Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.


802
HARE
Design devices to utilize these precepts involves the use of molecularly dense films
(highly uniform cross-linked thermosets and relatively crystalline thermoplastics).'* These
coatings are formulated with nonhydrophilic components and lamella pigments to give
coating systems of high electrical resistance that maintain adhesion even under wet condi-
tions.'"'' They are typified by fluoropolymers, highly cross-linked epoxies, and coal tar
modified systems, pigmented with pigments such as aluminum flake.
Barrier coatings can be used alone or in combination with impressed current driven
cathodic protection systems, where they act to minimize exposed cathode areas and thereby
reduce the cost of required electricity that maintains the direction of current flow." Barrier
coatings can also be used to minimize cathode-anode area ratios in mixed metal systems
where insulation is impractical, thereby eliminating pitting at exposed anodes.
Barrier coatings are widely used for extreme service such as the lining of water,
fuel, food, and chemical storage tanks, for marine coatings, etc.
2.3
Inhibitive Coatings
Inhibitive metal primers reduce corrosion by chemically modifying the interfacial condi-
tion against the metal.7-9 This is done by including pigments in the primer film that release
oxidizing ions and other passivating moieties into the aqueous phase against the interface.'
Reviews of pigments used in this manner may be noted in Refs.
21
and
22.
In
turn,
this
reduces the levels of electrolytic oxygen necessary to establish passive films on the metal5
beneath the coating (Fig.
5).
In order to minimize blistering and provide sufficient inhibitive
ions to achieve long term passivity without rapidly exhausting the available ionic reservoir
(provided by the pigment), selection of both binder and inhibitive pigment is critical.
Control
of
the PVCKPVC is also important in order to maintain permeability
so
that there
is sufficient moisture to access and dissolve enough inhibitive ions
from
the pigment in
the film, but not
so
much that the film becomes too porous. Highly porous films will
allow the transmission of depassivating ion species (chlorides and sulfates) into the film
Fllm
of
controlled
pomslly
allows
H20
02
NaCl
water through continuum
to
from soluble pigment
I
.I
..
...
rendering water
inhibitive.
leach inhibitive ions
H20
whlch largely remaln
outside film
so
that
ratio
of
inhibitbe ions
to corrosive ions at steel
surface is conducive to
formation of passivesurface.
Surface
of
steel beneath
inhibitive primer
is
comprised
of a passive
layer
which prevents
solubilization of metal as ions.
Figure
5
Corrosion protection
by
inhibitive primer.
CORROSION AND
ITS
CONTROL
BY
COATINGS
803
and through to the metal, there to compete with inhibitive ions for adsorption and
so
prevent passi~ation.~”
I
Inhibitive coatings are generally oleoresinous systems, oils, alkyds, epoxy esters,
phenolic varnishes, etc., although epoxies and many latex primers also function by this
mechanism. Inhibitive coatings are generally used for light to moderate duty atmospheric
