Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

up to 40% of their total body weight and has high mito-
chondrial capacity. Homologs of UCP1 have been identi-
fied: UCP2 occurs in many tissues including white adipose
tissue, whereas UCP3 occurs in brown adipose tissue, white
adipose tissue, and muscle. Leptin has been shown to up-
regulate UCP2. However, it has yet to be demonstrated
that UCP3 in muscle participates in diet-induced thermo-
genesis. ATP-hydrolyzing substrate cycles such as that be-
tween fatty acids and triacylglycerol in adipose tissue (Sec-
tion 27-2C) may also be involved.
H. Did Leptin Evolve as a Thrifty Gene?
The unusual behavior of leptin, which serves to control
weight in normal-weight individuals while its concentration
continues to climb without apparent effect in obese individu-
als, has led to the proposal that leptin evolved as a “thrifty
gene.” In hunter-gatherer societies,it was a distinct advantage
to be able to survive intermittent famines. In order to do this,
fat must be stored in adipose tissue in times of plenty, making
short-term obesity advantageous. However, the accumula-
tion of fatty acids and lipids in non-adipose tissue results in
coronary artery disease, insulin resistance,and diabetes (Sec-
tion 27-4B). Leptin, by directly stimulating the oxidation of
fatty acids as well as inhibiting the accumulation of lipids in
non-adipose tissue, is thought to protect against these dis-
eases during short-term obesity, thereby providing an evolu-
tionary advantage. However, in recent times in industrialized
nations, the unprecedented availability of food and lack of
famine has made obesity a long-term rather than a short-
term condition, which is a liability rather than a benefit.
4 METABOLIC ADAPTATION
In this section we consider the body’s responses to two
metabolically abnormal situations: (1) starvation and (2) the
disease diabetes mellitus.
A. Starvation
Glucose is the metabolite of choice of both brain and
working muscle. Yet, the body stores less than a day’s sup-
ply of carbohydrate (Table 27-1).Thus, the low blood sugar
caused by even an overnight fast results, through an in-
crease in glucagon secretion and a decrease in insulin se-
cretion, in the mobilization of fatty acids from adipose tis-
sue (Section 25-5). The diminished insulin level also
inhibits glucose uptake by muscle tissue. Muscles therefore
switch from glucose to fatty acid metabolism for energy
production. The brain, however, still remains heavily de-
pendent on glucose.
In animals, glucose cannot be synthesized from fatty
acids. This is because neither pyruvate nor oxaloacetate,
the precursors of glucose in gluconeogenesis (Section 23-1),
can be synthesized from acetyl-CoA (the oxaloacetate in
the citric acid cycle is derived from acetyl-CoA but the
cyclic nature of this process requires that the oxaloacetate
be consumed as fast as it is synthesized; Section 21-1A).
During starvation, glucose must therefore be synthesized
from the glycerol product of triacylglycerol breakdown
and, more importantly, from the amino acids derived from
the proteolytic degradation of proteins, the major source of
which is muscle.Thus, after a 40-hour fast, gluconeogenesis
supplies ⬃96% of the glucose produced by the liver. How-
ever,the continued breakdown of muscle during prolonged
starvation would ensure that this process became irre-
versible since a large muscle mass is essential for an animal
to move about in search of food.The organism must there-
fore make alternate metabolic arrangements.
After several days of starvation, gluconeogenesis has so
depleted the liver’s oxaloacetate supply that this organ’s
ability to metabolize acetyl-CoA via the citric acid cycle is
greatly diminished. Rather, the liver converts the acetyl-
CoA to ketone bodies (Section 25-3), which it releases into
the blood.The brain gradually adapts to using ketone bod-
ies as fuel through the synthesis of the appropriate en-
zymes: After a 3-day fast, only about one-third of the
brain’s energy requirements are satisfied by ketone bodies
but after 40 days of starvation, ⬃70% of its energy needs
are so met. The rate of muscle breakdown during pro-
longed starvation consequently decreases to ⬃25% of its
rate after a several-day fast.The survival time of a starving
individual is therefore much more dependent on the size of
his or her fat reserves than it is on his or her muscle mass.
Indeed, highly obese individuals can survive for over a year
without eating (and have occasionally done so in clinically
supervised weight reduction programs).
a. Caloric Restriction May Increase Longevity
Caloric restriction is a modified form of starvation
whereby energy intake is reduced 30–40%, while micronu-
trient (vitamin and mineral) levels are maintained. Rodents
kept on such a diet live up to 50% longer than rodents on
normal diets and exhibit fewer of the debilitating symptoms
of old age.The life spans of a large range of organisms from
yeast to primates are similarly extended. Considerable
research effort is being expended to determine the bio-
chemical basis of these observations.
Section 27-4. Metabolic Adaptation 1101
Table 27-1 Fuel Reserves for a Normal 70-kg Man
Fuel Mass (kg) Calories
a
Tissues
Fat (adipose triacyglycerols) 15 141,000
Protein (mainly muscle) 6 24,000
Glycogen (muscle) 0.150 600
Glycogen (liver) 0.075 300
Circulating fuels
Glucose (extracellular fluid) 0.020 80
Free fatty acids (plasma) 0.0003 3
Triacylglycerols (plasma) 0.003 30
Total 166,000
a
One (dieter’s) Calorie ⫽ 1 kcal ⫽ 4.184 kJ.
Source: Cahill, G.F., Jr., New Engl. J. Med. 282, 669 (1970).
JWCL281_c27_1088-1106.qxd 6/8/10 8:49 AM Page 1101
B. Diabetes Mellitus
The polypeptide hormone insulin acts mainly on muscle,
liver, and adipose tissue cells to stimulate the synthesis of
glycogen, fats, and proteins while inhibiting the breakdown
of these metabolic fuels. In addition, insulin stimulates the
uptake of glucose by most cells, with the notable exception
of brain and liver cells. Together with glucagon, which has
largely opposite effects, insulin acts to maintain the proper
level of blood glucose.
In the disease diabetes mellitus, which is the third leading
cause of death in the United States after heart disease and
cancer, insulin either is not secreted in sufficient amounts or
does not efficiently stimulate its target cells. As a conse-
quence, blood glucose levels become so elevated that the
glucose “spills over” into the urine, providing a convenient
diagnostic test for the disease. Yet, despite these high blood
glucose levels, cells “starve” since insulin-stimulated glucose
entry into cells is impaired. Triacylglycerol hydrolysis, fatty
acid oxidation, gluconeogenesis, and ketone body formation
are accelerated and, in a condition termed ketoacidosis, ke-
tone body levels in the blood become abnormally high.Since
ketone bodies are acids, their high concentration puts a
strain on the buffering capacity of the blood and on the kid-
ney, which controls blood pH by excreting the excess H
⫹
into the urine (Section 27-2E). This unusually high excess
H
⫹
excretion is accompanied by NH
4
⫹
,Na
⫹
,K
⫹
,P
i
, and H
2
O
excretion, causing severe dehydration (which compounds
the dehydration resulting from the osmotic effect of the high
glucose concentration in the blood; excessive thirst is a clas-
sic symptom of diabetes) and a decrease in blood volume—
ultimately life-threatening situations.
