Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

complex deadenylylates glutamine synthetase when P
II
is
uridylylated (also at a Tyr residue) and adenylylates gluta-
mine synthetase when P
II
lacks UMP residues.The level of P
II
uridylylation, in turn, depends on the relative activities of
two enzymatic activities located on the same protein: a
uridylyltransferase that uridylylates P
II
and a uridylyl-
removing enzyme that hydrolytically excises the attached
UMP groups of P
II
(Fig. 26-56).The uridylyltransferase is ac-
tivated by ␣-ketoglutarate and ATP and inhibited by gluta-
mine and P
i
, whereas uridylyl-removing enzyme is insensi-
tive to these metabolites. This complex metabolic cascade
therefore renders the activity of E. coli glutamine synthetase
extremely responsive to the cell’s nitrogen requirements.
d.
Glutamate Is the Precursor of
Proline, Ornithine, and Arginine
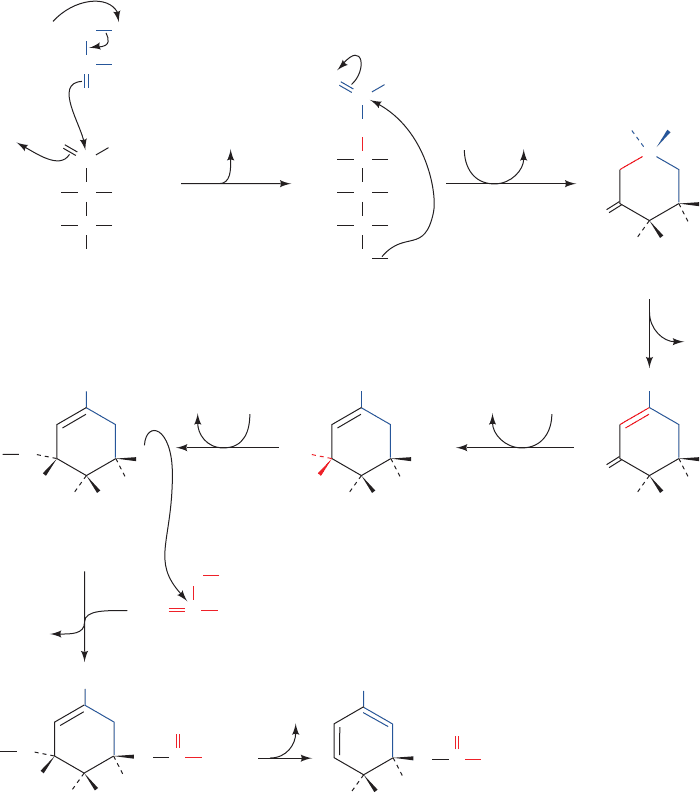
Conversion of glutamate to proline (Fig. 26-57, Reac-
tions 1– 4) involves the reduction of the ␥-carboxyl group to
an aldehyde followed by the formation of an internal Schiff
base whose further reduction yields proline. Reduction of
the glutamate ␥-carboxyl group to an aldehyde is an ender-
gonic process that is facilitated by the carboxyl group’s
prior phosphorylation by ␥-glutamyl kinase. The unstable
product,glutamate-5-phosphate, has not been isolated from
reaction mixtures but is presumed to be the substrate for
the reduction that follows. The resulting glutamate-5-
semialdehyde cyclizes spontaneously to form the internal
Schiff base ⌬
1
-pyrroline-5-carboxylate. The final reduction
to proline is catalyzed by pyrroline-5-carboxylate reductase.
Whether the enzyme requires NADH or NADPH is unclear.
The E. coli pathway from glutamate to ornithine and
hence to arginine likewise involves the ATP-driven reduc-
tion of the glutamate ␥-carboxyl group to an aldehyde (Fig.
26-57, Reactions 6 and 7). Spontaneous cyclization of this in-
termediate, N-acetylglutamate-5-semialdehyde, is prevented
by prior acetylation of its amino group by N-acetylglutamate
synthase to form N-acetylglutamate (Fig. 26-57, Reaction 5).
N-Acetylglutamate-5-semialdehyde, in turn, is converted to
the corresponding amine by transamination (Fig. 26-57, Re-
action 8). Hydrolysis of the acetyl protecting group finally
yields ornithine, which, as we have seen (Section 26-2),is con-
verted to arginine via the urea cycle. In humans, however, the
pathway to ornithine is more direct.The N-acetylation of glu-
tamate that protects it from cyclization does not occur.
Rather, glutamate-5-semialdehyde, which is in equilibrium
with ⌬
1
-pyrroline-5-carboxylate, is directly transaminated
to yield ornithine in a reaction catalyzed by ornithine-␦-
aminotransferase (Fig. 26-57, Reaction 10).
e. Serine, Cysteine, and Glycine Are Derived from
3-Phosphoglycerate
Serine is formed from the glycolytic intermediate
3-phosphoglycerate in a three-reaction pathway (Fig. 26-58):
1. Conversion of 3-phosphoglycerate’s 2-OH group to a
ketone yielding 3-phosphohydroxypyruvate, serine’s phos-
phorylated keto acid analog.
2. Transamination of 3-phosphohydroxypyruvate to
phosphoserine.
3. Hydrolysis of phosphoserine to yield serine.
Serine participates in glycine synthesis in two ways (Sec-
tion 26-3B):
1. Direct conversion of serine to glycine by serine hy-
droxymethyl transferase in a reaction that also yields
N
5
,N
10
-methylene-THF (Fig. 26-12, Reaction 4 in reverse).
2. Condensation of the N
5
,N
10
-methylene-THF with
CO
2
and by the glycine cleavage system (Fig. 26-12,
Reaction 3 in reverse).
We have already discussed the synthesis, in animals, of cys-
teine from serine and homocysteine, a breakdown product
of methionine (Section 26-3Ea). Homocysteine combines
with serine to yield cystathionine, which subsequently forms
cysteine and ␣-ketobutyrate (Fig. 26-18, Reactions 5 and 6).
Since cysteine’s sulfhydryl group is derived from the essen-
tial amino acid methionine, cysteine is really an essential
amino acid. In plants and microorganisms, however, cys-
teine is synthesized from serine in a two-step reaction in-
volving the activation of the serine ¬OH by converting it to
O-acetylserine followed by the displacement of acetate by
sulfide (Fig. 26-59a). The sulfide required is produced from
sulfate in an 8-electron reduction that occurs in E. coli as
shown in Fig. 26-59b. Sulfate is first activated by the
NH
⫹
4
Section 26-5. Amino Acid Biosynthesis 1071
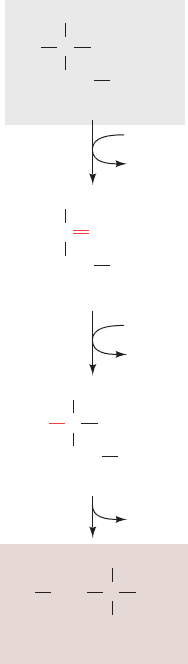
Figure 26-58 The conversion of 3-phosphoglycerate to serine.
The pathway enzymes are (1) 3-phosphoglycerate
dehydrogenase, (2) a PLP-dependent aminotransferase,
and (3) phosphoserine phosphatase.