service, although they may be part of relatively high performance systems such as automo-
tive primers, aircraft coatings, and coil coating primers. They are generally not favored
for long-term freshwater immersion service, however,
or
for continuous use under high-
temperature, highly humid conditions.
The formulation of inhibitive metal primer is considered in several texts and pa-
pers*8.Y.l
1,22.23
2.4
Zinc-Rich Coatings
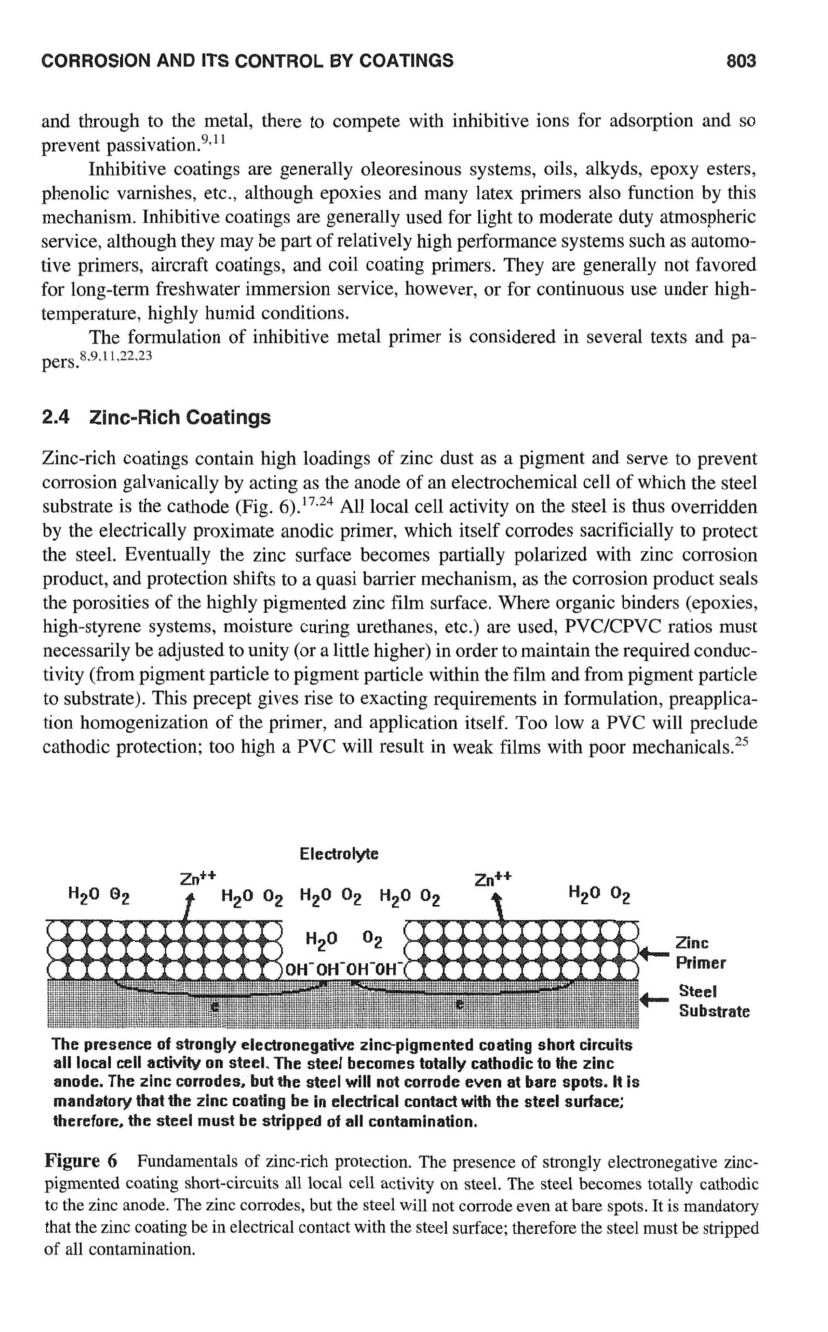
Zinc-rich coatings contain high loadings of zinc dust as a pigment and serve to prevent
corrosion galvanically by acting as the anode of an electrochemical cell of which the steel
substrate is the cathode (Fig.
6).’7.24
All local cell activity
on
the steel is thus overridden
by the electrically proximate anodic primer, which itself corrodes sacrificially to protect
the steel. Eventually the zinc surface becomes partially polarized with zinc corrosion
product, and protection shifts to a quasi barrier mechanism, as the corrosion product seals
the porosities of the highly pigmented zinc film surface. Where organic binders (epoxies,
high-styrene systems, moisture curing urethanes, etc.) are used, PVCKPVC ratios must
necessarily be adjusted to unity
(or
a little higher) in order to maintain the required conduc-
tivity (from pigment particle to pigment particle within the film and from pigment particle
to substrate). This precept gives rise to exacting requirements in formulation, preapplica-
tion homogenization of the primer, and application itself. Too low a PVC will preclude
cathodic protection: too high a PVC will result in weak films with poor mechanicals.2s

804
HARE
Inorganic binders such as alkaline silicates and alkyl silicates rely on chemical
reaction of the binder with the zinc atoms on the surface of the zinc dust pigment, and
probably the steel substrate also (during application and curing) to produce a wholly
continuous, chemically bound, inorganic matrix of zinc silicate.'7.'" While
PVC/CPVC
levels are somewhat less critical. high levels of zinc are still necessary to provide good
cathodic protection over the long term. Although these films are more porous than are
the organic zinc-rich systems, again the presence
of
water within the primer, even against
the metal surface. is not threatening to steel, for zinc corrosion is its only consequence.
As long as there remains sufficient zinc within the film, all oxidation must occur only
within the film itself. Eventually zinc corrosion product packs the porosities and seals the
film off as was the case with
the
surface
of
the organic zinc-rich film.
Zinc-rich primers are widely employed as nontopcoated systems in environments
where the zinc corrosion product is not easily dissolved (i.e., in neutral environments).
In
such applications, inorganic zinc primers have protected steel for half a century and more.
In other systems, especially where environments are either highly acidic or highly alkaline,
and therefore reactive with the metal, both organic and inorganic zinc-rich primers are
recoated with barrier topcoats (epoxies. vinyls, chlorinated rubbers, and urethanes) for
additional protection.
