Stacey F.D., Davis P.M. Physics of the Earth
Подождите немного. Документ загружается.

//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C21.3D
–
357
– [348–360] 13.3.2008 10:48AM
in terawatts. The expression in the table is the
integral over time , with a numerical factor to
give the integrated heat in units of 10
28
J, as for
other entries in the table. We consider two mod-
els, with A ¼0.2 terawatt and A ¼0.
The major item of heat loss listed in
Table 21.5, 54.0/
E
, is the conducted heat at the
top of the core, being 3.79 10
12
W for time ,
assuming a conductivity of 28.3 W m
1
K
1
(Section 19.6) and an adiabatic temperature gra-
dient (Eqs. (19.55) and (19.56)). Arguments about
the energy balance of the core, and the need to
consider radioactivity, are critically dependent
on this heat loss, and therefore on the conduc-
tivity. Most discussions have assumed a higher
value. The nature of the problem is seen by not-
ing that if the inner core has existed for most of
the life of the Earth (
E
), then the sources in
the Q column of Table 21.5 balance the heat loss
only with a radiogenic contribution, refrigerator
action, or both. Since refrigeration consumes
convective power that would otherwise be avail-
able for the dynamo (the fQ
R
entry in the E
column of the table), its availability is limited.
To determine the efficiency factor f with which it
operates we need to know the radial variation of
the core heat flux.
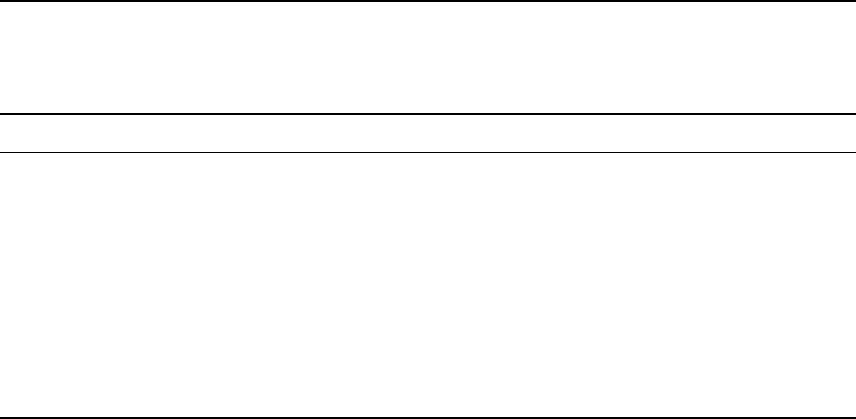
The heat flux within the core is plotted in
Fig. 21.3 for the two models considered, with
and without radiogenic heat. Conducted heat
diminishes strongly with depth mainly because
the adiabatic temperature gradient decreases
(Eq. 19.55). The total heat flux through any radius
is the total heat originating within it and varies
less with radius than the conducted heat, both
because of the latent heat of inner core solid-
ification and because the deep parts cool faster
in maintaining the adiabatic gradient. In the fig-
ure we see that the total heat comfortably
exceeds the conducted heat deep in the core
but that for both models the conducted heat is
greater in the outer part. Convected heat, shown
as the difference, is negative at the top of the
core (shaded for the A ¼ 0.2 model in Fig. 21.3),
where the adiabatic temperature gradient must
be maintained by reversed convection, that is
refrigerator action, the principle of which is dis-
cussed in Section 22.8. The refrigerator power
required is the product of heat carried down
and an efficiency, f, determined by the tempera-
ture range over which it operates. f is the Carnot
efficiency with which the same heat would gen-
erate power if convected upwards. For the A ¼0.2
model f ¼0.043. For the A ¼0 model not only
must more heat be transferred downwards but
the depth and temperature ranges are greater
and f ¼0.091. With these values of f we can
examine the models to see whether the refrige-
rator action leaves eno ugh conv ective energy
for a viable dynamo.
Table 21.5 Summary of components of the core energy balance during the lifetime of the
inner core. Q gives energy sources and E the corresponding convective energies, which are
related to Q by thermodynamic efficiencies.
