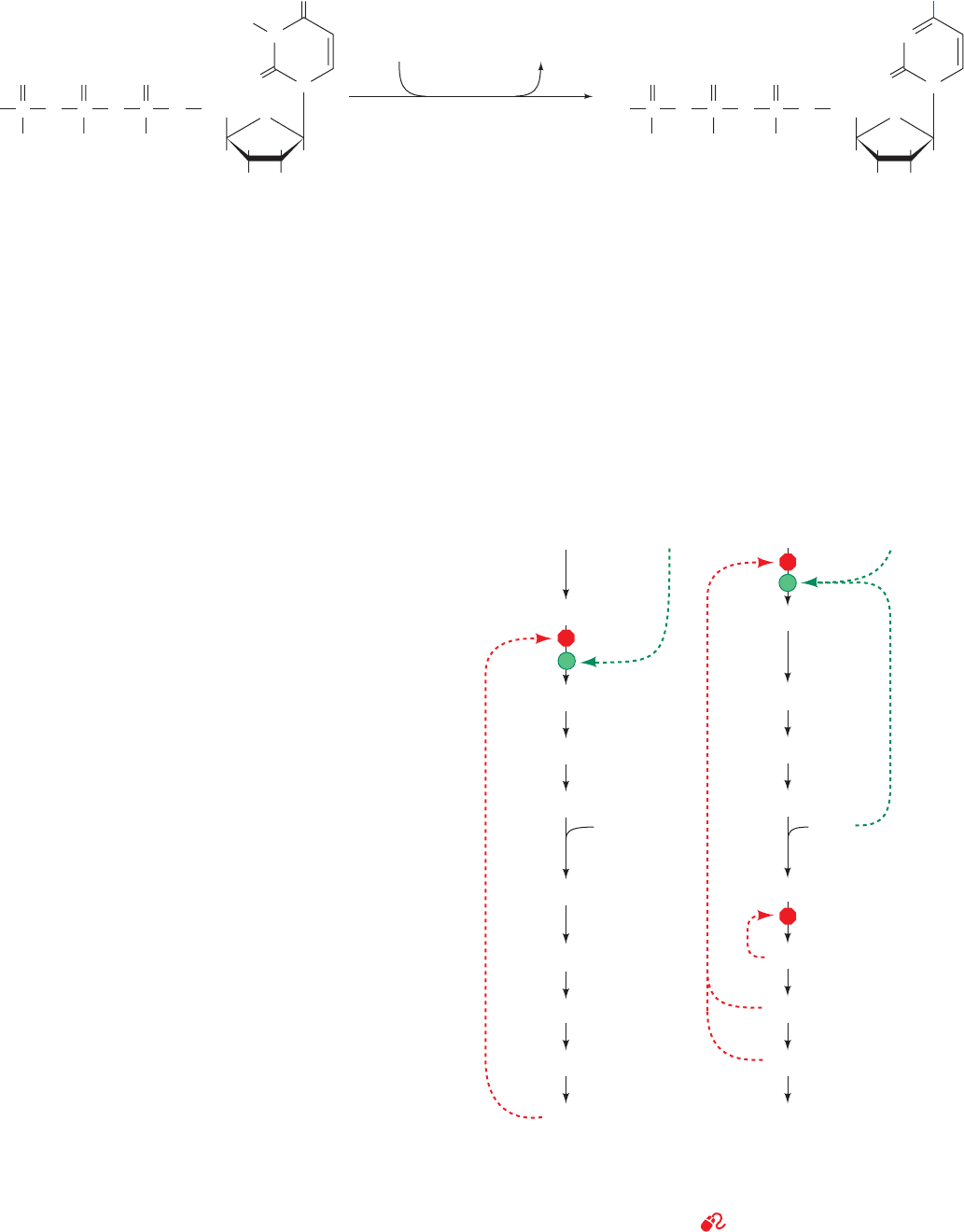
phosphate synthetase II, ATCase, and dihydroorotase,
occur on a single 2225-residue polypeptide chain known as
CAD, which forms 1400-kD homohexamers. Similarly,
Reactions 5 and 6 of the animal pyrimidine pathway are
catalyzed by a single 480-residue polypeptide named UMP
synthase that forms 102-kD homodimers.