There are two major forms of diabetes mellitus:
1. Insulin-dependent, type 1, or juvenile-onset diabetes
mellitus, which most often strikes suddenly in childhood.
2. Noninsulin-dependent, type 2, or maturity-onset
diabetes mellitus, which usually develops rather gradually
after the age of 40.
a. Insulin-Dependent Diabetes Is Caused by a
Deficiency of Pancreatic  Cells
In insulin-dependent (type 1) diabetes mellitus, insulin
is absent or nearly so because the pancreas lacks or has
defective  cells. This condition results, in genetically sus-
ceptible individuals (see below), from an autoimmune re-
sponse that selectively destroys their  cells. Individuals
with insulin-dependent diabetes, as Frederick Banting and
George Best first demonstrated in 1921, require daily
insulin injections to survive and must follow carefully bal-
anced diet and exercise regimens. Their life spans are,
nevertheless, reduced by up to one-third as a result of de-
generative complications such as kidney malfunction,
nerve impairment, and cardiovascular disease, which ap-
parently arise from the imprecise metabolic control pro-
vided by periodic insulin injections. The hyperglycemia
(high blood [glucose]) of diabetes mellitus also leads to
blindness through retinal degeneration and the glucosyla-
tion of lens proteins, which causes cataracts (Fig. 27-9).
Perhaps newly developed systems that monitor blood glu-
cose levels and continuously deliver insulin in the required
amounts will rectify this situation.
The usually rapid onset of the symptoms of insulin-
dependent diabetes had suggested that the autoimmune at-
tack on the pancreatic  cells responsible for this disease is
one of short duration. Typically, however, the disease
“brews” for several years as the aberrantly aroused immune
system slowly destroys the  cells. Only when ⬎80% of these
cells have been eliminated do the classic symptoms of dia-
betes suddenly emerge. Consequently, one of the most suc-
cessful treatments for insulin-dependent diabetes is a -cell
transplant, a procedure that became possible with the devel-
opment of relatively benign immunosuppressive drugs.
Why does the immune system attack the pancreatic 
cells? It has long been known that certain alleles (genetic
variants) of the Class II major histocompatibility complex
(MHC) proteins are particularly common in insulin-
dependent diabetics [MHC proteins are highly polymorphic
(variable within a species) immune system components to
which cell-generated antigens such as viral proteins must
bind in order to be recognized as foreign; Sections 35-2Aa
and 35-2E]. It is thought that autoimmunity against  cells is
induced in a susceptible individual by a foreign antigen, per-
haps a virus, which immunologically resembles some  cell
component. The Class II MHC protein that binds this anti-
gen does so with such tenacity that it stimulates the immune
system to launch an unusually vigorous and prolonged at-
tack on the antigen. Some of the activated immune system
cells eventually make their way to the pancreas, where they
initiate an attack on the  cells due to the close resemblance
of the  cell component to the foreign antigen.
b. Noninsulin-Dependent Diabetes Is
Characterized by Insulin Resistance as
Well as Impaired Insulin Secretion
Noninsulin-dependent (type 2) diabetes mellitus
(NIDDM), which accounts for over 90% of the diagnosed
cases of diabetes and affects 18% of the population over
65 years of age, usually occurs in obese individuals with a
genetic predisposition for this condition (although one
that differs from that associated with insulin-dependent
1102 Chapter 27. Energy Metabolism: Integration and Organ Specialization
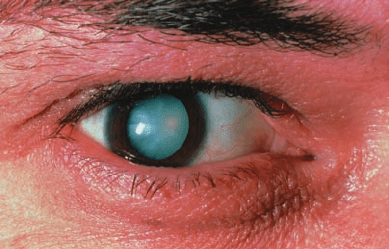
Figure 27-9 Photo of a diabetic cataract. The accumulation of
glucose in the lens leads to swelling and precipitation of lens
proteins.The resulting opacification causes blurred vision and
ultimately complete loss of sight. [© Sue Ford/Photo Researchers.]
JWCL281_c27_1088-1106.qxd 4/21/10 9:42 AM Page 1102
diabetes). These individuals may have normal or even
greatly elevated insulin levels. Their symptoms arise from
insulin resistance, an apparent lack of sensitivity to insulin
in normally insulin-responsive cells.
The hyperglycemia that accompanies insulin resistance
induces the pancreatic  cells to increase their production
of insulin. Yet the high basal level of insulin secretion di-
minishes the ability of the  cells to respond to further in-
creases in blood glucose. Consequently, the hyperglycemia
and its attendant complications tend to worsen over time.
A small percentage of cases of type II diabetes result
from mutations in the insulin receptor that affect its
insulin-binding ability or tyrosine kinase activity. However,
a clear genetic cause has not been identified in the vast ma-
jority of cases. It is therefore likely that many factors play a
role in the development of this disease. For example, the in-
creased insulin production resulting from overeating may
eventually suppress the synthesis of insulin receptors. This
hypothesis accounts for the observation that diet alone is
often sufficient to control this type of diabetes.
Insulin resistance, which may precede NIDDM by as
much as 10 to 20 years, appears to be caused by an inter-
ruption in the insulin signaling pathway (Section 19-4F).
Gerald Shulman has proposed that this interruption is
caused by a Ser/Thr kinase cascade that phosphorylates
proteins known as insulin receptor substrates (IRSs; Sec-
tion 19-3Cg) so as to decrease their ability to be phospho-
rylated on their Tyr residues by activated insulin receptor.
