Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.

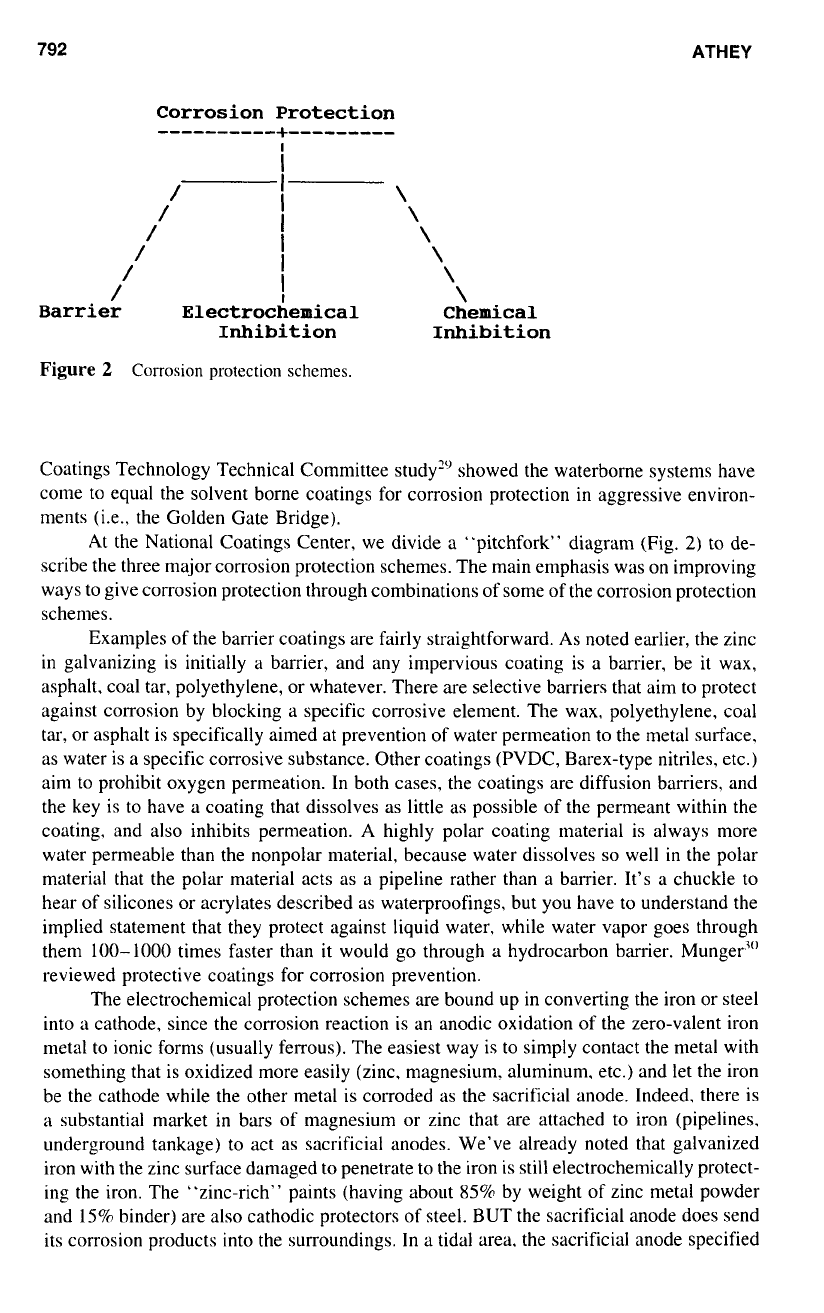
792
Corrosion Protection
I
I
"""""+""""-
l
/
ATHEY
/
/
i
/
I
I
I
/
/
I
Barrier Electrochemical
Inhibition
Figure
2
Corrosion protection
schemes.
\
\
\
\
\
\
Chemical
Inhibition
Coatings Technology Technical Committee study" showed the waterborne systems have
come to equal the solvent borne coatings for corrosion protection in aggressive environ-
ments (i.e., the Golden Gate Bridge).