E
is the age of the Earth and A is the present rate of
radiogenic heating by
40
K in terawatts. For a discussion of refrigerator action see Section 22.8
Sources Q (10
28
J) Efficiency E (10
28
J)
Heat capacity Cooling 17.75 0.139 2.47
Contraction 2.52 0.135 0.34
Freezing Latent heat 6.36 0.252 1.60
Contraction 2.76 0.190 0.52
Compositional separation 4.79 1.00 4.79
Precession 0.20 /
E
1.00 0.20 /
E
Radioactivity 5.69A[exp(2.5 /
E
) 1] 0.127 0.723A[exp(2.5 /
E
) 1]
Losses
Conduction 54.0 /
E
0.119 6.43 /
E
Refrigerator action Q
R
f fQ
R
21.4 ENERGY BALANCE OF THE CORE 357
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C21.3D
–
358
– [348–360] 13.3.2008 10:48AM
The sources and losses in the Q column of
Table 21.5 must balance. With A in terawatts
and other numerical values adjusted so that all
terms are expressed in units of 10
28
J, as in the
table,
34:14 þ 5:69A½expð2:5 =
E
Þ1¼53:8=
E
Q
R
:
(21:21)
Now we need also the entries in the E column
of the table. These are convective energies
related to corresponding entries in the Q col-
umn by thermodynamic efficiencies discussed
in Section 22.7. The sum of all the E entries
(subtracting the negative terms) is the energy,
E
D
, available for the dynamo. But the dynamo
itself generates heat by ohmic dissipation; by
assuming it to be uniformly distributed through
the core we assign an efficiency of 0.122 to its
generation of further dynamo power. This
cannot be counted as a heat source but it
means that we equate the available energy not
to E
D
but to (1 0.122) E
D
. Thus, in units of
10
28
J as before,
0:878E
D
¼ 9:72 þ 0:723A½expð2:5=
E
Þ1
6:23=
E
fQ
R
: (21:22)
Substituting for Q
R
by Eq. (21.21) we obtain an
expression for dynamo power in terms of A,
and f. For the two cases considered, (A ¼0,
f ¼0.091) and (A ¼0.2, f ¼0.043),
A ¼ 0 : E
D
¼ 14:61 12:67=
E
; (21:23)
A ¼ 0:2 : E
D
¼ 12:66 9:73=
E
þ0:2204½expð2:5=
E
Þ1: (21:24)
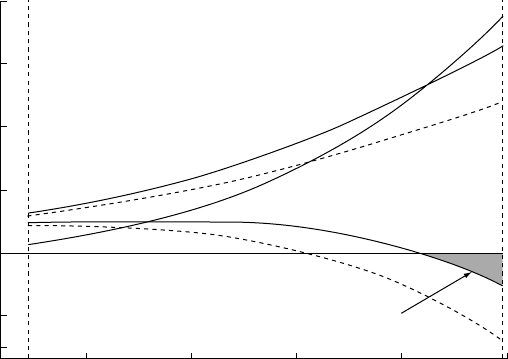
Dividing E
D
by , we have the average dynamo
power for the lifetime, , of the inner core as a
function of for each of the models, as plotted in
Fig. 21.4. The broken line in the figure shows the
estimated dynamo power requirement from
Section 24.7, 0.3 terawatt. Remembering that we
have deferred to Sections 22.7 and 23.5 the varia-
tions of core heat flux and dynamo power with
time, the figure shows the average power over
the inner core lifetime and not a time variation
of the power. The essential conclusion from this
figure is that the assumed core conductivity,
¼28.3 W m
1
K
1
at the top of the core and vary-
ing little with depth, marks a boundary between
alternative core models, with and without radio-
activity. With a lower conductivity there would be
no reason to consider radioactivity, but a higher
value would make it difficult to avoid. The uncer-
tainty in is the central doubt around which the
current debate about core physics revolves, as
reviewed by Nimmo et al. (2004).
3500
300025001500 20001100
Radius (km)
–1.5
4.0
3.0
2.0
1.0
–1.0
0
Average heat flux (terawatt)
Refrigerator action
Convected heat
(A
= 0.2 terawatt)
Conducted heat
Convected heat (A
= 0)
Total heat
(A
= 0.2 terawatt)
Total heat
(A
= 0)
Mantle
Inner core
FI G U R E 21.3 Radial variation of
the average core heat flux over the
life of the Earth for two models,
both of which assume that the
inner core began to form very
early. Conducted heat, calculated
assuming a thermal conductivity
which varies from 28.3 W m
1
K
1
at the top to 29.3 W m
1
K
1
at the
bottom of the outer core, exceeds
the plotted total heat flux in the
outermost part of the core for both
models. The difference is plotted
as convected heat, the negative
range indicating how much heat is
carried down by refrigerator
action, driven by compositional
convection. The model with 0.2
terawatt of present radiogenic heat
from
40
K(theA ¼0.2 model) is
shown by solid lines and the non-
radioactive model by broken lines.
358 THE GLOBAL ENERGY BUDGET
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C21.3D
–
359
– [348–360] 13.3.2008 10:48AM
21.5 Minor components of the
energy budget
By ‘minor components’ we mean contributions
that may be geophysically interesting and are
included in Table 21.4, but are smaller than the
uncertainties in the major components and are
not discussed elsewhere in this chapter. We con-
sider two: tidal friction and mantle differentia-
tion. Others that we can think of, such as
absorption of cosmic neutrinos, are certainly
insignificant.