H
3
N
+
COO
–
OHCH
OPO
3
2
–
P
i
CH
2
3-Phosphoglycerate
NADH
NAD
+
COO
–
C
OPO
3
2
–
CH
2
3-Phosphohydroxypyruvate
O
Glutamate
COO
–
CH
OPO
3
2
–
CH
2
3-Phosphoserine
HO
COO
–
C
H
NH
3
+
Serine
CH
2
3
2
1
α-Ketoglutarate
JWCL281_c26_1019-1087.qxd 7/21/10 6:26 PM Page 1071
enzymes ATP sulfurylase (which is used in the pyrosequenc-
ing of DNA; Section 7-2Ca) and adenosine-5ⴕ-phosphosulfate
(APS) kinase. The activated sulfate is then reduced to sulfite
by 3ⴕ-phosphoadenosine-5ⴕ-phosphosulfate (PAPS) reduc-
tase and to sulfide by sulfite reductase.
B. Biosynthesis of the Essential Amino Acids
Essential amino acids, like nonessential amino acids, are syn-
thesized from familiar metabolic precursors. Their synthetic
pathways are present only in microorganisms and plants,
however, and usually involve more steps than those of the
nonessential amino acids. For example, lysine, methionine,
and threonine are all synthesized from aspartate in pathways
whose common first reaction is catalyzed by aspartokinase,
an enzyme that is present only in plants and microorganisms.
Similarly, valine and leucine are formed from pyruvate;
isoleucine is formed from pyruvate and ␣-ketobutyrate; and
tryptophan, phenylalanine, and tyrosine are formed from
phosphoenolpyruvate and erythrose-4-phosphate. The en-
zymes that synthesize essential amino acids were apparently
lost early in animal evolution, possibly because of the ready
availability of these amino acids in the diet.
Time and space prevent a detailed discussion of the many
interesting reactions that occur in these pathways.The biosyn-
thetic pathways of the aspartate family of amino acids, the
pyruvate family, the aromatic family, and histidine are pre-
sented in Figs. 26-60 through 26-63 and 26-65 together with
lists of the enzymes involved. Several agriculturally useful
1072 Chapter 26. Amino Acid Metabolism
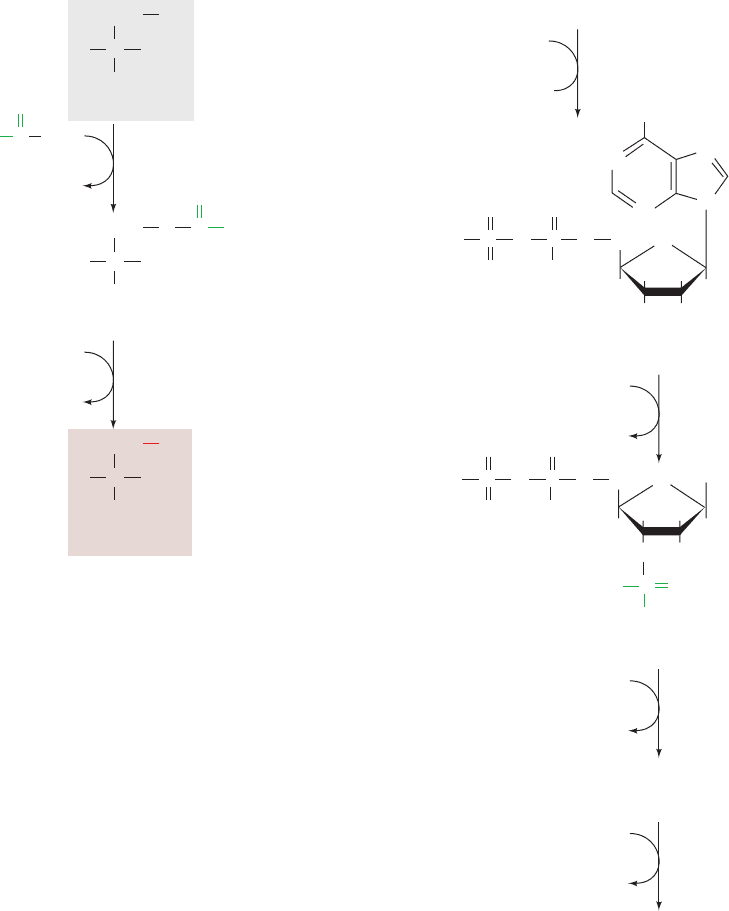
Figure 26-59 Cysteine biosynthesis. (a) The synthesis of
cysteine from serine in plants and microorganisms. (b) The
8-electron reduction of sulfate to sulfide in E. coli.
H
CH
2
Serine
serine acetyltransferase
NH
3
+
COO
–
CH
3
COO
–
H
+
+S
2
–
SCoA
CoASH
C
OH
H
CH
2
Cysteine
NH
3
+
COO
–
C
SH
H
CH
2
H
3
C
O-Acetylserine
O-acetylserine (thiol) lyase
NH
3
+
COO
–
C
O
C
O
CH
3
CH
2
NH
2
–
O
O
–
S
O
O
OPO
O
OH
N
N
N
N
H
HH
H
HO
O
C
O
(a) (b)
ATP sulfurylase
PP
i
H
+
+ATP SO
2
–
4
SO
2
–
S
2
–
3
ATP
APS kinase
ADP
NADPH
3⬘-Phospho-AMP
+ NADP
+
3NADP
+
PAPS reductase
sulfite reductase
Sulfite
3ⴕ-Phosphoadenosine-5ⴕ-phosphosulfate (PAPS)
Adenosine-5ⴕ-phosphosulfate (APS)
3NADPH
CH
2
–
O
–
O
O
–
S
O
O
OPO
O
OH
A
H
HH
H
O
O
–
PO
O
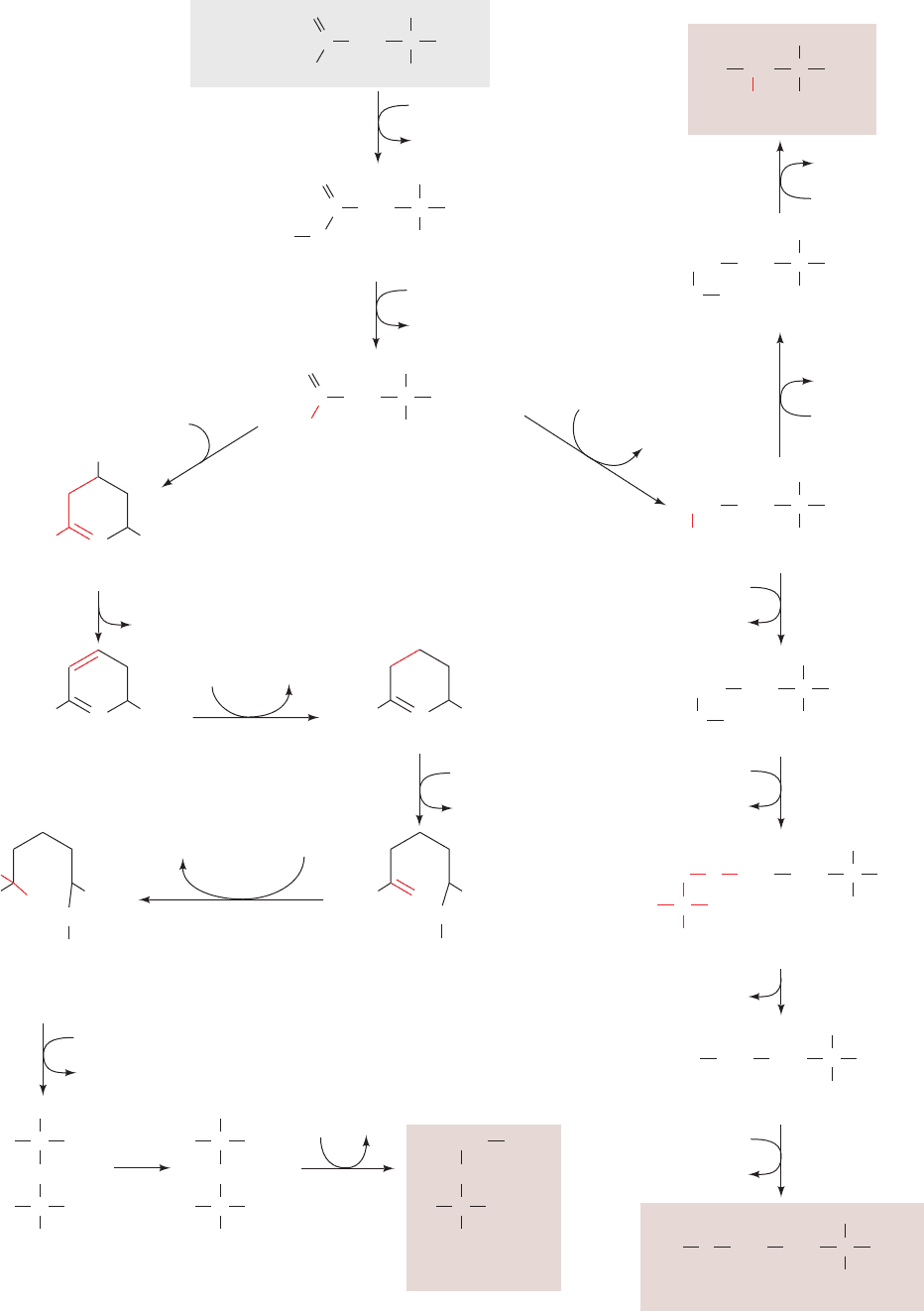
Figure 26-60 (Opposite) The biosynthesis of the “aspartate
family” of amino acids: lysine, methionine, and threonine. The
pathway enzymes are (1) aspartokinase, (2) -aspartate
semialdehyde dehydrogenase, (3) homoserine dehydrogenase,
(4) homoserine kinase, (5) threonine synthase (a PLP enzyme),
(6) homoserine acyltransferase, (7) cystathionine ␥-synthase,