Tyrosine phosphorylation is required for IRS activation
and communication with phosphoinositide 3-kinase (PI3K;
Section 19-4D), which subsequently activates the translo-
cation of GLUT4-containing vesicles to the cell surface for
increased glucose transport into cells (Section 20-2Ec).The
original Ser/Thr kinase cascade is triggered by the activa-
tion of an isoform of protein kinase C (PKC; Section 19-4C)
caused by an increase in fatty acyl-CoA,diacylglycerol,and
ceramides (Section 12-1D) resulting from elevated free
fatty acids (Fig. 27-10). The failure to activate IRSs de-
creases the cell’s response to insulin (Fig. 27-11).
c. Substances That Activate AMPK Attenuate the
Symptoms of Noninsulin-Dependent Diabetes
Other treatments for noninsulin-dependent diabetes
are drugs such as metformin and the thiazolidinediones
(TZDs),
which decrease insulin resistance by either suppressing
glucose release by the liver (metformin) or promoting
A thiazolidinedione (TZD)
Metformin
O
N
H
O
R
S
H
3
C
NH
2
NH
NH
NHN
H
3
C
Section 27-4. Metabolic Adaptation 1103
Figure 27-10 The mechanism through which high concentra-
tions of free fatty acids cause insulin resistance. Elevated concen-
trations of free fatty acids in the blood diffuse into muscle cells
where they are converted to fatty acyl-CoA, diacylglycerols, and
ceramides.These lipotoxic substances activate an isoform of
protein kinase C (PKC), triggering a Ser/Thr kinase cascade that
results in the phosphorylation of IRS-1 and IRS-2.This
phosphorylation inhibits the Tyr phosphorylation required for
transmission of the insulin signal, thereby decreasing the
activation of PI3K, which decreases the rate of fusion of
GLUT4-containing vesicles with the plasma membrane and
hence the amount of glucose entering the cell. [Modified from
Shulman, G.I., J. Clin. Invest. 106, 173 (2000).]
Figure 27-11 Twenty-four-hour plasma glucose profiles in nor-
mal and noninsulin-dependent diabetic subjects. The basal level
of glucose and the peaks following meals are higher in the
diabetic individuals. [After Bell, G.I., Pilkis, S.J., Weber, I.T., and
Polonsky, K.S., Annu. Rev. Physiol. 58, 178 (1996).]
Plasma
Glucose
Glucose
Insulin receptor
Insulin
IRS-1/IRS-2 Ser/Thr phosphorylation
PI3K
IRS-1/IRS-2 Tyr phosphorylation
Fatty acyl-CoA,
diacylglycerols,
ceramides
Fatty acids
Ser/Thr kinase
cascade
PKC
GLUT4
240
480
720
960
1200
1440
0
0
20
Plasma [Glucose] (mM)
Time (min)
Non-insulin-dependent
diabetes
Normal
breakfast
15
10
5
lunch dinner
JWCL281_c27_1088-1106.qxd 4/21/10 9:42 AM Page 1103

insulin-stimulated glucose disposal in muscle (TZDs).
These drugs act by increasing AMPK activity but by differ-
ent mechanisms.TZDs cause a large increase in the AMP to
ATP ratio in muscle cells, with the expected concomitant in-
crease in AMPK phosphorylation and activity. Metformin,
however, stimulates LKB1 to phosphorylate and hence ac-
tivate AMPK (LKB1 knockout mice are insensitive to met-
formin). In both cases, the increase in AMPK activity de-
creases gluconeogenesis in liver and increases glucose
utilization in muscle (Fig. 27-4). In addition, the TZDs de-
crease insulin resistance by binding to and activating a
transcription factor known as a peroxisome proliferator–
activated receptor ␥ (PPAR␥), primarily in adipose tissue.
Among other things, PPAR␥ activation induces the synthe-
sis of adiponectin (Section 27-3B), which leads to an in-
crease in AMPK activity. In adipose tissue, AMPK action
leads to a decrease in lipolysis and fatty acid export, de-
creasing the concentration of free fatty acids in the blood
and therefore decreasing insulin resistance (see above).
Intriguingly, Ronald Evans has shown that transgenic
mice expressing an activated form of PPAR␥ in their skele-
tal muscles can run around twice the distance of wild-type
mice and are resistant to weight gain, even on a high fat
diet.This activated PPAR␥ induces an increase in the num-
ber of the aerobic and hence fatty acid–oxidizing slow-
twitch (Type I) muscle fibers (Section 17-3Ca) relative to
the largely anaerobic and hence less energy-efficient fast-
twitch (Type II) muscle fibers.
Rodent adipocytes secrete a 108-residue polypeptide
hormone called resistin. The hormone is named for its abil-
ity to block the action of insulin on adipocytes. In fact, re-
sistin production is decreased by TZDs, a phenomenon
that led to the discovery of resistin. Overproduction of
resistin was proposed to contribute to the development of
noninsulin-dependent diabetes. An interesting difference
between rodents and humans is that in humans, resistin is
produced by macrophages, a divergence whose evolution-
ary and functional implications are unclear.
d. Obesity Is a Contributing Factor
in Metabolic Syndrome
Metabolic syndrome is a disturbance in metabolism
characterized by insulin resistance, inflammation, and a
predisposition to several disorders including type 2 dia-
betes, hypertension, and atherosclerosis. These disorders
are accompanied by an increase in coronary heart disease.
Obesity, physical inactivity, and possibly genetic determi-
nants have been implicated in its occurrence, which affects
as many as 65 million people in the United States alone.
Exercise, calorie/weight reduction, adiponectin, leptin,
metformin, and TZDs have all been successfully used to
treat metabolic syndrome. Similarly, the PPAR␥ agonist
known as GW1516
GW1516
F
3
C
CH
3
N
S
S
O COOH
1104 Chapter 27. Energy Metabolism: Integration and Organ Specialization
alleviates the symptoms of metabolic syndrome in obese
men, probably by stimulating fatty acid oxidation.
Evans has shown that GW1516 greatly increases exer-
cise endurance in mice, particularly when it is administered
together with the AMPK agonist 5-aminoimidazole-4-
carboxamide ribotide [AICAR];
which is also a product of histidine biosynthesis (Section
26-5Be) and an intermediate in purine ribonucleotide
biosynthesis (Section 28-1A)]. This treatment mimics the
effects of the expression of activated PPAR␥, which sug-
gests that the administration of GW1516 and AICAR can
confer some of the benefits of exercise without actually ex-
ercising. Indeed, the World Anti-Doping Agency has
placed these compounds on the list of performance-
enhancing drugs that athletes are forbidden from taking.
e. DNA Chip Technology Permits the Integrated
Study of Metabolic Regulation
Our ability to understand the integrated nature of me-
tabolism and its genetic regulation in health and disease
has taken a giant step forward with the advent of DNA
chips (microarrays; Section 7-6B). For example, Ronald
Kahn has used this technology to study the genetic basis of
the metabolic abnormalities underlying both obesity and
diabetes. To do so, he isolated the mRNA from the skeletal
muscle of normal, diabetic, and insulin-treated diabetic
mice and reverse-transcribed it to cDNA (Section 5-5Fa),
which was then hydridized to oligonucleotide microarrays
that represented 14,288 mouse genes. Thereby, 129 up-
regulated and 106 down-regulated genes were identified in
diabetic mice. Not surprisingly, the expression of the mRNAs
encoding enzymes of the fatty acid -oxidation pathway
were increased, whereas those for GLUT4, glucokinase,
the E1 component of the pyruvate dehydrogenase multien-
zyme complex, and the subunits of all four mitochondrial
electron-transport chain complexes were coordinately de-
creased. Intriguingly, only about half of these changes in
gene expression could be reversed by insulin treatment.