The tidal energy dissipation, calculated from
the slowing rotation in Section 8.3, with a small
allowance for the increasing orbital energy of
the Moon, is 3.7 10
12
W. Most of this is due to
marine tides and has no impact on the solid
Earth. The internal dissipation can be estimated
from the elastic strain energy of the solid tide, of
which a fraction, 2p/Q
S
, is lost per tidal cycle by
anelastic effects. We adopt the value of Q
S
¼280
for shear deformation of the mantle at tidal fre-
quencies obtained by Ray et al. (2001), who com-
bined satellite observations of the total tidal
deformation of the Earth, as deduced from its
gravitational effect, with altimeter data on the
deformation of the ocean surface. Although
measuring a small effect as a difference between
much larger ones, they claimed 25% accuracy.
Their tidal Q
S
value appears high compared
with what might be expected from extrapolation
from seismic frequencies, but it is the only
direct observation. The tidal strain of the solid
Earth, estimated from the Love number h
(Section 10.5), is " ¼5 10
8
so the strain energy
is
1
/
2
"
2
V ¼2 10
17
J, with rigidity modulus,
¼176 GPa, averaged over the volume, V,of
the mantle. With Q
S
¼280, the fraction of this
energy that is dissipated each tidal cycle (12.4
hours or 44 700 s) is 2.25%, giving a rate of
1.0 10
11
W, about 2.7% of the total tidal dissi-
pation but of little consequence to the present
thermal state of the Earth. However, it could
have exceeded 10
12
W very early in the history
of the Earth, when the Moon was closer and the
Earth was rotating faster.
The crust is a light differentiate that has sep-
arated from the mantle, releasing gravitational
energy in the process. We consider only the con-
tinental crust, which has progressively accumu-
lated, and not the oceanic crust, which is
continuously recycled back into the mantle.
The continental crust, volume V ¼8 10
18
m
3
,
is less dense than the underlying mantle by an
average D ¼470 kg m
3
, so that VD ¼3.8 10
21
kg. The average gravitational potential through
which this mass has moved depends on whether
the crust is believed to have been derived uni-
formly from the whole mantle or only from
the upper mantle. We calculate each case and
adopt a compromise, because the upper mantle
appears to have been more affected. It is conven-
ient to the calculation that the density profile of
4.5
4.03.53.02.5
Inner core lifetime (10
9
Years)
0.3
1.3
1.0
0.5
Mean dynamo power (terawatt)
0
A = 0
A
= 0.2 terawatt
FIG U R E 21.4 Average dynamo power
over the inner core lifetime, t,asa
function of t for models with 0.2
terawatt of radiogenic heat from
40
Kat
the present time and no radiogenic
heat (A ¼0). The broken line indicates
the power requirement for a
geomagnetic field of the present
strength.
21.5 MINOR COMPONENTS OF THE ENERGY BUDGET 359

//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C21.3D
–
360
– [348–360] 13.3.2008 10:48AM
the Earth happens to give almost constant grav-
ity, g 10 m s
2
, over the whole depth of the
mantle (see Appendix F). Since at radius r,
g ¼Gm(r)/r
2
, the mass inside r is
mðr Þ¼ðg=GÞr
2
; (21:25)
where G ¼6.67 10
11
m
3
kg
1
s
2
is the gravita-
tional constant, so that, in this approximation,
(g/G) is constant. It allows a simple calculation of
the average gravitational potential difference
between radius r
1
and the surface at r
2
, weighted
according to the distribution of mass in this
range. By Eq. (21.23) the mass in the range r to
r þdr is a fraction of the mass M between r
1
and
r
2
, given by
dm=M ¼ 2rdr=ðr
2
2
r
2
1
Þ; (21:26)
and its gravitational potential relative to the
surface is g(r
2
r), so that the gravitational
energy released by separation of the mass differ-
ence VD uniformly from the mass in the range
r
1
to r
2
is
E ¼
Vg
ðr
2
2
r
2
1
Þ
ð
r
2
r
1
ðr
2
rÞr dr
¼ Vg
r
2
3
2r
2
1
3ðr
1
þ r
2
Þ
: (21: 27)
For the two cases, separation from the whole
mantle or from the upper mantle, the values of
this energy are 4.9 10
28
J and 1.2 10
28
J.
Accretion of the continental crust is a conse-
quence of convection, which was faster in the
past than at present. We assume that the rate of
accretion is proportional to the rate of convec-
tive heat transport, calculated in Chapter 23, and
that the present rate is half of the average over
the life of the Earth. On this basis the present
rates of energy release for the two cases are
1.7 10
11
W and 4.3 10
10
W. We should prob-
ably add an energy of accumulation of dense
differentiates in the D
00
layer, but have no quan-
titative estimate and presume it to be smaller. In
Tables 21.1 and 21.4 we take rounded averages of
the alternative estimates.
360 THE GLOBAL ENERGY BUDGET
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C22.3D
–
361
– [361–375] 13.3.2008 10:47AM
22
Thermodynamics of convection
22.1 Preamble
We have a good measure of the rate of heat loss
from the solid Earth to the atmosphere and
oceans, 44.2 10
12
W (Pollack et al. 1993).