(8) cystathionine -lyase, (9) methionine synthase (alternatively
homocysteine methyltransferase, which also occurs in mammals;
Section 26-3Ec), (10) dihydrodipicolinate synthase,
(11) dihydrodipicolinate reductase, (12) N-succinyl-2-amino-6-
ketopimelate synthase, (13) succinyl-diaminopimelate
aminotransferase (a PLP enzyme), (14) succinyl-diaminopimelate
desuccinylase, (15) diaminopimelate epimerase, and
(16) diaminopimelate decarboxylase.
JWCL281_c26_1019-1087.qxd 6/8/10 9:38 AM Page 1072
Section 26-5. Amino Acid Biosynthesis 1073
CH
2
NH
3
+
C
CH
2
COO
–
NH
3
+
H
C
O
–
O
1
ADP
ATP
CoASH
C
CH
2
COO
–
NH
3
+
H
C
O
O
–2
O
3
P
2
NADP
H
NADP
+
+ P
i
14
Glutamate
α-Ketoglutarate
Aspartate
Aspartyl-
β
-phosphate
C
CH
2
COO
–
NH
3
+
H
C
O
H
β
-Aspartate-semialdehyde
Pyruvate
spontaneous
H
2
O
NADP
NADPH
CH
2
COO
–
NH
3
+
H
C
Homoserine
C
OH
4
ADP
ATP
CH
2
COO
–
NH
3
+
H
C
Phosphohomoserine
CH
2
O
PO
3
2
–
5
P
i
H
2
O
CH
COO
–
NH
3
+
H
C
Threonine
H
3
C
OH
3
Succinyl-CoA
6
CH
2
COO
–
NH
3
+
H
C
CH
2
O
Succ
O-Succinylhomoserine
7
Succinate
Cysteine
CH
2
COO
–
NH
3
+
H
C
CH
2
S
H
2
C
NH
3
+
CH
COO
–
Cystathionine
8
Pyruvate
+ NH
3
CH
2
COO
–
NH
3
+
H
C
CH
2
HS
Homocysteine
9
THF
CH
2
COO
–
NH
3
+
H
C
CH
2
SH
3
C
Methionine
COO
––
OOC
N
Dihydrodipicolinate
4-Hydroxy-tetrahydrodipicolinate
11
NADPH
NADP
+
COO
––
OOC
N
Tetrahydrodipicolinate
COO
–
OH
–
OOC
N
COO
––
OOC
O
N-Succinyl-2-amino-
6-keto-
L-pimelate
12
CoASH
Succinyl
-CoA + H
2
O
NH
Succ
COO
––
OOC
N-Succinyl-L,L-
α
,
ε
-diamino-
pimelate
NH
3
+
Succ
NH
2
H
H
2
O
Succinate
CH
2
)
3
NH
3
+
C H
COO
–
(
NH
3
+
CH
COO
–
L,L
-α
,
ε
-Diamino-
pimelate
CH
2
)
3
NH
3
+
CH
COO
–
(
NH
3
+
CH
COO
–
15
(CH
2
)
3
NH
3
+
CH
COO
–
meso-
α
,
ε
-Diamino-
pimelate
16
H
+
CO
2
13
10
Lysine
N
5
-Methyl-THF
H
2
JWCL281_c26_1019-1087.qxd 4/20/10 9:26 AM Page 1073
1074 Chapter 26. Amino Acid Metabolism
NH
3
+
2
NAD(P)H
NAD(P)
+
Glutamate
α-Ketoglutarate
C
COO
–
NH
3
+
H
C
Threonine
H
3
C
HO
CH
2
C
α-Ketobutyrate
H
3
C
O
COO
–
COO
–
C
H
3
C
O
Pyruvate
COO
–
C
H
3
C
O
Pyruvate
+
TPP
CO
2
C
–
H
3
C
TPP
OH
NH
3
CH
3
COO
–
C
H
3
C
O
C
OH
COO
–
C
H
3
C
O
C
HO
α-Acetolactate
H
3
C
COO
–
C
H
3
C
C
HO
H
OH
α,β-Dihydroxy-
isovalerate
H
3
C
COO
–
C
H
3
C
C
H
α-Ketoisovalerate
O
H
3
C
COO
–
C
H
3
C
C
H
Valine
NH
3
+
H
H
3
C
COO
–
C
H
3
C
C
H
α-Isopropylmalate
OH
CH
2
COO
–
H
3
C
COO
–
C
H
3
C
C
H
β-Isopropylmalate
H
CH
COO
–
OH
H
3
C
H
3
C
C
H
α-Ketoisocaproate
CH
2
COO
–
C
O
H
3
C
H
3
C
C
H
Leucine
CH
2
COO
–
C
H
COO
–
C
H
3
C
O
C
OH
α-Aceto-α-
hydroxybutyrate
CH
3
COO
–
C
H
3
C
O
C
HO
CH
3
H
2
C
COO
–
C
H
3
C
C
HO
CH
3
H
OH
α,β-Dihydroxy-
β-methylvalerate
α-Keto-β-
methylvalerate
H
2
C
COO
–
C
H
3
C
C
H
CH
3
O
Isoleucine
H
2
C
COO
–
C
H
3
C
C
H
CH
3
H
NH
3
+
H
2
O
Acetyl-CoA
CoA
NAD
+
NADH + CO
2
2
NAD(P)H
NAD(P)
+
H
2
O
α-Ketoglutarate
Glutamate
Glutamate
α-Ketoglutarate
11
3
3
4
5
6
7
8
9
5
4
H
10
H
2
C
H
3
C
H
2
C
JWCL281_c26_1019-1087.qxd 4/20/10 9:26 AM Page 1074
herbicides are specific inhibitors of some of these enzymes.