REFERENCES
l.
2.
3.
4.
S.
6.
7.
X.
9.
10.
11.
12.
13.
14.
IS.
F.
L. LaQue and N. D. Greene, Basics
of
corrosion, in
Corrosion
Brr,sit~.s"A~~
Introducrior~
(A. des. Brasunas, ed.). Houston, TX: NACE, 1984, chap.
2.
J. C. Scully, Aqueous corrosion, in
The
Furdtrrrw.rltal,s
of
Corrosiorl.
2nd ed. New York:
Pergamon Press, 1975, chap.
2.
U.
R. Evans, Electrochemical corrosion. in
,411
Intrntluctiorr
/I)
Metrrllic
Corrosiorr.
London:
Edward Arnold. 1963, chap.
11.
C. H. Hare. Metallic corrosion,
Jourml
of'
Protective
Cotrtirl,q.s
trr~d
Linir~,g.s,
l4(
12),
74 (Dec.
1997).
Z.
W. Wicks, Corrosion protection by coatings, Federation Series on Coatings Technology,
Federation of Societies for Coatings Technology,
M. Stern, The mechanism of passivating type inhibitors,
Jour~l
o/
Electroc~he~nicnl
Society,
lOS(
1
l),
638
(Nov.
1958).
H.
Leidheiser, Mechanism of corrosion inhibition with special attention
to
inhibition in organic
coatings,
Jourrml
of
Cotrtings
Techolog~,
S3
(678).
29
(July 198
1
).
M. Svohoda and J. Mlcziva. Properties of coatings determined by anti-corrosive pigments,
Pro,grc~s.s
in
Urgtrnic
Contirlgs,
12,
25
I
(
1984).
G.
Salensky, Corrosion inhibition,
in
Hrtntlhook
ofCocrtincq.s
Aclditi\v.s
(L. J. Calho. ed.). New
York: Marcel Dekker, 1987. chap.
12.
H. H. Uhlig. Passivity,
in
Corrosion
rtml
Corrosion
Control,
2nd ed. New York: John Wiley,
C.
H.
Hare. Anticorrosive barrier and inhibitive primers. Federation Series on Coatings Tech-
nology. Philadelphia, PA: Federation of Societies
for
Coatings Technology, 1979.
J. E.
0.
Mayne, Paints for the protection of steel-A review
of
research into their modes of
action,
British
Corro.sio/~
Jourmrl.
May 1970.
p.
106.
R. C. Bacon, J.
J.
Smith, and F. M. Rugg, Electrolytic resistance
in
evaluating protective merits
of
coatings on metal,
Irdustritrl
Engirrerrirlg
Chemistry,
January 1948,
p.
161.
S.
Gumviah, The relation between the permeation of oxygen and water through paint films
and corrosion of painted steel,
JUCCA.
S3
(g),
669 (August 1970).
N. L. Thomas, The barrier properties of paint coatings.
Pro,grr.ss
irz
Orp~r~ic.
Coatings.
19,
101
(1991).
1971,
pp.
76-79.

CORROSION AND
ITS
CONTROL
BY
COATINGS
805
16.
17.
18.
19.
20.
21.
22.
23.
24.
2.5.
26.
W. Funke, Towards environmentally acceptable corrosion protection by organic coatings,
Jour-
rl~l
of
Cocctirlgs
Tec~hr~ology.
Oct. 1983.
p.
3
1.
A. C. Elm, Zinc dust metal protective coatings. Palmenton, PA: New Jersey Zinc Company,
1968.
A.
S.
Michaels, Water and the barrier film,
Officirrl
Digest.
June 196.5,
p.
638.
H. Leidheiser and W. Funke, Water disbondment and wet adhesion of organic coatings on
metal: A review and interpretation,"
JOCCA.
May 1987,
p.
121.
W. Funke, The role of adhesion in conosion protection by organic coatings,
JOCCA,
Sept.
1985,
p.
229.
M.
J. Austin, Anticorrosive inorganic pigments, in
Sur:frrc.e
Corhgs-Vol.
I--Rtrn*
Mrrterictls
crud
Their
Uscrp
(P. Parson. ed.) London: Chapman and Hall, 1993, chap. 2.5,
p.
409.
A. Smith, Inorganic primer pigments, Federation Series
on
Coatings Technology. Philadelphia,
PA: Federation of Societies for Coatings Technology. 1988.
C.
H.
Hare, A review of inhibitive metal primers.
Mnclerrl
Pcrirlt
nrld
Cotrtinys,
July 1996,
p.
26.