B. Synthesis of UTP and CTP
The synthesis of UTP from UMP is analogous to the syn-
thesis of purine nucleotide triphosphates (Section 28-1B).
The process occurs by the sequential actions of a nucleo-
side monophosphate kinase and nucleoside diphosphate
kinase:
CTP is formed by amination of UTP by CTP synthetase
(Fig. 28-10). In animals, the amino group is donated by
glutamine, whereas in bacteria it is supplied directly by
ammonia.
C. Regulation of Pyrimidine
Nucleotide Biosynthesis
In bacteria, the pyrimidine biosynthesis pathway is prima-
rily regulated at Reaction 2, the ATCase reaction
(Fig. 28-11a). In E. coli, control is exerted there through
the allosteric stimulation of ATCase by ATP and its inhibi-
tion by CTP (Section 13-4). In many bacteria, however,
UTP is the major ATCase inhibitor.
In animals, ATCase is not a regulatory enzyme. Rather,
pyrimidine biosynthesis is controlled by the activity of
carbamoyl phosphate synthetase II, which is inhibited by
UDP and UTP and activated by ATP and PRPP (Fig.
28-11b). A second level of control in the mammalian path-
way occurs at OMP decarboxylase, for which UMP and to
a lesser extent CMP are competitive inhibitors. In all or-
ganisms, the rate of OMP production varies with the avail-
ability of its precursor, PRPP. The PRPP level, it will be re-
called, depends on the activity of ribose phosphate
pyrophosphokinase, which is inhibited by ADP and GDP
(Section 28-1A).
UDP ATP Δ UTP ADP
UMP ATP Δ UDP ADP
a. Orotic Aciduria Results from an
Inherited Enzyme Deficiency
Orotic aciduria, an inherited human disease, is charac-
terized by the excretion of large amounts of orotic acid in
the urine, retarded growth, and severe anemia. It results
from a deficiency in the bifunctional enzyme catalyzing
1118 Chapter 28. Nucleotide Metabolism
Figure 28-10 Synthesis of CTP from UTP.
Figure 28-11 Regulation of pyrimidine biosynthesis. The
control networks are shown for (a) E. coli and (b) animals. Red
octagons and green dots indicate control points. Feedback
inhibition is represented by dashed red arrows and activation is
indicated by dashed green arrows.
See the Animated Figures
–
O
O
–
O
–
POP
O
O
–
O OP
OO
H
2
C
O
O
N
O
N
H
H
OHOH
H
–
O
O
–
O
–
POP
O
O
–
O OP
OO
H
2
C
O
O
N
NH
2
N
H
OHOH
H
UTP
Glutamine
+ ATP + H
2
O
Glutamate
+ ADP + P
i
CTP synthetase
HH HH
CTP
pyrimidine
HCO
3
(a) (b)
E. coli
biosynthesis
Animal pyrimidine
biosynthesis
–
+ Glutamine +
Carbamoyl phosphate
Carbamoyl aspartate
Dihydroorotate
Orotate
OMP
UMP
UTP
CTP
PRPP
HCO
3
–
+ Glutamine +
Carbamoyl phosphate
Carbamoyl aspartate
Dihydroorotate
Orotate
OMP
UMP
UTP
CTP
PRPP
Inhibition
ATP
Activation
UDP
UDP
Inhibition
ATP
Activation
JWCL281_c28_1107-1142.qxd 4/22/10 9:16 AM Page 1118
Reactions 5 and 6 of pyrimidine nucleotide biosynthesis.