Such herbicides have little toxicity toward animals and hence
pose minimal risk to human health and the environment.
a. The Aspartate Family: Lysine, Methionine,
and Threonine
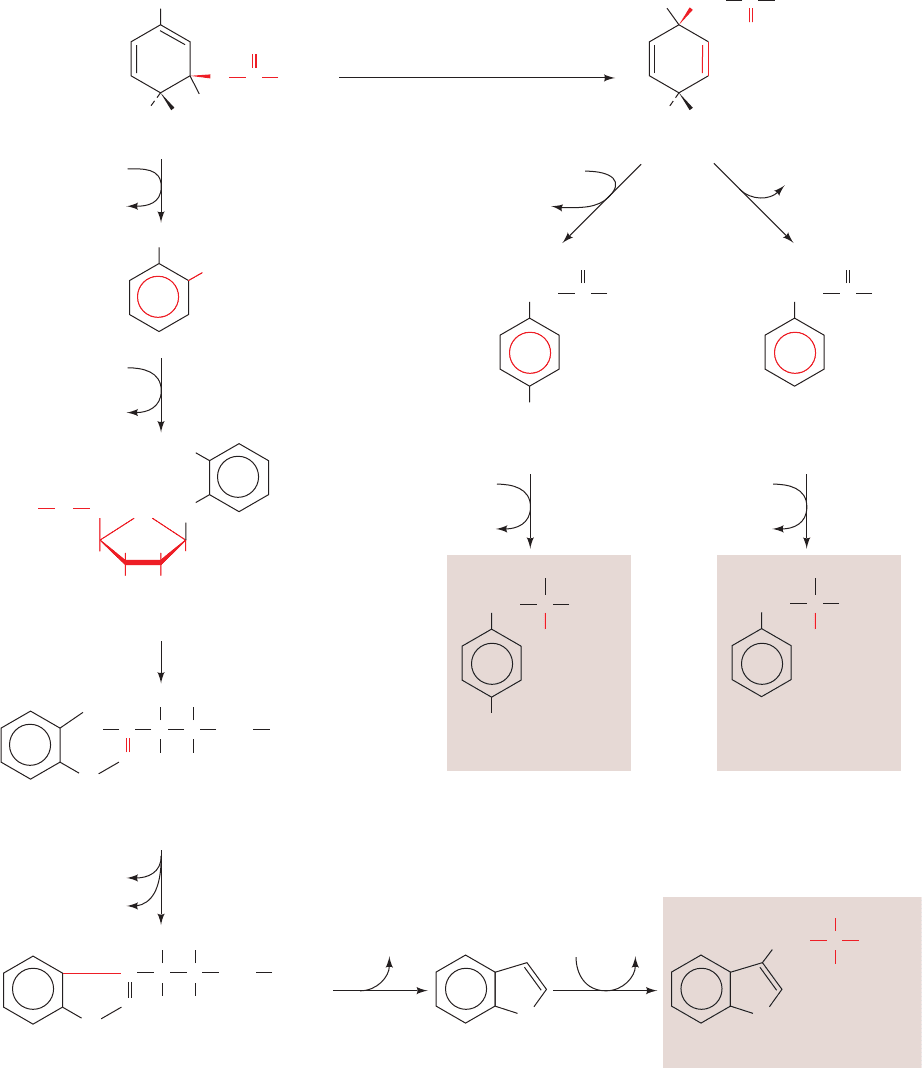
In bacteria, aspartate is the common precursor of lysine,
methionine, and threonine (Fig. 26-60).The biosyntheses of
these essential amino acids all begin with the aspartoki-
nase-catalyzed phosphorylation of aspartate to yield
aspartyl--phosphate. We have seen that the control of
metabolic pathways commonly occurs at the first commit-
ted step of the pathway. One might therefore expect lysine,
methionine, and threonine biosynthesis to be controlled as
a group. Each of these pathways is, in fact, independently
controlled. E. coli does so via three isozymes of aspartoki-
nase that respond differently to the three amino acids in
terms both of feedback inhibition of enzyme activity and
repression of enzyme synthesis. Table 26-3 summarizes this
differential control. In addition, the pathway direction is
controlled by feedback inhibition at the branch points by
the individual amino acids. Thus methionine inhibits the
O-acylation of homoserine (Fig. 26-60, Reaction 6), and
lysine inhibits dihydrodipicolinate synthase (Fig. 26-60,
Reaction 10).
b.
The Pyruvate Family: Leucine, Isoleucine,
and Valine
Valine and isoleucine are both synthesized via the same
five-step pathway (Fig. 26-61), the only difference being in
the first step of the series. In this TPP-dependent reaction,
which resembles those catalyzed by pyruvate decarboxy-
lase (Section 17-3Ba) and transketolase (Section 23-4Ca),
pyruvate forms an adduct with TPP, which is decarboxy-
lated to hydroxyethyl-TPP. This resonance-stabilized car-
banion adds either to the keto group of a second pyruvate
to form acetolactate on the way to valine, or to the keto
group of threonine-derived ␣-ketobutyrate to form
␣-aceto-␣-hydroxybutyrate on the way to isoleucine. The
leucine biosynthetic pathway branches off from the valine
pathway at ␣-ketoisovalerate (Fig. 26-61, Reaction 6). Re-
actions 6 to 8 in Fig. 26-61 are reminiscent of the first three
reactions of the citric acid cycle (Sections 21-3A–C). Here,
acetyl-CoA condenses with ␣-ketoisovalerate to form
␣-isopropylmalate, which then undergoes a dehydration/
hydration reaction, followed by oxidative decarboxylation
and transamination, to yield leucine.
c. The Aromatic Amino Acids: Phenylalanine,
Tyrosine, and Tryptophan
The precursors to the aromatic amino acids are the gly-
colytic intermediate phosphoenolpyruvate (PEP) and
erythrose-4-phosphate (an intermediate in the pentose
phosphate pathway; Section 23-4Cb). Their condensation
forms 2-keto-3-deoxy-
D-arabinoheptulosonate-7-phosphate,
a C
7
compound that cyclizes and is ultimately converted to
chorismate (Fig. 26-62), the branch point for tryptophan
biosynthesis. Chorismate is converted either to anthrani-
late and then on to tryptophan, or to prephenate and on to
either tyrosine or phenylalanine (Fig. 26-63). Although
mammals synthesize tyrosine by the hydroxylation of
phenylalanine (Section 26-3Ha), many microorganisms
synthesize it directly from prephenate.
Since the synthesis of aromatic amino acids only occurs
in plants and microorganisms, this pathway is a natural tar-
get for herbicides that will not be toxic to animals. For ex-
ample, glyphosate,
the active ingredient in one of the most widely used weed
killers, Roundup, is a competitive inhibitor with respect to
PEP in the 5-enolpyruvylshikimate-3-phosphate (EPSP)
synthase reaction (Reaction 6 of Fig. 26-62).
d. A Protein Tunnel Channels the Intermediate
Product of Tryptophan Synthase between
Two Active Sites
The final two reactions in tryptophan biosynthesis, Re-
actions 5 and 6 in Fig. 26-63, are both catalyzed by trypto-
phan synthase:
1. The ␣ subunit (268 residues) of this ␣
2

2
bifunctional
enzyme cleaves indole-3-glycerol phosphate, yielding in-
dole and glyceraldehyde-3-phosphate (Reaction 5).