C. G. Munger, Corrosion resistant zinc coatings, in
Cm-osiorl
Preverltiorl
/>y
~VOteCtilJt'
Cocrt-
irzgs
Houston. TX: NACE, 1984, chap. 6, p. 129.
C. H. Hare et
al..
Geometrics
of
organic zinc rich primers and their effect on pigment loadings.
Moderr1
Ptritlt
rrnd
Cocrtirlgs,
73
(6),
30
(June 1983).
C. G. Munger, Inorganic zinc coatings-past, present and future,
Merterirrls
Per;firrmrlc,e,
May
1975,
p.
25.
This Page Intentionally Left Blank

93
Marine Coatings
Industry
Marine coatings are special-purpose coatings that are supplied to the shipbuilding and
repair, offshore, and pleasure craft markets. The products used are diverse and unique and
are formulated for severe climatic and immersion conditions.
As
a result of these conditions, the coatings used must have maximum resistance
properties
to
salt
spray, constant sea water, and in the case of tankers,
a
broad range of
chemicals. For these reasons, a substantial volume
of
products sold today are two-compo-
nent epoxy primers, intermediate high builds, and tank linings.
Above-the-waterline finishes are still predominantly single-package alkyds or
acrylics on commercial ships and offshore platforms. This is due to the subsequent ease
of maintenance required. Similarly, single-pack alkyds, urethane-alkyds, and silicone-
alkyds are predominant in the pleasure craft market, at least for hulls up to the
30-35
foot class.
Larger pleasure crafts are still painted with the single-pack finishes, but many such
craft (yachts) are coated today with two-part aliphatic polyurethanes
to
achieve the best
in gloss and gloss retention, abrasion resistance, and long-term durability.
The use of two-component products. whether applied to
a
ship's tanks or
a
yacht's
topside, requires more professional applicators to achieve the best result. Such applicators
must be familiar with multiple spray application equipment from the simple siphon cup
to the sophisticated twin-feed heated airless spray.
Whether coating deep-sea ships, offshore platforms, or pleasure craft, one unique
characteristic of the marine coatings industry is the need
to
protect the underwater surfaces
from the attachment and growth of marine fouling organisms. These are living animals,
algae or slime, that will adhere, colonize, and grow rapidly if not controlled through the
use of antifouling coatings.
Antifouling paints are unique to this industry and make up approximately
50%
of
the total volume of coatings used. By their nature,
in
order to mitigate fouling attachment.
807

808
HICKEY
antifouling paints contain biocides. which are registered with the federal EPA as pesticides
under the Federal Insecticide. Fungicide and Rodenticide Act.
Subsequently, all antifouling paints must be both federally registered with the EPA
and registered with the state EPA in which they are sold.
This unique class of product is expensive to develop, test. and register and thus is
expensive for the customer. Most antifouling paints contain rosin (gum or wood) as part
of the vehicle and a copper compound-cuprous oxide being the most common-as the
biocide.
Some antifoulings are based
on
organotin-copolymer resins, which are biocidally
active polymers along with a copper compound. These are generally the best-in-class for
complete fouling control.
Unfortunately, the new ship construction industry in the U.S. is dramatically reduced
compared to
20-25
years ago. Although there are still new construction shipyards, the
volume of construction is primarily military with
most
commercial construction being
done elsewhere.
Where ship construction is still active, zinc-rich primers, silicates, and epoxies are
still prevalent for steel protection from corrosion.
In the pleasure craft industry, the most prevalent construction material is fiber-
reinforced polyester (FRP) and polyester resin gelcoats.
In the
shipbuilding/repair/offshore
and pleasure craft market sectors, today’s product
technology is not driven simply by performance requirements but jointly with regulatory
demands. The Clean Air Act and its amendments now control the volume of organic
solvents (volatile organic compounds. VOCs) emitted, the chemical composition of the
emitted compound (hazardous air pollutants, HAPS; ozone depleting substance,
ODs),
and airborne particulates.
These regulatory demands, coupled with the ever-increasing desire to minimize
coating frequency, place heavy technical emphasis on the marine coatings industry and
will continue to do
so
in
the near future.
The marine coatings industry represents less than
10%
of the total volume of paints
sold
in
North America. Of this, the volume
is
split between commercial marine (primarily
maintenance, military, pleasure craft and offshore. The trends for volume increase are
overall fair, with the bulk of the increase due to maintenance and repair.
There are several major suppliers that provide the majority of the marine coatings
sold
in
North America. These include Courtaulds’ Coatings, DeVoe
&
Reynolds, Ameron,
Kopcoat, Hempel, and the latest entry. Sherwin Williams.