Although the final stage is observed as conduc-
tion through the crust and hydrothermal circu-
lation of sea water through young ocean floor,
most of the heat comes from the deep interior.
Thermal diffusion in a body the size of the Earth
is too slow to have cooled the lower mantle
noticeably in its lifetime. The deep heat is trans-
ported upwards by hot material, driven by
the buoyancy of its thermal dilation relative to
the cooler material that sinks to replace it. The
global scale motion is evident at the surface as
tectonic activity, including continental drift and
earthquakes, with volcanism as a side effect.
Chapter 13 considers the stresses involved in
this process and relates them to the mechanical
energy derived from convection. The calculation
of the energy is presented in this chapter. It is an
application of classical thermodynamics.
The mechanical power of convection is the
product of heat transport and a thermodynamic
efficiency that we can calculate in two ways.
First, we recognize that the mantle is a heat
engine in the classical thermodynamic sense
and that we can apply the Carnot theorem to
derive the mechanical power of convection with-
out an explicit consideration of the forces
involved. Then, we relate the conclusion more
directly to the driving forces of convection, as
outlined in Chapter 13, by calculating the
efficiency from the buoyancy forces acting on
the rising and falling limbs of a convective cell.
The two calculations are complementary. The
mechanical model shows not only how convec-
tive power is generated, but that its efficiency is
that of a Carnot cycle operating over an adiabatic
temperature ratio between its heat source and
sink. We point out here that this is a completely
general result. We are calculating the power that
is inevitably generated, not merely may be gen-
erated, and show that no more power is possible,
in principle. This is a fundamental theorem on
which a discussion of tectonics must be based, as
well as being an essential consideration in the
thermal history of the mantle (Section 23.4).
The thermodynamic theorem is first pre-
sented in a special, limited form by applying
physical restrictions that make the principle
easy to see, and then we show that the restric-
tions can be removed without affecting the con-
clusions. We consider a homogeneous layer with
an adiabatic temperature gradient. As pointed
out in Section 19.5, such a layer is neutrally buoy-
ant and a steeper gradient would be required to
make it convect, but we can nevertheless postu-
late that a parcel of material at the bottom of the
layer is given an infinitesimal amount of heat
which it carries up and releases at the top. By
making the heat infinitesimal we can ignore the
thermodynamically irreversible processes of
heat conduction into and out of the parcel and
treat them as isothermal. Then, by allowing the
parcel to return to its original position and tem-
perature, we take it round a Carnot cycle
between two adiabats and two isotherms. In
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C22.3D
–
362
– [361–375] 13.3.2008 10:47AM
fact, the return path is not really necessary
because the parcel considered and the material
it displaces by its return are identical. So, we can
appeal to the standard theorem that the mechan-
ical energy, W, generated by the cycle, being the
area of a loop in a P–V diagram, is the heat input,
Q
1
, multiplied by a thermodynamic efficiency, ,
given by
¼
W
Q
1
¼
T
1
T
2
T
1
¼ 1
T
2
T
1
; (22:1)
where T
1
and T
2
are the temperatures of the heat
source and sink at the bottom and top of the
layer (assumed to be the equilibrium tempera-
tures at those levels). By restricting the applied
temperature increment to an infinitesimal
value, and the temperature profile of the
medium to be precisely adiabatic, we can adopt
the ideal Carnot efficiency, given by Eq. (22.1). In
the following section we show how this is gener-
alized to arbitrary temperature increments and
gradients.
22.2 Thermodynamic efficiency,
buoyancy forces and
convective power
Conservation of energy (the first law of thermo-
dynamics) means that the heat exhausted to the
sink is
Q
2
¼ Q
1
W; (22:2)
so that we can write Eq. (22.1) in a less familiar
form,
0
¼
W
Q
2
¼
T
1
T
2
T
2
¼
T
1
T
2
1 ¼
1
: (22:3)
A reason for interest in
0
, the efficiency referred
to the heat exhausted to the sink, rather than the
conventional , referred to the source, is that in
the case of the Earth we observe the heat
exhausted at the surface. The mechanical
power generated is consumed in deforming and
so heating the mantle, being thereby put back
into the heat source, although not necessarily
distributed in the same way, and effectively
reduces the net heat input to Q
2
. Note that it is
not the difference in the temperatures of the
heat source and sink that appears in the effi-
ciency, it is their ratio.