2. The  subunit (396 residues) joins indole with
L-serine in a PLP-dependent reaction to form L-tryptophan
(Reaction 6).
Either subunit alone is enzymatically active, but when they
are joined in the ␣
2

2
tetramer, the rates of both reactions
Glyphosate
⫺2
O
3
P ¬ CH
2
¬ NH ¬ CH
2
¬ COO
⫺
Section 26-5. Amino Acid Biosynthesis 1075
Table 26-3 Differential Control of Aspartokinase
Isoenzymes in E. Coli
Feedback
Enzyme Inhibitor Corepressor(s)
a
Aspartokinase I Threonine Threonine and isoleucine
Aspartokinase II None Methionine
Aspartokinase III Lysine Lysine
a
Compounds whose presence results in the transcriptional repression of
enzyme synthesis (Section 31-3G).
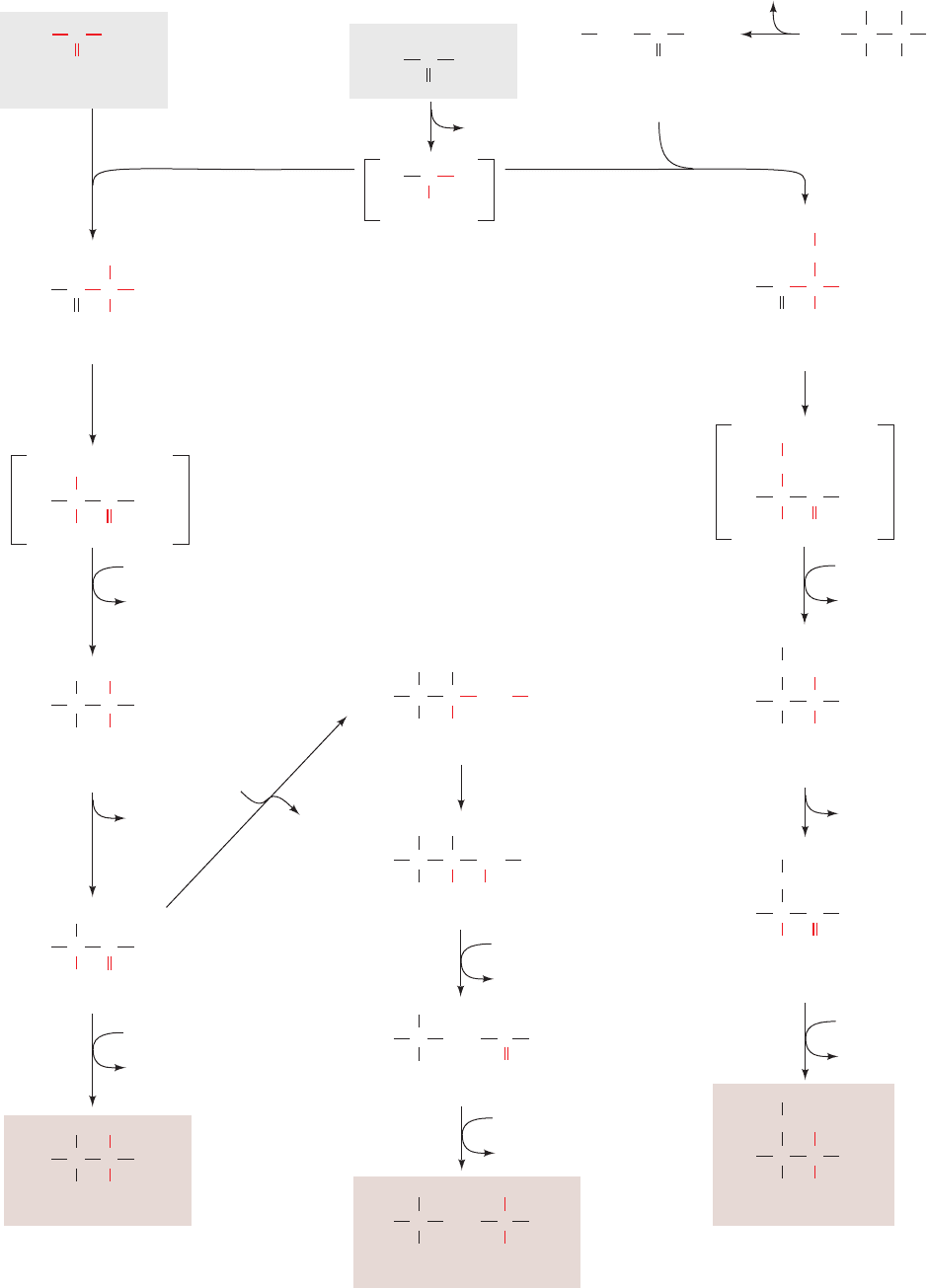
Figure 26-61 (Opposite) The biosynthesis of the “pyruvate
family” of amino acids: isoleucine, leucine, and valine. The
pathway enzymes are (1) acetolactate synthase (a TPP enzyme),
(2) acetolactate mutase, (3) reductase, (4) dihydroxy acid
dehydratase, (5) valine aminotransferase (a PLP enzyme),
(6) ␣-isopropylmalate synthase, (7) ␣-isopropylmalate
dehydratase, (8) isopropylmalate dehydrogenase, (9) leucine
aminotransferase (a PLP enzyme), and (10) threonine deaminase
(serine dehydratase, a PLP enzyme).
JWCL281_c26_1019-1087.qxd 6/8/10 9:38 AM Page 1075
and their substrate affinities are increased by 1 to 2 orders of
magnitude. Indole, the intermediate product, does not ap-
pear free in solution; the enzyme apparently sequesters it.
The X-ray structure of tryptophan synthase from Sal-
monella typhimurium, determined by Craig Hyde, Edith
Miles, and David Davies, explains the latter observation.
The protein forms a 150-Å-long, 2-fold symmetric
␣–––␣ complex (Fig. 26-64) in which the active sites of
neighboring ␣ and  subunits are separated by ⬃25 Å.
These active sites are joined by a solvent-filled tunnel that is
wide enough to permit the passage of the intermediate sub-
strate, indole. This structure, the first in which the presence
of a tunnel between active sites was observed, suggests
the following series of events. The indole-3-glycerol phos-
phate substrate binds to the ␣ subunit through an opening
into its active site, its “front door,” and the glyceraldehyde-
3-phosphate product leaves via the same route. Similarly,
the  subunit active site has a “front door” opening to the
solvent through which serine enters and tryptophan leaves.
Both active sites also have “back doors” that are connected
1076 Chapter 26. Amino Acid Metabolism
H
C
HO
C
OH
OH
C
H
H
CH
2
OPO
3
2
–
BH
+
O
COO
–
C
CH
2
PO
3
2
–
HO
–
+
C
COO
–
O
C
OH
OH
C
H
H
H
2
C
OP
O
3
2
–
CH
2
Phosphoenol-
pyruvate (PEP)
Erythrose-4-
phosphate
P
i
C
HO
H
2-Keto-3-deoxyarabino-
heptulosonate-7-
phosphate (DAHP)
P
i
+ NAD
+
NAD
+
O
H
OH
HO
COO
–
HO
C
5-Dehydroquinate
H
2
O
H
O
H
OH
HO
COO
–
5-Dehydroshikimate
NADHNAD
+
H
H
OH
HO
COO
–
COO
–
COO
–
COO
–
Shikimate
H
HO
ATPADP
H
H
OH
HO
Shikimate-5-
phosphate
H
O
2–
O
3
P
O
COO
–
C
H
2
C
PO
3
2
–
PEP
P
i
..