Consideration of the biochemistry of this situation led to
its effective treatment: the administration of uridine and/or
cytidine. The UMP formed through the phosphorylation of
these nucleosides, besides replacing that normally synthe-
sized, inhibits carbamoyl phosphate synthetase II so as to
attenuate the rate of orotic acid synthesis. Few other ge-
netic deficiencies in pyrimidine nucleotide biosynthesis are
known in humans, presumably because most such defects
are lethal in utero.
3 FORMATION OF
DEOXYRIBONUCLEOTIDES
DNA differs chemically from RNA in two major respects:
(1) its nucleotides contain 2¿-deoxyribose residues rather
than ribose residues, and (2) it contains the base thymine
(5-methyluracil) rather than uracil. In this section we con-
sider the biosynthesis of these DNA components.
A. Production of Deoxyribose Residues
Deoxyribonucleotides are synthesized from their correspon-
ding ribonucleotides by the reduction of their C2¿ position
rather than by their de novo synthesis from deoxyribose-
containing precursors.
This pathway was established through Irwin Rose’s study
of how rats metabolize cytidine that is
14
C-labeled in both
its base and ribose components.The dCMP recovered from
the rats’ DNA had the same labeling ratio in its cytosine
and deoxyribose residues as had the original cytidine, indi-
cating that the DNA’s components remained linked during
DNA synthesis. If the cytosine and the ribose residues had
become separated, dilution of the labeled cytosine and ri-
bose residues with unlabeled residues, which are present in
rat tissues in different amounts, would have altered this
ratio.
The enzymes that catalyze the formation of deoxyribonu-
cleotides by the reduction of the corresponding ribonu-
cleotides are named ribonucleotide reductases (RNRs).
Three classes of RNRs are known that differ in their
NDP
dNDP
O
H
2
C
HH
OHOH
HH
O
O
PO
O
–
O
P
–
O
O
–
Base
O
H
2
C
HH
HOH
HH
O
O
PO
O
–
O
P
–
O
O
–
Base
substrates (NDP or NTP), the cofactors they employ, and in
the way they obtain reducing equivalents (see below).
Class I and II RNRs are widely distributed among prokary-