At the National Coatings Center, we divide a "pitchfork" diagram (Fig.
2)
to de-
scribe the three major corrosion protection schemes. The main emphasis was on improving
ways to give corrosion protection through combinations of some of the corrosion protection
schemes.
Examples of the barrier coatings are fairly straightforward. As noted earlier, the zinc
in galvanizing is initially a barrier, and any impervious coating is a barrier, be it wax,
asphalt, coal tar, polyethylene, or whatever. There are selective barriers that aim to protect
against corrosion by blocking a specific corrosive element. The wax. polyethylene, coal
tar, or asphalt is specifically aimed at prevention of water permeation to the metal surface,
as water is a specific corrosive substance. Other coatings (PVDC, Barex-type nitriles, etc.)
aim to prohibit oxygen permeation. In both cases, the coatings are diffusion barriers, and
the key is to have a coating that dissolves as little as possible of the permeant within the
coating. and also inhibits permeation. A highly polar coating material is always more
water permeable than the nonpolar material, because water dissolves
so
well
in
the polar
material that the polar material acts as a pipeline rather than a barrier. It's a chuckle to
hear
of
silicones or acrylates described as waterproofings, but you have to understand the
implied statement that they protect against liquid water, while water vapor goes through
them 100-1000 times faster than it would go through a hydrocarbon barrier. Munger3"
reviewed protective coatings for corrosion prevention.
The electrochemical protection schemes are bound up in converting the iron or steel
into a cathode, since the corrosion reaction is an anodic oxidation of the zero-valent iron
metal to ionic forms (usually ferrous). The easiest way is to simply contact the metal with
something that is oxidized more easily (zinc, magnesium. aluminum, etc.) and let the iron
be the cathode while the other metal is corroded as the sacrificial anode. Indeed, there is
a
substantial market
in
bars of magnesium or zinc that are attached
to
iron (pipelines.
underground tankage) to act as sacrificial anodes. We've already noted that galvanized
iron with the zinc surface damaged
to
penetrate to the iron is still electrochemically protect-
ing the iron. The "zinc-rich" paints (having about
85%
by weight of zinc metal powder
and
15%
binder) are also cathodic protectors of steel.
BUT
the sacrificial anode does send
its corrosion products into the surroundings.
In
a tidal area, the sacrificial anode specified

METAL COATINGS
793
by some regulatory agencies feeds metal ions into the underground water while
it
is
protecting the underground storage tank.
An alternative
in
cathodic protection is to impress
a
current onto the potentially
corroding metal to make it cathodic. Here a battery. or
a
step down transformer is used
with another power source. This technique is used on shipboard for iron hulled vessels,
and the battery can be recharged when needed. Some pipelines are cathodically protected
with impressed current. The advantage is that there are no ions lost to the surroundings.
hence subsequent contamination of water.
The chemical inhibitors are many and varied items of commerce and may work
in
many ways. Most are sold because they’ve been shown
to
work. though no mechanism
is proposed. Some of the surfactant type materials (oleyl sarcosine, for instance) may only
add
a
physical barrier by adsorbing
to
the surface and blocking approach of oxygen, water.
or catalytic ion. Other materials may adsorb on the metal and act as a pH modifier or buffer.
as an amine would inhibit acid-catalyzed attack. Some inhibitors modify the electromotive
potential at which corrosion occurs and are said to have “passivated” the surface. Most
of the chemical inhibitors are low molecular weight compounds and can be washed away
or otherwise rendered ineffective by chemical attacks or reactions. They are effective over
short periods of time, and can be Jtabilized against erosions by formulation
to
some degree.
For instance, there are oils into which corrosion inhibitors are formulated.