H
H
O
HO
5-Enolpyruvylshikimate-
3-phosphate
H
O
2–
O
3
P
COO
–
C
CH
2
P
i
H
H
O
HO
Chorismate
COO
–
C
CH
2
12
3
45
6
7
Figure 26-62 The biosynthesis of chorismate, the aromatic
amino acid precursor. The pathway enzymes are (1) 2-keto-3-
deoxy-
D-arabinoheptulosonate-7-phosphate synthase,
(2) dehydroquinate synthase (an NAD
⫹
-requiring reaction that
yields an unchanged NAD
⫹
product and is thereby indicative of
an oxidized intermediate as similarly occurs in the
UDP–galactose-4-epimerase reaction; Section 17-5B),
(3) 5-dehydroquinate dehydratase, (4) shikimate dehydrogenase,
(5) shikimate kinase, (6) 5-enolpyruvylshikimate-3-phosphate
synthase, and (7) chorismate synthase.
JWCL281_c26_1019-1087.qxd 4/20/10 9:26 AM Page 1076
by the tunnel.The indole intermediate presumably diffuses
between the two active sites via the tunnel and hence does
not escape to the solvent.
Allosteric interactions between the subunits to control
the activity of the ␣ subunit also serve to ensure that indole
is only released when the  subunit is ready to accept it.
Section 26-5. Amino Acid Biosynthesis 1077
Serine
H
H
O
HO
COO
–
Chorismate
COO
–
C
CH
2
COO
–
NH
2
Anthranilate
CH
2
HH
HH
HO OH
O
O
HN
–
OOC
2–
O
3
P
N
-(5ⴕ-Phosphoribosyl)-
anthranilate
N
H
COO
–
C
H
CH
2
C
HO
C
OH
H
C
OH
H
OPO
3
2
–
Enol-1- -carboxyphenylamino-
1-deoxyribulose phosphate
o
N
H
C
H
CH
2
C C
OH
H
C
O
H
H
OPO
3
2
–
Indole-3-glycerol
phosphate
Indole
N
H
Tryptophan
N
H
COO
–
C
H
CH
2
NH
3
+
H
O
HO
–
OOC
Prephenate
COO
–
C
CH
2
OH
–
+ CO
2
NAD
+
4-Hydroxyphenyl-
pyruvate
O
COO
–
C
CH
2
Phenylpyruvate
O
COO
–
C
CH
2
Tyrosine
H
COO
–
C
CH
2
OH
NH
3
+
Phenylalanine
COO
–
C
CH
2
H
NH
3
+
Glutamate
α-Ketoglutarate
Glutamate
α-Ketoglutarate
CO
2
+ NADH
Glutamine
Pyruvate + Glutamate
5-Phosphoribosyl-
α-pyrophosphate (PRPP)
PP
i
CO
2
H
2
O
H
2
O
Glyceraldehyde-
3-phosphate
65
4
3
2
1
7
810
911
OH
Figure 26-63 The biosynthesis of phenylalanine, tryptophan,
and tyrosine from chorismate. The pathway enzymes are
(1) anthranilate synthase, (2) anthranilate
phosphoribosyltransferase, (3) N-(5¿-phosphoribosyl)
anthranilate isomerase, (4) indole-3-glycerol phosphate synthase,
(5) tryptophan synthase, ␣ subunit, (6) tryptophan synthase,
 subunit, (7) chorismate mutase, (8) prephenate dehydrogenase,
(9) aminotransferase, (10) prephenate dehydratase, and
(11) aminotransferase.
JWCL281_c26_1019-1087.qxd 6/8/10 9:38 AM Page 1077

Michael Dunn has shown that the elimination of water
from the serine–PLP Schiff base on the  subunit to form
an aminoacrylate–PLP Schiff base intermediate
triggers a conformational change that activates the ␣ sub-
unit to produce indole. The diffusion of the indole to the 
N
+
C
C
2–
O
3
PO
CH
3
A
minoacrylate–PLP Schiff base
O
–
H
N
+
H
H
2
C
COO
–
H
subunit to react with this intermediate then results in the
formation of tryptophan.
Channeling may be particularly important for indole
since this nonpolar molecule otherwise can escape the bac-
terial cell by diffusing through its plasma and outer mem-
branes. We have seen similar phenomena in reactions
involving glutamine amidotransferases (Sections 26-2Aa
and 26-5Aa), as well as in the series of reactions catalyzed
by fatty acid synthase, in which the growing product is kept
in the vicinity of the multifunctional enzyme’s active site by
covalent attachment to the enzyme’s flexible phosphopan-
tetheine arm (Section 25-4Ca). Channeling is also implicated
in the multistep biosyntheses of purines and pyrimidines
(Sections 28-1A and 28-2A).
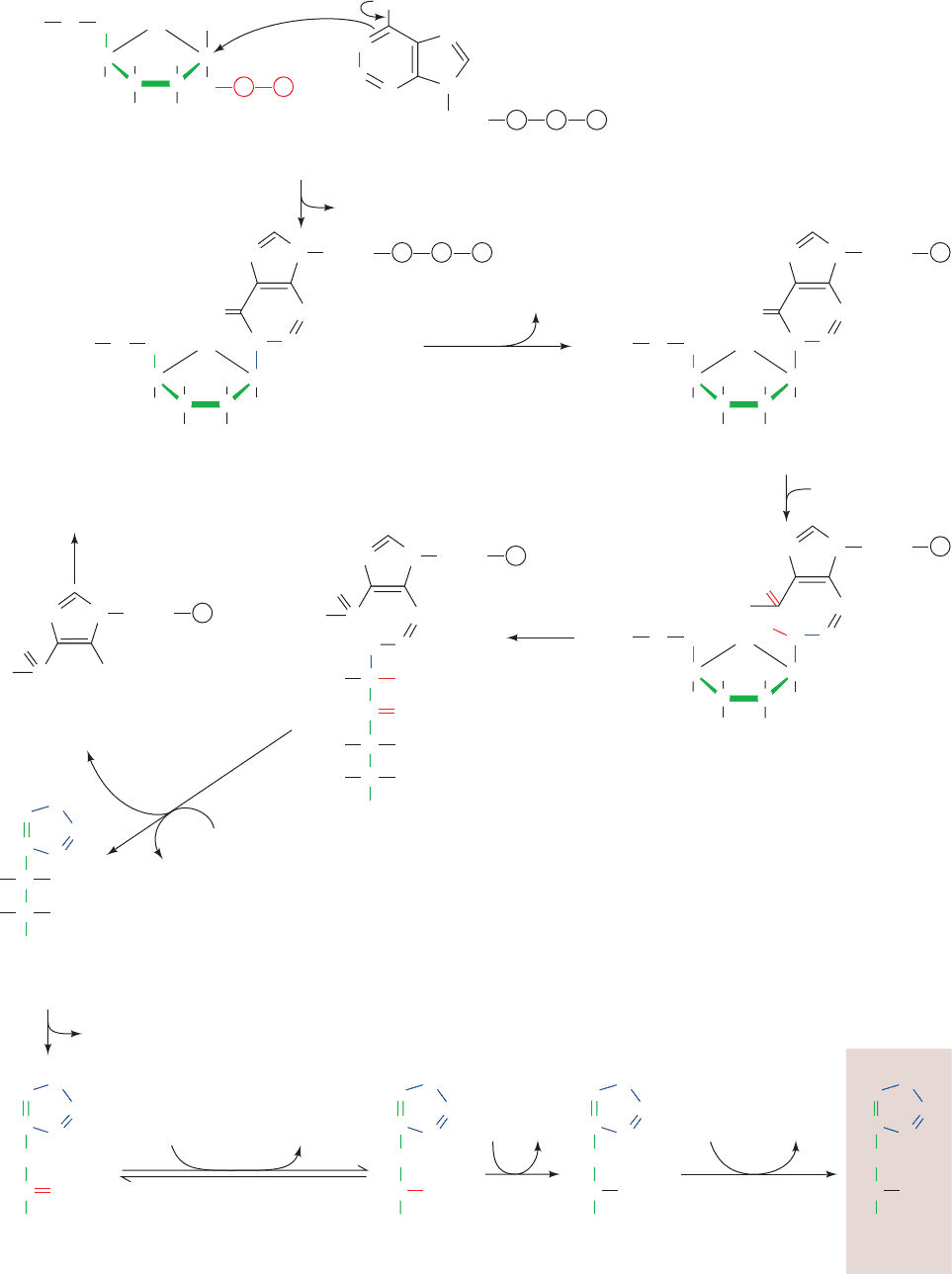
e. Histidine Biosynthesis
Five of histidine’s six C atoms are derived from 5-phos-
phoribosyl-␣-pyrophosphate (PRPP; Fig. 26-65), an inter-