BIBLIOGRAPHY

Decorative Surface Protection
Products
Jaykumar (Jay) J. Shah
Decoru,
Fort
Edwnrrl,
NW
York
1
.O
INTRODUCTION
Decorative surface protection products, as the term suggests, are products that provide
both decoration and protection to a wide variety of surfaces. These products are available
to consumers
in
a variety of colors, decorative designs, and surface finishes. They are
attractive, durable. washable, waterproof, and resistant to stains from food, beverages, and
common household items. They must be conformable to a wide variety
of
surfaces, requir-
ing no tools, water, or paste to be applied to any plain surface.
In
most cases. they have informative and instructional carriers traditionally known
as printed release liners, which are affixed by a flexible pressure-sensitive adhesive
to
a
base sheet, also known as a primary substrate or a face stock. In some cases, the release
liner has been eliminated by using an embossed primary substrate. Alternatively, the
primary substrate may have been coated on one side with a release coating and on the
other side with a low tack adhesive.' These products are known as self-wound adhesive
coated decorative sheets. They are made
in
solid colors, decorative prints, and textured
woodgrains.
2.0
HISTORY
Pressure-sensitive adhesive usage in making decorative surface protection products goes
back to the early
1950s.
Similar types of
vinyl
film were used by consumers
on
products
such as printed and laminated table cloths and printed draperies. The idea of printed film
led to the making of decorative surface protection products by a low tack, pressure-sensitive
adhesive
on
printed film.
809
81
0
SHAH
BASE SHEET
ADHESIVE COATING
SILICONE COATING
RELEASE LINER
Figure
1
Construction
of
decorative products.
The construction of decorative products is shown in Figure
l.
3.0
PRODUCTS
The manufacturer planning
to
bring out a new product has many varieties to choose from.
He should consider not only the physical, chemical, and thermal properties, but also the
cost and ease of the processing method. The key to selecting the right material may be
good communication between the processor and the material suppliers. The more rigid
the specifications covering end use, the easier selection becomes.
3.1
Primary Substrate
or
Base Sheet
Typical substrates used are calendered plasticized vinyl films, extruded homo- or copoly-
mers of polyolefin films, metallized polyolefins and polyester films. preimpregnated pa-
pers, treated cloth, and nonwovens.
Plasticized vinyl films have a dominant position as a decorative film product. These
films are usually manufactured by the calendering process. The method
of
making such
films
is
discussed in detail by Reip.’ Abrasion and stain resistance coupled with unlimited
coloring and design possibilities have made vinyl films one of the largest base sheets
in
the decorative coated product field.
Films extruded from homo- or copolymers of polyolefin are flexible but tough and
durable, moisture proof, and grease resistant. The film industry, through recent coextrusion

DECORATIVE SURFACE PROTECTION PRODUCTS
81
1
Table
1
Criteria
to
Select a Qualified Supplier
for
Base Sheet
Composition
of
film
Phssicd properties
Tensile strength
Elongation
Modulus
Tear strength
Dimensional stability ASTM D-
1204
methanol (both machine
Opacity
Volatiles content
Surface finish (gloss)
Hardness
Color
Routirw
tesls
Opacity
Color
Gloss
General observation to check hand, voids, pinholes. gel particles.
Width and length
of
roll
direction and cross direction)
dimensional stability, roll counter, roll profile
techniques, is producing single- and multiple-ply laminated polyolefin films that are printa-
ble. dimensionally stable, and opaque or clear. These materials are becoming more popular
because of their lower cost.
Cotton or cotton-polyester fabrics were used earlier because they are more durable,
tough, moistureproof. and washable, as well as more easily printable than films. However,
they are not as flexible and conformable as films. Their higher cost have made them
less popular. They have been replaced in some applications by supported or unsupported
nonwovens made from natural or synthetic fibers.
Decorative products made with printed and metallized polyester or polypropylene
films provide brightness and contemporary design. Their use is limited because of more
difficult handling and higher cost.
Impregnated paper has been used more recently because of its
low
cost. Self-wound
decorative products have been made using such paper. Because impregnated papers are
printable and can be made self-wound, they have more applications. However, papers tear
easily and are
not
as flexible as films. limiting their application.
The criteria to be considered to qualify a supplier for the base sheet and routine
tests performed
in
accepting base sheets from qualified suppliers are described in Table
1.
3.2
Pigmented Coating for Decorative Printing
An organic coating is made up of two principal components, a printable vehicle and
a
pigment. A vehicle is a film forming ingredient that enables the coating
to
convert from
a mobile liquid to a solid film. It acts as a carrier and suspending agent for the pigment.
The pigments are the coloring agents.