Thus, the post-genomic era will almost certainly witness an
explosion in our knowledge of metabolic regulation that
should yield major health benefits. Nevertheless, our ability
to sensibly interpret this huge influx of information may
prove to be the greatest challenge.
N
CCH
C
C
O
N
CH
2
O
2–
O
3
P
HH
HH
OH OH
O
H
2
N
H
2
N
5-Aminoimidazole-4-carboxamide ribotide (AICAR)
JWCL281_c27_1088-1106.qxd 4/21/10 9:42 AM Page 1104

References 1105
1 Major Pathways and Strategies of Energy Metabolism:
A Summary
The complex network of processes involved in
energy metabolism are distributed among different compart-
ments within cells and in different organs of the body. These
processes function to generate ATP “on demand,” to generate
and store glucose, triacylglycerols, and proteins in times of
plenty for use when needed, and to keep the concentration of
glucose in the blood at the proper level for use by organs such
as the brain, whose sole fuel source, under normal conditions,
is glucose.The major energy metabolism pathways include gly-
colysis, glycogen degradation and synthesis, gluconeogenesis,
the pentose phosphate pathway, and triacylglycerol and fatty
acid synthesis, which are cytosolically based, and fatty acid ox-
idation, the citric acid cycle, and oxidative phosphorylation,
which are confined to the mitochondrion. Amino acid degra-
dation occurs, in part, in both compartments. The mediated
membrane transport of metabolites therefore also plays an es-
sential metabolic role.
2 Organ Specialization The brain normally consumes
large amounts of glucose. Muscle, under intense ATP demand
such as in sprinting, degrades glucose and glycogen anaerobi-
cally, thereby producing lactate, which is exported via the
blood to the liver for reconversion to glucose through gluco-
neogenesis. During moderate activity, muscle generates ATP
by oxidizing glucose from glycogen, fatty acids, and ketone
bodies completely to CO
2
and H
2
O via the citric acid cycle and
oxidative phosphorylation. Adipose tissue stores triacylglyc-
erols and releases fatty acids into the bloodstream in response
to the organism’s metabolic needs. These metabolic needs are
communicated to adipose tissue by means of the hormones in-
sulin, which indicates a fed state in which storage is appropri-
ate, and glucagon, epinephrine, and norepinephrine,which sig-
nal a need for fatty acid release to provide fuel for other
tissues. The liver, the body’s central metabolic clearinghouse,
maintains blood glucose concentrations by storing glucose as
glycogen in times of plenty and releasing glucose in times of
need both by glycogen breakdown and by gluconeogenesis. It
also converts fatty acids to ketone bodies for use by peripheral
tissues. During a fast, it breaks down amino acids resulting
from protein degradation to metabolic intermediates that can
be used to generate glucose. The kidney filters out urea from
the blood, recovers important metabolites, and maintains pH
balance. To do so, glutamine is broken down to produce NH
⫹
4
for H
⫹
excretion.The resulting ␣-ketoglutarate product is con-
verted to CO
2
to resupply HCO
⫺
3
to the blood to maintain
its buffering capacity. During starvation, the kidney uses the
␣-ketoglutarate from glutamine breakdown for gluconeo-
genesis.
3 Metabolic Homeostasis: Regulation of Appetite,
Energy Expenditure, and Body Weight
AMP-dependent
protein kinase (AMPK), the cell’s fuel gauge, senses the cell’s
need for ATP and activates metabolic breakdown pathways
while inhibiting biosynthetic pathways. Adiponectin, an
adipocyte hormone that increases insulin sensitivity, acts by
activating AMPK. Appetite is suppressed by the actions of
leptin, a hormone produced by adipose tissue, insulin, pro-
duced by the  cells of the pancreas, and PYY
3–36
, produced by
the gastrointestinal tract, which act in the hypothalamus to in-
hibit the secretion of neuropeptide Y (NPY) and stimulate the
secretion of ␣-MSH and CART. This decreases the appetite
and hence food intake. Ghrelin, a hormone secreted by the
empty stomach, opposes the actions of leptin, insulin, and
PYY
3–36
, stimulating appetite and food intake. Leptin also acts
in peripheral tissues to stimulate energy expenditure by fatty
acid oxidation and thermogenesis.
4 Metabolic Adaptation During prolonged starvation,
the brain slowly adapts from the use of glucose as its sole fuel
source to the use of ketone bodies, thereby shifting the meta-
bolic burden from protein breakdown to fat breakdown. Dia-
betes mellitus is a disease in which insulin either is not se-
creted or does not efficiently stimulate its target tissues,
leading to high concentrations of glucose in the blood and
urine. Cells “starve” in the midst of plenty since they cannot
absorb blood glucose and their hormonal signals remain those
of starvation.Abnormally high production of ketone bodies is
one of the most dangerous effects of uncontrolled diabetes.
Metabolic syndrome is caused by obesity, physical inactivity,
and possibly genetic determinants. It symptoms can be re-
lieved by substances that activate AMPK.
CHAPTER SUMMARY
Chapters 17 to 26 of this text.
Batterham, R.L., et al., Gut hormone PYY
3–36
physiologically in-
hibits food intake, Nature 418, 650–654 (2002).
Brüning, J.C., Gautam, D., Burks, D.J., Gillette, J., Schubert, M.,
Orban, P.C., Klein, R., Krone, W., Müller-Weiland, D., and
Kahn, C.R., Role of brain insulin receptor in control of body
weight and reproduction, Science 289, 2122–2125 (2000).
Carling, D., The AMP-activated protein kinase cascade—a unify-
ing system for energy control, Trends Biochem. Sci. 29, 18–24
(2004).
Coll,A.P., Farooqi, I.S., and O’Rahilly, S.,The hormonal control of
food intake, Cell 129, 251–262 (2007).
Evans, J.L., Goldfine, I.D., Maddux, B.A., and Grodsky, G.M.,
Oxidative stress and stress-activated signaling pathways: a
unifying hypothesis of type 2 diabetes, Endocrine Rev. 23,
599–622 (2002).