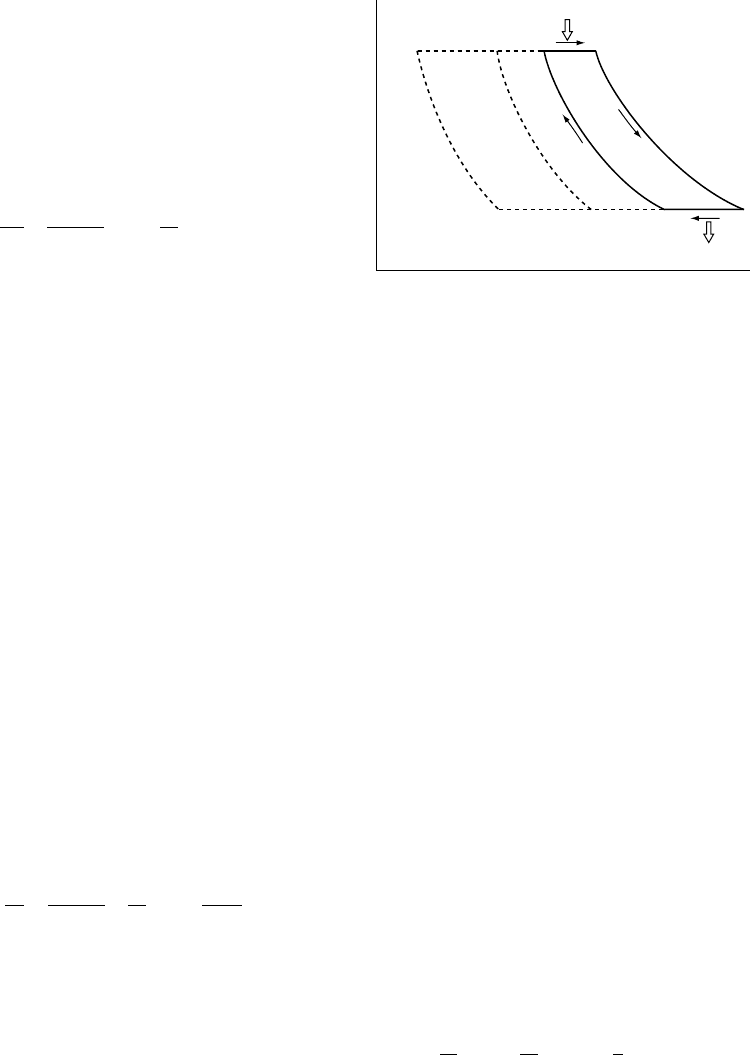
We can extend the loop with an indefinite
number of adjacent loops, each of which is a
Carnot cycle, as in Fig. 22.1. The limbs at pres-
sures P
1
and P
2
then involve finite temperature
changes and cannot be regarded as isotherms,
but each point at P
1
is adiabatically related to a
point at P
2
and gives an efficiency corresponding
to that section of the total loop. This is given by
the temperature ratio, calculated by Eq. (19.19),
which integrates to give
ln
T
1
T
2
¼
ð
P
1
P
2
K
S
dP ¼
ð
1
2
d: (22:4)
In general and K
S
are temperature dependent
so that these component efficiencies are not all
equal, but the temperature variations in phys-
ical properties are quite small, especially at
P
V
P
1
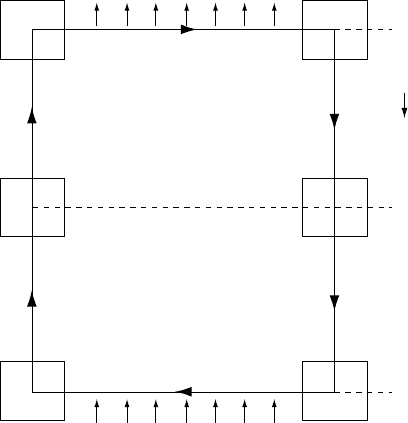
FA'AB
Q
1
S
1
CDD'E
P
2
S
2
T
1
T
1
'
Q
2
T
2
T
2
'
FI G U R E 22.1 A thermodynamic cycle between
pressures P
1
and P
2
. The solid loop is of infinitesimal
width, involving infinitesimal heat input, Q
1
, on limb AB
and output, Q
2
, on limb CD. The temperature changes on
these limbs are, therefore, infinitesimal and they can be
treated as isothermal. The other two limbs are adiabats of
entropies S
1
and S
2
(also infinitesimally different),
making the loop ABCDA a Carnot cycle. When an adjacent
cycle, A
0
ADD
0
A is added, the limb DA is cancelled, but
temperatures T
1
0
and T
2
0
cannot be treated as equal to T
1
and T
2
. They are, however, adiabatically related to each
other in the same way as T
1
and T
2
. This allows a finite
extension of the loop to EF, with limbs FB and CE
involving finite temperature changes, but with each point
on FB adiabatically related to a point on CE by the same
pressure difference. The mechanical work done on one
cycle is the area of the loop on the P–V diagram.
362 THERMODYNAMICS OF CONVECTION
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C22.3D
–
363
– [361–375] 13.3.2008 10:47AM
high pressure, and there is no difficulty in cal-
culating the average efficiency for the complete
loop. Even a large temperature difference
between B and F (or C and E) in Fig. 22.1 does
not compromise the efficiency argument. Of
course, to get heat into the medium on limb
FB the source may need to be at a higher tem-
perature, and to get heat out on limb CE the
sink must certainly be at a lower temperature,
but these excess temperature differences con-
tribute nothing to the convective cycle per se.