mediate also involved in the biosynthesis of tryptophan
(Fig. 26-63, Reaction 2), purine nucleotides (Section 28-1A),
and pyrimidine nucleotides (Section 28-2A). The histi-
dine’s sixth carbon originates from ATP. The ATP atoms
that are not incorporated into histidine are eliminated as
5-aminoimidazole-4-carboxamide ribonucleotide (AICAR;
Fig. 26-65, Reaction 5), which is also an intermediate in
purine biosynthesis (Section 28-1A).
The unusual biosynthesis of histidine from a purine has
been cited as evidence supporting the hypothesis that life
was originally RNA based (Section 1-5Ca). His residues, as
we have seen, are often components of enzyme active sites,
where they act as nucleophiles and/or general acid–base
catalysts. The discovery that RNA can have catalytic prop-
erties (Section 31-4Ae) therefore suggests that the imida-
zole moiety of purines plays a similar role in these RNA
enzymes (ribozymes). This further suggests that the histi-
dine biosynthesis pathway is a “fossil” of the transition to
more efficient protein-based life-forms.
6 NITROGEN FIXATION
The most prominent chemical elements in living systems
are O, H, C, N, and P. The elements O, H, and P occur widely
in metabolically available forms (e.g., H
2
O, O
2
, and P
i
).
However, the major available forms of C and N, CO
2
and
N
2
, are extremely stable (unreactive); for example, the
N‚N triple bond has a bond energy of 945 kJ ⴢ mol
⫺1
1078 Chapter 26. Amino Acid Metabolism
Figure 26-64 A ribbon diagram of the bifunctional enzyme
tryptophan synthase from S. typhimurium. Only one ␣
protomer of this 2-fold symmetric ␣␣ heterotetramer is
shown.The ␣ subunit is blue, the  subunit’s N-terminal domain
is orange, its C-terminal domain is red-orange, and all  sheets
are tan.The active site of the ␣ subunit is located by its bound
competitive inhibitor, indolepropanol phosphate (IPP; red
ball-and-stick model), whereas that of the  subunit is marked by
its PLP coenzyme (yellow ball-and-stick model). The
solvent-accessible surface of the ⬃25-Å-long “tunnel” connecting
the active sites of the ␣ and  subunits is outlined by a yellow dot
surface. Several indole molecules (green ball-and-stick models)
have been modeled into the tunnel in head to tail fashion,
thereby demonstrating that the tunnel has sufficient width to
permit the indole product of the ␣ subunit to pass through the
tunnel to the  subunit’s active site. [Courtesy of Craig Hyde,
Edith Miles, and David Davies, National Institutes of Health.]
See Interactive Exercise 25
Figure 26-65 (Opposite) The biosynthesis of histidine. The
pathway enzymes are (1) ATP phosphoribosyltransferase,
(2) pyrophosphohydrolase, (3) phosphoribosyl–AMP
cyclohydrolase, (4) phosphoribosylformimino-5-aminoimidazole
carboxamide ribonucleotide isomerase, (5) imidazole glycerol
phosphate synthase (a glutamine amidotransferase),
(6) imidazole glycerol phosphate dehydratase, (7)
L-histidinol
phosphate aminotransferase, (8) histidinol phosphate
phosphatase, and (9) histidinol dehydrogenase.
JWCL281_c26_1019-1087.qxd 10/19/10 9:46 AM Page 1078
Section 26-6. Nitrogen Fixation 1079
CH
2
H O
HH
HO OH
O
O
2–
O
3
P
2–
O
3
P
2–
O
3
P
2–
O
3
P
5-Phosphoribosyl-
α-pyrophosphate (PRPP)
H
C
CC
C
P
P
+
N
N
N
N
Ribose
NH
2
:
P P P
ATP
PP
i
C
N
N
N
N
Ribose
HN
P P P
CH
2
H H
HH
OH OH
O
O
N
1
-5ⴕ-Phosphoribosyl-ATP
C
CC
C
H
PP
i
C
N
N
N
N
Ribose
HN
P
CH
2
H H
HH
OH OH
O
O
N
1
-5ⴕ-Phosphoribosyl-AMP
H
C
N
N
N
N
Ribose
H
2
N
P
CH
2
H H
HH
HO OH
O
O
N
1
-5ⴕ-Phosphoribosylformimino-
5-aminoimidazole-4-
carboxamide ribonucleotide
H
O
H
CH
N
N
N
Ribose
P
N
1
-5ⴕ-Phosphoribulosylformimino-
5-aminoimidazole-4-
carboxamide ribonucleotide
O
HN
C
C
O
C
OH