Flier, J.S., Obesity wars: Molecular progress confronts an expand-
ing epidemic, Cell 116, 337–350 (2004).
Kadowaki, T. and Yamauchi, T., Adiponectin and adiponectin
receptors, Endocrine Rev. 26, 439–451 (2005).
Lowell, B.B. and Spiegelman, B.M., Towards a molecular under-
standing of adaptive thermogenesis, Nature 404, 652–660 (2000).
Montague, C.T., et al., Congenital leptin deficiency is associated
with severe early-onset obesity in humans, Nature 387, 903–908
(1997).
Moreno-Aliaga, M.J., Marti, A., García-Foncillas, J. and Martínes,
J.A., DNA hybridization arrays: a powerful technology for
nutritional and obesity research, Br. J.Nutr. 86, 119–122 (2001).
REFERENCES
JWCL281_c27_1088-1106.qxd 6/8/10 8:49 AM Page 1105

1106 Chapter 27. Energy Metabolism: Integration and Organ Specialization
Nakar, V.A., et al., AMPK and PPAR␥ agonists are exercise
mimetics, Cell 134, 405–415 (2008).
Nakazato, M., Murakami, N., Date, Y., Kojima, M., Matsuo, H.,
Kangawa, K., and Matsukara, S., A role for ghrelin in the
central regulation of feeding, Nature 409, 194–198 (2001).
Schwartz, M.W. and Morton, G.J., Keeping hunger at bay, Nature
418, 595–597 (2002).
Shaw, R.J., Lamia, K.A., Vasquez, D., Koo, S.-H., Bardeesy, N.,
DePinho, R.A., Montminy, M., and Cantley, L.C., The kinase
LKB1 mediates glucose homeostasis in liver and therapeutic
effects of metformin, Science 310, 1642–1646 (2005).
Shulman, G.I., Cellular mechanisms of insulin resistance, J. Clin.
Invest. 106, 171–176 (2000).
Towler, M.C. and Hardie, D.G., AMP-Activated protein kinase in
metabolic control and insulin signaling, Circ. Res. 100, 328–341
(2007).
Tshöp, M., Smiley, D.L., and Heiman, M.L., Ghrelin induces
adiposity in rodents, Nature 407, 908–913 (2000).
Type 2 Diabetes, Science 307, 369–387 (2005). [A series of inform-
ative articles on the origins of type 2 diabetes and its relation-
ship to obesity.]
Unger, R.H., Leptin physiology: a second look, Regul. Pept. 92,
87–95 (2000).
Wang, Y.-X., Zhang, C.L., Yu, R.T., Cho, H.K., Nelson, M.C.,
Bayuga-Ocampo, C.R., Ham, J., Kang, H., and Evans, R.M.,
Regulation of muscle fiber type and running endurance by
PPAR␥, PLoS Biol. 2, e294 (2004).
Yechoor, V.K., Patti, M.-E., Saccone, R., and Kahn, C.R., Coordi-
nated patterns of gene expression for substrate and energy
metabolism in skeletal muscle of diabetic mice, Proc. Natl.
Acad. Sci. 99, 10587–10592 (2002).
Zhang,F., et al., Crystal structure of the obese protein leptin-E100,
Nature 387, 206–209 (1997).
Zick, Y., Insulin resistance: a phosphorylation-based uncoupling
of insulin signaling, Trends Cell Biol. 11, 437–441 (2001).
1. Describe the metabolic effects of liver failure.
2. What is the basis of the hypothesis that athletes’ muscles
are more heavily buffered than those of normal individuals?
3. Experienced runners know that it is poor practice to ingest
large amounts of glucose prior to running a long-distance race
such as a marathon. What is the metabolic basis of this apparent
paradox?
4. Explain why urea output is vastly decreased during
starvation.
5. Explain why people survive longer by total fasting than on
a diet consisting only of carbohydrates.
6. Explain why the breath of an untreated diabetic smells of
acetone.
7. Among the many eat-all-you-want-and-lose-weight diets
that have been popular for a time is one that eliminates all carbo-
hydrates but permits the consumption of all the protein and fat
desired.Would such a diet be effective? (Hint: Individuals on such
a diet often complain that they have bad breath.)
8. Pancreatic  cells express a receptor for fatty acids. Fatty
acid binding to this protein appears to stimulate insulin secretion.
(a) Does this phenomenon make metabolic sense? (b) Fatty acids
appear to stimulate insulin secretion to a much greater extent
when glucose is also present.Why is this significant?
9. High concentrations of free fatty acids in the blood are
known to cause insulin resistance in muscle, but only after
5 hours. This suggests that a metabolite of these fatty acids may
be responsible for this phenomenon. It is also known that an iso-
form of protein kinase C is activated during the process and that
high concentrations of free fatty acids result in intramuscular
accumulation of triacylglycerols. With this information, review
the mechanism of activation of PKC and the pathway of triacyl-
glycerol biosynthesis and suggest a metabolite that may be
responsible for PKC activation.
10. Discuss, in molecular terms, how physical inactivity might
lead to insulin resistance.
PROBLEMS
JWCL281_c27_1088-1106.qxd 4/21/10 9:42 AM Page 1106
N
C
C
C
C
C
N
N
N
H
1
2
3
4
5
6
7
8
9
Glutamine amide
Formate
A
spartate
amine
HCO
–
3
Glycine
Formate
1107
CHAPTER 28
Nucleotide
Metabolism
1 Synthesis of Purine Ribonucleotides
A. Synthesis of Inosine Monophosphate
B. Synthesis of Adenine and Guanine Ribonucleotides
C. Regulation of Purine Nucleotide Biosynthesis
D. Salvage of Purines
2 Synthesis of Pyrimidine Ribonucleotides
A. Synthesis of UMP
B. Synthesis of UTP and CTP
C. Regulation of Pyrimidine Nucleotide Biosynthesis
3 Formation of Deoxyribonucleotides
A. Production of Deoxyribose Residues
B. Origin of Thymine
4 Nucleotide Degradation
A. Catabolism of Purines
B. Fate of Uric Acid
C. Catabolism of Pyrimidines
5 Biosynthesis of Nucleotide Coenzymes
A. Nicotinamide Coenzymes
B. Flavin Coenzymes
C. Coenzyme A
Nucleotides, as we have seen, are biologically ubiquitous
substances that participate in nearly all biochemical
processes: They are the monomeric units of DNA and
RNA; the hydrolysis of ATP and GTP drives many free
energy–requiring processes; the levels of ATP, ADP, and
AMP regulate numerous metabolic pathways; cAMP and
cGMP mediate hormonal signals; and NAD
, NADP
,
FMN, FAD, and coenzyme A are essential coenzymes in a
great variety of enzymatic reactions. The importance of
nucleotides in cellular metabolism is indicated by the ob-
servation that nearly all cells can synthesize them both
de novo (anew) and from the degradation products of nu-
cleic acids. In this chapter, we consider the nature of these
biosynthetic pathways. In doing so, we shall examine how
they are regulated and the consequences of their blockade,
both by genetic defects and through the administration of
chemotherapeutic agents. We then discuss how nucleotides
are degraded. Finally, we outline the biosynthesis of the
nucleotide coenzymes.