They merely drive thermal diffusion, which is
thermodynamically irreversible and cannot
affect the calculated power or efficiency.
Convective power is determined by the adia-
batic temperature ratio, whatever higher ratio
actually drives the convection.
Now we can also ask whether it is necessary
for limbs BC and DA in Fig. 22.1 to be adiabats. If
they are not, then it means that heat leaks into or
out of the medium on these limbs. If it leaks from
one limb of the cycle to the other then it is
simply returned to its source and contributes
nothing to either heat transfer or convective
power. If it leaks into some non-participating
part of the medium then we do not have a closed
cycle and additional material must be brought
into the energy balance. Inevitably some of the
heat from the source, or equivalently coolness
from the sink, gets only part way round a cycle.
But, if we disallow time variations of the thermal
structure, this only means that part of the heat
participates in a shortened cycle, with its own
thermodynamic efficiency. This is directly rele-
vant to mantle convection for which it is conven-
ient to describe convection in terms of coolness
transported downwards by subducting slabs,
rather than heat transported upwards. The
assumption of a uniform distribution of heat
sources requires that the subducting coolness is
distributed through the mantle and does not all
end up at the bottom.
We now examine a mechanical model that is
more obviously related to the forces driving con-
vection in the Earth. The cycle ABCDA in Fig. 22.2
is the physical path of a mass m between depths
z
1
and z
2
and corresponds to the elementary
cycle shown by the solid line in Fig. 22.1. On
limb AB of the path it absorbs (or acquires by
its own radioactivity) heat Q
1
, so that the temper-
ature rises from T
A
to T
B
and
Q
1
¼ mC
P1
ðT
A
T
B
Þ: (22:5)
As before, we assume initially that (T
B
T
A
)is
infinitesimal, but we allow the surrounding
medium to have any arbitrary temperature pro-
file. The assumption of an infinitesimal temper-
ature increment means that physical properties,
specific heat, C
P
, volume expansion coefficient,
, and Gr ¨uneisen parameter, , are taken as inde-
pendent of temperature over the small incre-
ment, although they may vary arbitrarily with
pressure. The mass element then rises from B to
C, cooling to temperature T
C
, and we make the
further assumption, removed later, that this
limb of the path is adiabatic. It loses heat on
limb CD, cooling to temperature T
D
, which is so
adjusted that with adiabatic recompression on
limb DA it returns to temperature T
A
.
The buoyancy forces at arbitrary depth z,at
positions X and Y in Fig. 22.2, are
F
X
¼ mgðT
X
TÞ; (22:6)
C
X
B
A
Y
D
Q
1
Q
2
z
2
z
z
z
1
FI G U R E 22.2 The physical path of an element of
material in a convective cycle.
22.2 EFFICIENCY AND CONVECTIVE POWER 363
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C22.3D
–
364
– [361–375] 13.3.2008 10:47AM
F
Y
¼ mgðT
Y
TÞ; (22:7)
where g is gravity and the surrounding ambient
temperature, T, is assumed to be the same at X
and Y. Then, since no mechanical work is done
on the horizontal limbs AB and CD, the net
work done by buoyancy forces over the whole
cycle is
W ¼
ð
z
1
z
2
ðF
X
F
Y
Þdz ¼ m
ð
gðT
X
T
Y
Þdz: (22:8)
This result does not depend on T and it is the
same whether the convection is considered to be
driven by upwelling at X or subduction at Y.
Since BC and DA are adiabats the entropy differ-
ence, DS, between any point on one and any
point on the other is the same. Thus
S ¼
ð
dQ
T
¼
ð
T
B
T
A
mC
P
1
T
dT ¼
ð
T
X
T
Y
mC
P
T
dT
¼
ð
T
C
T
D
mC
P
2
T
dT (22:9)
and, with the assumption of an infinitesimal
temperature difference,
S ¼ mC
P
lnðT
X
=T
Y
ÞmC
P
ðT
X
=T
Y
1Þ
¼ mC
P
lnðT
A
=T
B
ÞmC
P
ðT
A
=T
B
1Þ
¼ Q
1
=T
A
: (22:10)
Using this result to substitute for (T
X
T
Y
)in
Eq. (22.8),
W ¼
Q
1
T
A
ð
z
1
z
2
gT
Y
C
P
dz ¼
Q
1
T
A
ð
z
1
z
2
gT
Y
K
S
dz: (22:11)
The integrand in Eq. (22.11) is the adiabatic tem-
perature gradient (see Eqs. (19.55) and (19.56)), so
we can write Eq. (22.11) in a form coinciding with
the conclusion of Section 22.1:
W ¼ Q
1
T
A
T
D
T
A
¼ Q
1
1
T
D
T
A
¼ Q
1
1 exp
ð
2
1
d
2
6
4
3
7
5
8
>
<
>
:
9
>
=
>
;
Q
1
1
2
1
:
(22:12)
The efficiency, ¼W/Q
1
, is calculated knowing
only the density variation and the Gr ¨uneisen
parameter. It is not necessary to know the tem-
peratures. depends only the adiabatic temper-
ature ratio between the heat source and sink.