otes; some species have a Class I RNR, whereas other,
sometimes related species have a Class II RNR. However,
all eukaryotes except a few unicellular species have Class I
RNRs. Class III RNRs occur in prokaryotes that can grow
anaerobically. (Class III RNRs are O
2
-sensitive whereas
Class I RNRs require O
2
for activation; see below.) In fact,
E. coli, which can grow both aerobically and anaerobically,
expresses a Class I and a Class III RNR. In what follows,
we shall mainly discuss the mechanism of Class I RNRs but
end with a consideration of the evolutionary relationships
among the different classes of RNRs.
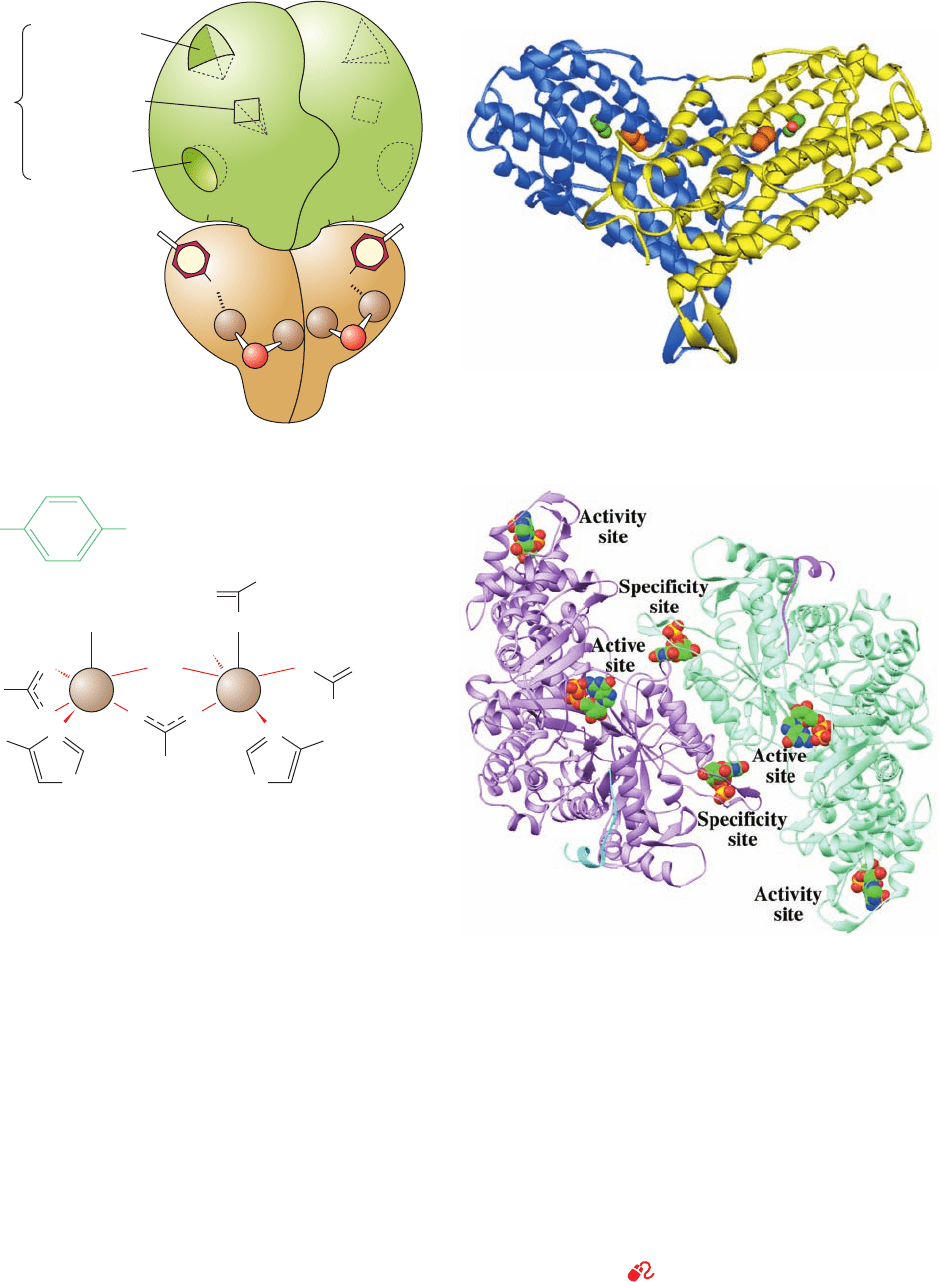
a. Class I Ribonucleotide Reductase:
Structure and Mechanism
The E. coli Class I RNR, as Peter Reichard demon-
strated, is mainly present in vitro as a heterotetramer that
can be decomposed to two catalytically inactive homo-
dimers, R1
2
(761-residue subunits) and R2
2
(375-residue sub-
units), which together form the enzyme’s two active sites
(Fig. 28-12a). Each R1 subunit contains a substrate binding
site as well as three independent effector binding sites that
control both the enzyme’s catalytic activity and its sub-
strate specificity (see below). R1’s catalytic residues in-
clude several redox-active thiol groups.
The X-ray structure of R2
2
(Fig. 28-12b), determined by
Hans Eklund, reveals that each of its subunits contains a
novel binuclear Fe(III) prosthetic group whose two Fe(III)
ions are bridged by both an O
2
ion (a -oxo bridge) and
the carboxyl group of Glu 115 (Fig. 28-12c). Each Fe(III) is
further liganded by two carboxyl O atoms from Asp or Glu
residues, a His N
atom, and a water molecule. The Fe(III)
complex interacts with Tyr 122 to form, as EPR measure-
ments indicate, an unusual tyrosyl free radical (TyrO) that
is 5 Å from the closest Fe atom and is buried 10 Å beneath
the surface of the protein, where it is out of contact with
solvent and any oxidizable side chain [tyrosyl radicals have
also been observed in cytochrome c oxidase (Section 22-
2C5c) and in Photosystem II (Section 24-2Cd)].