1 SYNTHESIS OF PURINE
RIBONUCLEOTIDES
In this section we commence our considerations of how
nucleic acids and their components are synthesized by
describing the synthesis of purine ribonucleotides. In 1948,
John Buchanan obtained the first clues as to how this
process occurs de novo by feeding a variety of isotopically
labeled compounds to pigeons and chemically determining
the positions of the labeled atoms in their excreted uric
acid (a purine).
He used birds in these experiments because they ex-
crete waste nitrogen almost entirely as uric acid, a water-
insoluble and therefore easily isolated substance. The results
of his studies, which are summarized in Fig. 28-1, demon-
strated that N1 of purines arises from the amine group of
aspartate; C2 and C8 originate from formate; N3 and N9
are contributed by the amide group of glutamine; C4, C5,
and N7 are derived from glycine (strongly suggesting that
this molecule is wholly incorporated into the purine ring);
and C6 comes from HCO
3
(CO
2
).
N
N
H
O
O
N
O
N
H
H
H
Uric acid
Figure 28-1 The biosynthetic origins of purine ring atoms.
Note that C4, C5, and N7 come from a single glycine molecule
but each of the other atoms is derived from an independent
precursor.
JWCL281_c28_1107-1142.qxd 4/22/10 9:16 AM Page 1107

The actual pathway by which these precursors are incor-
porated into the purine ring, the subject of Section 28-1A,
was elucidated in subsequent investigations performed
largely by Buchanan and by G. Robert Greenberg. These
investigations showed that the initially synthesized purine
derivative is inosine monophosphate (IMP),
the nucleotide of the base hypoxanthine. AMP and GMP
are subsequently synthesized from this intermediate via
separate pathways (Section 28-1B).Thus, contrary to naive
expectation, purines are initially formed as ribonucleotides
rather than as free bases. Additional studies have demon-
strated that such widely divergent organisms as E. coli,
yeast, pigeons, and humans have virtually identical path-
ways for the biosynthesis of purine nucleotides, thereby
further demonstrating the biochemical unity of life.
A. Synthesis of Inosine Monophosphate
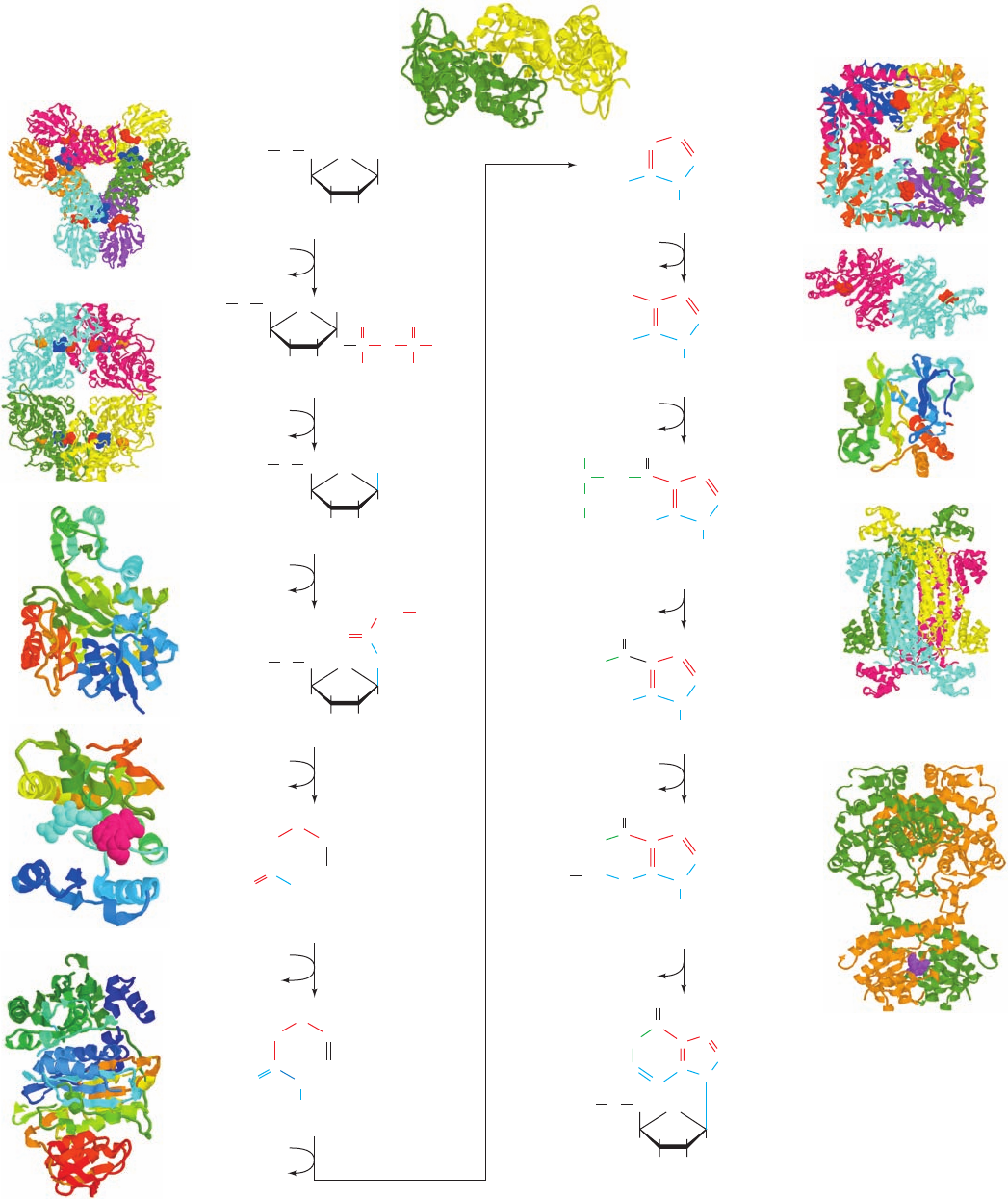
IMP is synthesized in a pathway comprising 11 reactions
(Fig. 28-2):
1. Activation of ribose-5-phosphate. The starting mate-
rial for purine biosynthesis is -
D-ribose-5-phosphate
(R5P), a product of the pentose phosphate pathway (Sec-
tion 23-4). In the first step of de novo purine biosynthesis,
ribose phosphate pyrophosphokinase (also known as
phosphoribosylpyrophosphate synthetase) activates R5P
by reacting it with ATP to form 5-phosphoribosyl--
pyrophosphate (PRPP). This reaction, which occurs via the
nucleophilic attack of the R5P’s C1¬H group on the P
of
ATP, is unusual in that a pyrophosphoryl group is directly
transferred from ATP to C1 of R5P and that the product
has the anomeric configuration. PRPP is also a precursor
in the biosynthesis of pyrimidines (Section 28-2A) and the
amino acids tryptophan and histidine (Section 26-5Bd,e).