This is given by Eq. (22.4) and does not depend
on the actual temperatures unless the thermal
properties are temperature dependent.
As mentioned, the assumption of an infinites-
imal temperature increment is removed by con-
sidering a large number of infinitesimally
displaced cycles and allowing for the tempera-
ture variations of properties by taking the aver-
age efficiency over any temperature range. If we
postulate that limb BC or DA departs from adia-
bats by leakage of heat to or from the surround-
ing medium, then we must include additional
material in a more complicated cycle to main-
tain a closed system, and if heat leaks from X to Y
it is merely returned to the source at A and con-
tributes nothing. The rising and sinking limbs of
a convective cycle do not need to be adiabats. The
essential conclusion is that the thermodynamic
efficiency of convection, that is, the ratio of
mechanical power generated to the convective
heat transport, is the Carnot efficiency corre-
sponding to the adiabatic temperature ratio of
the heat source and sink. Excess temperature
gradients are required to make convection
occur, but they do not yield more power. They
drive the thermodynamically irreversible pro-
cess of conduction, by which heat enters and
leaves the convecting medium. This makes
numerical calculations simple because we need
only the thermal properties in Appendix G and
not the absolute temperatures. However, before
discussing numerical results, we consider a com-
plication arising from phase transitions.
22.3 Convection through phase
transitions
At depths between 200 km and 700 km, mineral
phase transitions complicate convection in ways
that have been subject to much debate. The tran-
sitions obey the Clausius–Clapeyron equation,
which is considered in the special case of melt-
ing in Section 19.4 (Eq. (19.40)), but is quite
general. We apply it here to the solid–solid tran-
sitions of mantle minerals, whose crystal
364 THERMODYNAMICS OF CONVECTION

//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C22.3D
–
365
– [361–375] 13.3.2008 10:48AM
structures adjust to higher pressure forms at
depth. We write the variation with pressure of
the temperature of any transition, T
C
, in terms of
the volume and entropy changes of the transi-
tion by the Clausius–Clapeyron equation:
dT
C
dP
¼
V
S
: (22:13)
The principle of LeChatelier demands that, for
any phase transition caused by increasing pres-
sure, the volume change, DV, is negative but the
entropy change, DS, may have either sign, and
both positive and negative values are observed
for mantle minerals. If DS is positive, that is the
higher pressure form has the higher entropy,
then the mineral absorbs heat in converting to
this form. The transition is referred to as endo-
thermic and if external heat is not supplied then
the mineral is cooled. The phase boundary at
660 km depth in the mantle is due to an endo-
thermic transition. Conversely, with positive DS,
we have an exothermic transition of the kind
occurring at 220 km and 410 km.
If convection proceeds adiabatically through a
transition, with no thermodynamic irreversi-
bility or thermal diffusion resulting from the
temperature change, then Eqs. (22.1) and (22.12)
still apply, where (T
1
T
2
)or(T
A
T
D
)now
include the temperature increment or decre-
ment of the phase transition. An exothermic
transition increases the adiabatic temperature
ratio between the heat source and sink, increas-
ing the thermodynamic efficiency of the convec-
tion, whereas an endothermic transition reduces
the temperature ratio and the efficiency. The
660 km transition has received particular atten-
tion because of its inhibiting effect on whole
mantle convection. Also, the assumption of per-
fect thermodynamic reversibility and neglect of
thermal diffusion are unrealistic, and all such
irreversible effects reduce the efficiency for all
transitions, whether endothermic or exother-
mic. The effect of thermal diffusion in this sit-
uation is considered in Section 22.5.
Table 22.1 gives basic numerical data for the
transitions that we must consider. The depths
and absolute densities as well as values of bulk
modulus that are needed for calculations are
taken from PREM, but the density increments,
D, do not coincide with the PREM values, being
estimated from mineralogical data, along with
the entropy increments, DS. Other thermal pro-
perties are taken from Appendix G. DT is the
temperature increment corresponding to DS,
that is
T ¼ TS=C
P
: (22:14)
It is a notional quantity, not directly observable,
but its significance is that it adds to or subtracts
from the adiabatic temperature difference
(T
A
T
D
) in Eq. (22.12). Its magnitude, relative
to the total adiabatic temperature range of the
mantle, about 1100 K, is a measure of the impor-
tance of phase transitions to the total mechan-
ical energy generated by convection (5% to 8%,
see Table 22.1). However, the total energy does
not determine what happens locally. The detail
of what happens is of greatest interest in the case
of the endothermic 660 km transition, for which
DT is negative, because this presents an obstacle
to convection through it.