The E. coli RNR is inhibited by hydroxyurea, which
specifically quenches (destroys) the tyrosyl radical, and by
8-hydroxyquinoline, which chelates Fe
3
ions.
Mammalian RNRs have similar characteristics to the
E. coli enzyme. Indeed, hydroxyurea is in clinical use as an
antitumor agent.
If E. coli RNR is incubated with [3¿-
3
H]UDP, a small
but reproducible fraction of the
3
H is released as
3
H
2
O.
This observation, together with kinetic, spectroscopic, and
site-directed mutagenesis studies, led JoAnne Stubbe to
OH
OHNHC
O
H
2
N
N
Hydroxyurea 8-Hydroxyquinoline
Section 28-3. Formation of Deoxyribonucleotides 1119
JWCL281_c28_1107-1142.qxd 6/8/10 10:39 AM Page 1119
R2 dimer
R1 dimer
(a)
Substrate-binding site
(ATP, GDP, UDP, CDP)
Specificity site
(ATP, dATP,
dGTP, dTTP)
Activity site
(ATP, dATP)
Hexamerization
site (ATP)
Allosteric
sites
Fe
3+
Fe
3+
SH
SH
Tyr
O
•
O
•
Tyr
SH
SH
O
O
Fe
3+
Fe
3+
O•
Tyr 122
Asp 84
Glu 115
H
2
O
H
2
O
O
2–
O
–
–
O
N
O
Fe 1
(c)
N
H
His 118
Glu 204
Glu 238
O
–
O
O
–
O
O
N
Fe 2
N
H
His 241
1120 Chapter 28. Nucleotide Metabolism
Figure 28-12 (Opposite) Class I ribonucleotide reductase from
E. coli. (a) A schematic diagram of its quaternary structure.The
enzyme consists of two pairs of identical subunits, R1
2
and R2
2
.
Each R2 subunit contains a binuclear Fe(III) complex that
generates a phenoxy radical at its Tyr 122. The R1 subunits each
contain three different allosteric effector sites and five
catalytically important Cys residues.The enzyme’s two active
sites are located near the interface between neighboring R1 and
R2 subunits. (b) The X-ray structure of R2
2
as viewed
perpendicular to its 2-fold axis with the dimer’s longest
dimension in the horizontal plane. One subunit of the
homodimeric protein is shown in blue and the other in yellow.
The Fe(III) ions of its binuclear Fe complexes are represented by
orange spheres and the radical-harboring Tyr 122 side chains are
drawn in space-filling form with their C and O atoms green and
red. Note that each subunit consists mainly of a bundle of eight
unusually long helices. (c) The binuclear Fe(III) complex of R2.
Each Fe(III) ion is octahedrally coordinated by a His N
␦
atom
and five O atoms, including those of the O
2⫺
ion and the Glu
carboxyl group that bridge the two Fe(III) ions. (d) The X-ray
structure of the R1 dimer, each subunit of which is in complex
with the 20-residue C-terminal peptide of R2 together with GDP
in the active site and dTTP in the specificity site. The ATP analog
AMPPNP bound in the activity site of the closely similar
complex of R1 with the 20-residue peptide and AMPPNP has
been superimposed on this structure. The structure is viewed
along its 2-fold axis with its two subunits lavender and light
green, the two R2 peptides cyan and magenta, and the GDP,
dTTP, and ATP shown in space-filling form colored according to
atom type (C green, N blue, O red, and P gold). [Parts b and d
based on X-ray structures by Hans Eklund, Swedish University
of Agricultural Sciences, Uppsala, Sweden. PDBids (b) 1RIB and
(d) 3R1R and 4R1R.]
See the Animated Figures and Interactive
Exercise 28
(b)
(d)
JWCL281_c28_1107-1142.qxd 10/19/10 9:58 AM Page 1120