OH
C
H
H
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
CH
2
OH
C
H
H
N
N
Ribose
P
O
H
2
N
H
2
N
C
Imidazole glycerol
phosphate
OH
OH
H
H
NH
2
5-Aminoimidazole-
4-carboxamide
ribonucleotide (AICAR)
Glutamine
Glutamate
To purine biosynthesis
Imidazole acetol
phosphate
C
CH
2
C
N
N
H
HC
O
L-Histidinol
phosphate
NH
3
+
NH
3
+
HC
P
i
Glutamate α-Ketoglutarate
L-Histidinol Histidine
NH
3
+
2NADH2NAD
+
1
2
3
4
5
6
789
CH
C
CH
2
N
N
H
HC
C
H
H
C
C
H
C
N
C
N
H
H
2
C
H
H
C
C
CH
2
COO
–
N
H
N
HC
C
H
C
C
C
N
C
N
H
H
C
H
HC
H
2
O
H
2
O
H
2
O
C C
CC
C
CC
C
JWCL281_c26_1019-1087.qxd 4/20/10 9:26 AM Page 1079
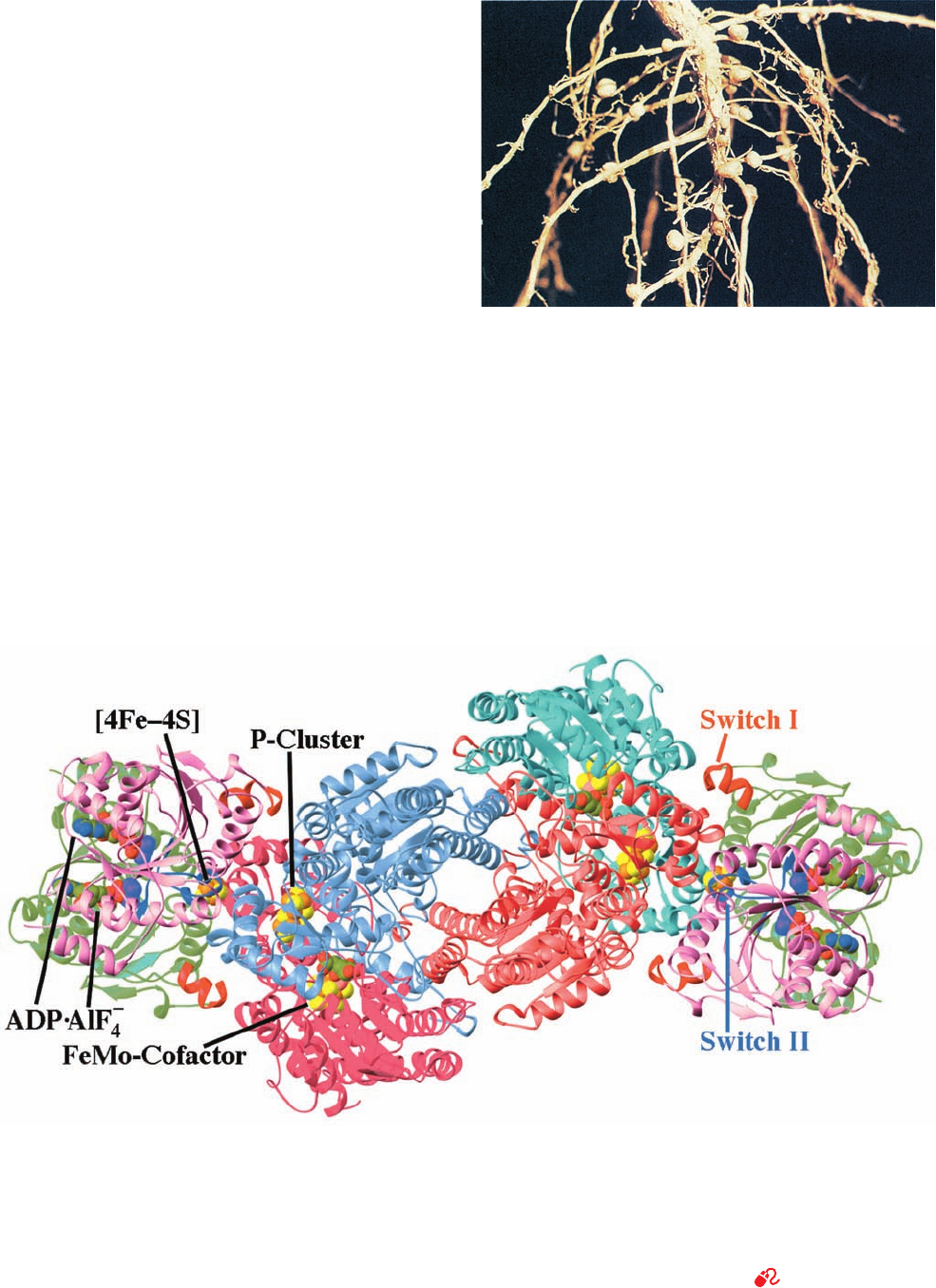
2. The MoFe-protein, an ␣
2

2
heterotetramer that con-
tains Fe and Mo.
The X-ray structure of Azotobacter vinelandii nitrogenase
in complex with the inhibitor ADP ⴢ AIF
⫺
4
(which mimics
the transition state in ATP hydrolysis), determined by
Douglas Rees, reveals that each MoFe-protein associates
with two molecules of Fe-protein (Fig. 26-67).
Each Fe-protein dimer’s single [4Fe–4S] cluster is lo-
cated in a solvent-exposed cleft between the two subunits
1080 Chapter 26. Amino Acid Metabolism
Figure 26-66 Photograph showing the root nodules of the
legume bird’s-foot trefoil. [Vu/Cabisco/Visuals Unlimited.]
Figure 26-67 X-ray structure of the A. vinelandii nitrogenase
in complex with ADP ⴢ AIF
ⴚ
4
. The enzyme, which is viewed along
its molecular 2-fold axis, is an (␣␥
2
)
2
heterooctamer in which
the –␣–␣– assembly, the MoFe-protein, is flanked by two ␥
2
Fe-proteins whose 289-residue subunits are related by local
2-fold symmetry. The homologous ␣ subunits (cyan and magenta;
491 residues) and  subunits (light red and light blue; 522
residues) are related by pseudo-2-fold symmetry. The two ␥
subunits forming each Fe-protein (pink and green with their
Switch I and Switch II segments red and blue) bind to the
MoFe-protein with the 2-fold axis relating them coincident with
the pseudo-2-fold axis relating the MoFe-protein’s ␣ and 
subunits.The ADP ⴢ AIF
⫺
4
, [4Fe–4S] cluster, FeMo-cofactor, and
P-cluster are drawn in space-filling form with C green, N blue, O
red, S yellow, Fe orange, Mo pink, and the AlF
⫺
4
ion purple.
[Based on an X-ray structure by Douglas Rees, California
Institute of Technology. PDBid 1N2C.]
See Interactive
Exercise 26
(versus 351 kJ ⴢ mol
⫺1
for a C¬O single bond). CO
2
, with
only minor exceptions, is metabolized (fixed) only by pho-
tosynthetic organisms (Chapter 24). N
2
fixation is even less
common; this element is converted to metabolically useful
forms by only a few strains of bacteria, named diazatrophs.
Diazatrophs of the genus Rhizobium live in symbiotic
relationship with root nodule cells of legumes (plants be-
longing to the pea family, including beans, clover, and al-
falfa; Fig. 26-66) where they convert N
2
to NH
3
:
The NH
3
thus formed can be incorporated either into glu-
tamate by glutamate dehydrogenase (Section 26-1B) or
into glutamine by glutamine synthetase (Section 26-5Ab).
This nitrogen-fixing system produces more metabolically
useful nitrogen than the legume needs; the excess is ex-
creted into the soil, enriching it. It is therefore common
agricultural practice to plant a field with alfalfa every few
years to build up the supply of usable nitrogen in the soil
for later use in growing other crops.
a. Nitrogenase Contains Novel Redox Centers
Nitrogenase, which catalyzes the reduction of N
2
to
NH
3
, is a complex of two proteins:
1. The Fe-protein, a homodimer that contains one
[4Fe–4S] cluster and two ATP binding sites.
2NH
3
⫹ H
2
⫹ 16ADP ⫹ 16P
i
N
2
⫹ 8H
⫹
⫹ 16ATP ⫹ 16H
2
O ⫹ 8e
⫺
¡
JWCL281_c26_1019-1087.qxd 10/19/10 9:48 AM Page 1080