Also listed in Table 22.1 is the Clapeyron slope,
dT
C
/dP, given by Eq. (22.13). From this we obtain
the ‘overshoot’, z, that is, the difference between
the depths at which the transition occurs in
upgoing and downgoing material, according to
their temperature difference, T. In the table
this is given as a ratio, that is, the depth of over-
shoot per degree temperature difference,
Table 22.1 Characteristics of mantle phase
transitions (olivine composition)
Depth (km) 220 410 660
D (kg m
3
) 212 94 301
DS (J K
1
kg
1
) 40 35 þ49
DT (K)
(Eq. (22.14))
þ61 þ54 79
dT
C
/dP (K MPa
1
) þ0.44 þ0.21 0.36
z/ T (m K
1
)
(Eq. (22.15))
þ69 þ135 70
(MPa)
(dT ¼200K)
39
Dz (km)
(Eq. (22.24))
4.3 7.6 þ5.4
v(critical)(cm/year)
(Eq. (22.29))
1.47 0.83 1.17
22.3 CONVECTION THROUGH PHASE TRANSITIONS 365

//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C22.3D
–
366
– [361–375] 13.3.2008 10:48AM
z
T
¼
1
gdT
C
=dP
: (22:15)
Bina and Helffrich (1994) discuss the topography
of mantle phase boundaries that result from this
effect. For subducting material, which is sinking
because it is cooler and denser, T is negative, so
the 660 km phase boundary, for which dT
C
/dP is
negative, is depressed by 70 m per degree temper-
ature difference. This means 14 km if the subduct-
ing slab is 200 K cooler, on average, than its
surroundings. It pushes (or pulls) lower density
material into the higher density phase, producing
abuoyancyforce,(gzD) per unit cross section of
slab, opposing its subduction. To estimate the
stress we must subtract the driving force of ther-
mal shrinkage, g(T )z per unit cross section,
where is the volume expansion coefficient. For
a temperature contrast T the net force per unit
area of the slab cross section is therefore
¼
T
ðdT
C
=dPÞ
T
; (22:16)
where D is the density increment of the transi-
tion. Note that the second term in Eq. (22.16)
depends on the square of the temperature decre-
ment, T, because the thermal contraction and
the depth of penetration are each proportional
to T. However, the first term is dominant for any
plausible value of T.
We can refer to s, as calculated by Eq. (22.16),
as the virtual stress. It is a rough estimate of the
boundary stress caused by subduction through
an endothermic transition, but the actual stress
depends on geometrical factors, especially the
slab thickness relative to the depth overshoot.
If the initial maximum temperature deficit of
subducting material is 1000 K, with an average
of 500 K, diffusion of heat from the surrounding
mantle produces a thicker slab with T 200 K. A
value of stress, , for dT ¼200 K is given in
Table 22.1 for the 660 km transition. Even
acknowledging that we have probably overesti-
mated the stress by assuming a 100% olivine
composition of the subducting material, this
stress exceeds the average tectonic stress esti-
mated in Section 13.2. Thus, subduction through
this transition presents a mechanical problem.
Seismological evidence of horizontal deflections
of slabs and accumulations of subducted mate-
rial at the boundary is referred to in Chapter 12,
but some slabs appear to pass straight through.
Numerical simulations (e.g. Solheim and Peltier,
1994; Tackley et al., 1994) have suggested that
accumulated material may avalanche into the
lower mantle after a delay.
22.4 Thermodynamic efficiency
of mantle convection and
tectonic power
The mechanical power,
E
, generated by the con-
vection of heat,
Q
, from a source at pressure P
1
to
a sink at pressure P
2
is given by rewriting
Eq. (22.12) in a more general way:
_
E ¼
_
Q
ð
P
1
P
2
ð@ ln T @P=
Þ
S
dP ¼
_
Q
ð
P
1
P
2
K
S
=
Þ
S
dP:
(22:17)
We use this to calculate the tectonic power from
the distribution of heat sources. It is not neces-
sary to know the temperatures, but only adia-
batic ratios that are expressed in terms of
physical properties. The intervention of phase
transitions is handled by introducing discontinu-
ities in the adiabatic temperature profile accord-
ing to the entropies of the transitions in
Table 22.1:
T=T ¼ ln T ¼S=C
P
: (22:18)
There are several ways of representing the
results of such calculations. A straightforward
calculation of the thermodynamic efficiency of
convective heat transport between any two
depths is simply ¼
_
E=
_
Q by Eq. (22.17) and this
is the fundamental result. Figure 22.3 is a plot of
this efficiency for convection of heat to the sur-
face as a function of the depth from which it
originates. The efficiency corresponding to the
transport of heat between any two depths is the
difference between the values at those depths.
The calculations assume that convective heat
transport stops at the base of the lithosphere
and that heat loss from the lithosphere to the
366 THERMODYNAMICS OF CONVECTION