Thus, as is expected for an enzyme at such an important
biosynthetic crossroads, the activity of ribose phosphate py-
rophosphokinase varies with the concentrations of numer-
ous metabolites, including PP
i
and 2,3-bisphosphoglycerate,
which are activators, and ADP and GDP, which are mixed
inhibitors (Section 14-3C). The regulation of purine nu-
cleotide biosynthesis is further discussed in Section 28-1C.
2. Acquisition of purine atom N9. Amidophosphoribo-
syltransferase (alternatively, glutamine PRPP aminotrans-
ferse or PurF; the latter being named for the E. coli gene
encoding it, purF) catalyzes the displacement of PRPP’s
HN
N
O
N
N
Inosine monophosphate (IMP)
Hypoxanthine
base
O
H
2
C
HH
OHOH
HH
O
O
P
–
O
O
–
pyrophosphate group by glutamine’s amide nitrogen to
yield -5-phosphoribosylamine (PRA). This is the first re-
action in the pathway that is unique to de novo purine
biosynthesis (and hence some sources refer to it as the first
reaction of the pathway, which is then said to consist of 10
reactions).This process occurs in two consecutive reactions
that take place on separate active sites on the enzyme:
1.
2.
Step 1 is catalyzed by a member of the N-terminal nucle-
ophile (Ntn) amidotransferase family (Section 26-5Aa).
Step 2 occurs with inversion of configuration about ribose
C1 and hence establishes the anomeric form of the future
nucleotide. The NH
3
passes between the two active sites
through a 20-Å-long tunnel that is lined with conserved
NH
3
PRPP S PRA PP
i
Glutamine H
2
O S glutamic acid NH
3
1108 Chapter 28. Nucleotide Metabolism
Figure 28-2 (Opposite) The metabolic pathway for the de
novo biosynthesis of IMP. The purine residue is built up on a
ribose ring in 11 enzymatically catalyzed reactions.The X-ray
structures for all enzymes are shown to the outside of the
corresponding reaction arrow. The peptide chains of monomeric
enzymes are colored in rainbow order from their N-termini
(blue) to their C-termini (red).The oligomeric enzymes, all of
which consist of identical polypeptide chains, are viewed along a
rotation axis with their various chains differently colored. Bound
ligands are shown in space-filling form. Enzyme 1, determined by
Sine Larsen, University of Copenhagen, Denmark, is a D
3
hexamer from B. subtilis that binds ,-methylene-ADP at its
catalytic (red) and allosteric (blue) sites; PDBid 1DKU. Enzyme
2, determined by Janet Smith, Purdue University, is a D
2
tetramer
from B. subtilis that binds GMP (blue), ADP (red), and a
[4Fe–4S] cluster (orange, which appears to have a regulatory
rather than a redox function); PDBid 1AO0. Enzymes 3 and 6,
both from E. coli, were determined by JoAnne Stubbe, MIT, and
Steven Ealick, Cornell University; PDBids 1GSO and 1CLI.
Enzyme 4, from E. coli, determined by Robert Almassy, Agouron
Pharmaceuticals, San Diego, California, binds GAR (cyan) and
5-deazatetrahydrofolate (red); PDBid 1CDE. Enzyme 5, from
Thermatoga maritima, was determined by Ian Wilson, Scripps
Research Institute, La Jolla, California; PDBid 1VK3. Reaction
7, in E. coli, is catalyzed by two sequentially acting enzymes,
Class I PurE (above) and PurK (below). Class I PurE,
determined by JoAnne Stubbe, MIT, and Steven Ealick, Cornell
University, is a D
4
octamer that binds AIR (red); PDBid 1D7A.
PurK, determined by JoAnne Stubbe, MIT, and Hazel Holden,
University of Wisconsin, is a C
2
dimer that binds ADP (red);
PDBid 1B6S. Enzyme 8, from yeast, was determined by Victor
Lamzin,Academy of Sciences, Moscow, Russia, and Keith
Wilson, EMBL, Hamburg, Germany; PDBid 1A48. Enzyme 9,
from Thermatoga maritima, determined by Todd Yeates, UCLA,
is a D
2
tetramer; PDBid 1C3U. Reactions 10 and 11 in chicken
are catalyzed by a bifunctional enzyme that was determined by
Stephen Benkovic, Pennsylvania State University, and Ian
Wilson, The Scripps Research Institute, La Jolla, California. It
forms a C
2
dimer shown with its AICAR transformylase function
above and its IMP cyclohydrolase function, which binds GMP
(purple), below; PDBid 1G8M.
See the Animated Figures
JWCL281_c28_1107-1142.qxd 4/22/10 9:16 AM Page 1108
H
2
C
H OH
HH
OH OH
O
O
–2
O
3
P
–2
O
3
P
H
α
α-D-Ribose-5-phosphate (R5P)
CH
2
H O
HH
OH OH
O
O
–2
O
3
P
–2
O
3
P
H
α
5-Phosphoribosyl-
α-pyrophosphate (PRPP)
O
–
O
O
–
PPO
O
O
–
CH
2
CH
2
HH
HH
OH OH
O
O
β
-5-Phosphoribosylamine (PRA)
β
NH
2
HH
HH
OH OH
O
O
Glycinamide ribotide (GAR)
NH
C
CH
2
O
NH
2
C
H
N
CH
O
NH
O
H
2
C
Ribose-5-phosphate
Formylglycinamide ribotide (FGAR)
C
H
N
CH
O
NH
HN
H
2
C
Ribose-5-phosphate
Formylglycinamidine ribotide (FGAM)
C
5
N
CH
N
HC
H
2
N
Ribose-5-phosphate
5-Aminoimidazole ribotide (AIR)
C
5
N
CH
N
C
4
H
2
N
Ribose-5-phosphate
Carboxyaminoimidazole ribotide (CAIR)
–
OOC
ADP
+ P
i
PP
i
ATP
AMP
ribose phosphate
pyrophosphokinase
Glutamine
Glutamate
amidophosphoribosyl
transferase
+
+
H
2
O
Glycine + ATP
ADP
+ P
i
GAR synthetase
FGAM synthetase
N
10
-Formyl-THF
THF
GAR transformylase
ATP + Glutamine + H
2
O
ADP + Glutamate + P
i
AIR synthetase
ATP
ADP
+ P
i
AIR carboxylase
ATP + HCO
–
3
1
2
3
4
5
6
7
C
5
N
CH
N
C
4
H
2
N
Ribose-5-phosphate
5-Aminoimidazole-4-(N-succinylocarboxamide)
ribotide (SAICAR)
C
HC
O
CH
2
NH
COO
–
COO
–
C
5
N
CH
N
C
4
H
2
N
Ribose-5-phosphate
5-Aminoimidazole-4-carboxamide ribotide (AICAR)
C
O
H
2
N
N
H
C
5
N
CH
N
C
4
Ribose-5-phosphate
5-Formaminoimidazole-4-carboxamide
ribotide (FAICAR)
C
O
H
2
N
CHO
SAICAR synthetase
ADP
+ P
i
8
Aspartate
+ ATP
adenylosuccinate lyase
Fumarate
9
THF
AICAR
transformylase
N
10
-Formyl-THF
10
IMP
cyclohydrolase
11
H
2
O
HN
N
N
N
CH
2
HH
HH
OH OH
O
O
O
HC
C
C
CH
C
Inosine monophosphate (IMP)
–2
O
3
P
6
Section 28-1. Synthesis of Purine Ribonucleotides 1109
JWCL281_c28_1107-1142.qxd 4/22/10 9:16 AM Page 1109
1110 Chapter 28. Nucleotide Metabolism
attacked by an amine nitrogen atom to yield a tetrahedral
adduct that, in turn, expels P
i
to form product.
6. Formation of the purine imidazole ring. The purine
imidazole ring is closed in an ATP-requiring intramolecu-
lar condensation that yields 5-aminoimidazole ribotide
(AIR) in a reaction catalyzed by AIR synthetase (PurM).
nonpolar residues that lack hydrogen bonding groups and
hence do not impede the diffusion of the NH
3
[we have
seen that NH
3
generated by glutamine hydrolysis is simi-
larly channeled to the active site that uses it in carbamoyl
phosphate synthetase (Section 26-2Aa) and glutamate syn-
thetase (Section 26-5Aa)]. These reactions, which are
driven to completion by the subsequent hydrolysis of the
released PP
i
, constitute the pathway’s flux-generating step.
Not surprisingly, therefore, amidophosphoribosyltrans-
ferase is subject to feedback inhibition by purine nu-
cleotides (Section 28-1C).
3. Acquisition of purine atoms C4, C5, and N7.
Glycine’s carboxyl group forms an amide with the amino
group of PRA, yielding glycinamide ribotide (GAR) in a
reaction, catalyzed by GAR synthetase (PurD), that oc-
curs via the intermediate phosphorylation of glycine’s car-
boxyl group. The reaction, which is reversible despite its
concomitant hydrolysis of ATP to ADP P
i
, is the only
step of the purine biosynthesis pathway in which more
than one purine ring atom is acquired. The observation
that PRA is chemically unstable (it is hydrolyzed to R5P
and NH
3
with a half-life of 5 s at 37°C) suggests that GAR
synthetase and amidophosphoribosyltransferase associate
in a way that channels PRA between them. Indeed, a ster-
ically and electrostatically plausible model of such a com-
plex has been built based on the X-ray structures of these
two enzymes.
4. Acquisition of purine atom C8. GAR’s primary -
amino group is formylated by GAR transformylase (PurN)
to yield formylglycinamide ribotide (FGAR). The formyl
donor in this reaction is N
10
-formyltetrahydrofolate
(N
10
-formyl-THF), a cofactor that transfers C
1
units from
such donors as serine, glycine, and formate to various ac-
ceptors in biosynthetic reactions (Section 26-4D). Struc-
tural and enzymological studies indicate that the reaction
proceeds via the nucleophilic attack of the GAR primary
amine group on the formyl carbon of N
10
-formyl-THF to
yield a tetrahedral intermediate.
5. Acquisition of purine atom N3. The amide amino
group of a second glutamine is transferred to the growing
purine ring to form formylglycinamidine ribotide (FGAM).
This reaction, which is catalyzed by FGAM synthetase
(PurL), is driven by the coupled hydrolysis of ATP to
ADP P
i
. It is thought to proceed by the mechanism dia-
grammed in Fig. 28-3. Here the oxygen of the FGAR
isoamide form reacts with ATP to yield a phosphoryl ester
intermediate. This intermediate then reacts with NH
3
(the
glutamine amide nitrogen as labilized through the tran-
sient formation of an enzyme thioester) to form a tetra-
hedral adduct. The adduct then eliminates P
i
to yield the
imine product, FGAM. Such reactions, in which a carbox-
amide oxygen is replaced by an imino group, are common
in the biosynthesis of nucleotides. For example, Reaction 6
of this pathway and the reactions converting IMP to AMP
(Section 28-1B) and UTP to CTP (Section 28-2B) follow
similar mechanisms, that is, conversion of a carboxamide
oxygen to a phosphoryl ester that is nucleophilically
+
R
R'
R'
R'
R"
R"
H
2
O
O
C
R"
O
C
–
O
O
–
CSE
SHE
SHE
R
CO
O
O
–
PO
3
2
–
PO
3
2–
NH
+
R
R'
C
O
R'
C
NH
+
R
NH
ADP
ATP
FGAR (isoamide form)
FGAR (amide form)
Glutamine
Glutamate
FGAM
P
i
C
C
R
NH
NH
HN
H
2
N
.
.
NH
3
NH
3
NH
2
.
.
Figure 28-3 The proposed mechanism of formylglycinamide
ribotide (FGAM) synthetase. The glutaminase domain of the
enzyme contains an active site Cys residue that catalyzes the
release of NH
3
with the transient formation of an enzyme
thioester (not shown) whose hydrolysis produces glutamate. The
isoamide form of FGAR is phosphorylated by ATP and then
reacts with “NH
3
” to form a tetrahedral intermediate whose
collapse yields FGAM P
i
.
JWCL281_c28_1107-1142.qxd 6/8/10 10:39 AM Page 1110