There are corrosion-inhibitive pigments. Seldom is there discussion of the mecha-
nism by which these chemical inhibitive pigments work, and such characterization could
extend their utility. It has been shown that nominally corrosion-inhibitive pigments don’t
work in some formulations (see SSPC literature and corrosion studies by the technical
committees of the Northwest, New England, and Golden Gate Societies for Coatings
Technology). Indeed. Nadim Ghanem (American University of Cairo) told of
a
basic lead
carbonate formulation study
in
which the solvent-borne
vinyl
binder gave
no
corrosion
protection, while a water-borne vinyl gave excellent protection. His hypothesis was that
the coating needs
to
be permeable
to
water
to
get the lead ions
to
migrate
to
where they
need to be to act
as
corrosion protectors. That may have also been the message
in
the
Los
Angeles Society for Coatings Technology work with aminosilane-treated talcs in latex
formulations. Though many demonstrations of the effectiveness of the corrosion-inhibitive
pigments exist, the mechanisms should be more thoroughly described to aid the formulator.
REFERENCES
1.
2.
3.
4.
S.
6.
7.
8.
9.
IO.
11.
12.
F. N. Longo, Ed.
Tlrtwrrol
Sprq
CoLrtirI,gs:
Nett.
Mtrtc~ritr1.s.
Proct~xses
trrrr1Applic~tctiorl.s.
Amcri-
can Society for Metals Conference Book. Metals Park,
OH:
ASM, 1985.
R.
T.
Sorg,
Prod.
Fi,ri.sh.
p.
72 July 1978.
Anon..
hrd.
Finish.
p.
24 January 1978.
British Patent 1,369.056:
Chcwl.
Ahstr..
83.
116382~.
M.
Rogers,
Pltrst.
Trchol..
p.
20, November 1982.
R.
H.
Hochman et
al..
Eds.
lorr
lnrplrrrlfrrfiorl
rrrrd
Plrrsr~~cc
Assisfrd
Procr.sses.
Amencwn Society
for Metals Conference Book.
Metals
Park,
OH:
ASM.
1988.
Fujitsu Ltd., Japanese Patent 8,089,833
Dec.
28, 1978:
Cllrrrl.
A1)str.
5.
39579m.
A.
K.
Sharma et
d.,
J.
AppI.
fo/yru.
Sci.
26. 2197 (1981).
R. Athey et
al.,
J.
Cocrtirrsq.s
Techol.,
57(726). 71 (July
19x5).
A. Moriknnn and
Y.
Asnno,
J.
Appl.
Po/vru.
Si.,
27, 2139
(
1982).
C.
Arnold, Jr., et al.,
J.
AppL
Po/,vrlr.
Sci..
27, 821
(
1982).
M. R. Havens et
nl..
J.
Appl.
Po/vrr~.
Sci.
22
(IO),
2793 (1978).
794
ATHEY
13.
14.
15.
16.
17.
1
8.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
D.
T.
Clark and M.
Z.
Abraham,
J.
Polw.
Sei.,
Polyl.
Cllern.
Ed.
IY,
2129 (198
I
).
N.
Inagaki et
al.,
J.
Po/w.
Sci..
Polyrrl.
Lett.
Ed.,
19,
335
(
198
I
).
H.
A.
Bcalc,
hd.
Res.
Dell..
p.
135.
July 198
I.
M. Murphy,
Ed.,
Metd
Firrisllirlg
H~rnrhok.
Hackcnsack. NJ.
E. B.
Saubestre. in
Modern
Elrc~/ro/~ltr/ir~~~.
3rd
ed., F. Lowenheim, Ed. New York; Wilcy-
Interscicnce. Chaptcr 28.
G.
Muller et
al.,
Plrhg
or1
Plastics.
Surrey, England; Portcullis
Prcss.
J. Sprmgcr et
al.,
Angeu,.
Mrrkrord
C/wtrl.,
89,
81
(
1980).
J. Springcr et
al.,
A~rgeu,.
Mrrkromd.
C/wtf.,
XY.
8
1
(
1980).
J. Springcr et
al.,
Angrw.
Mrrkmrlzol.
Chm..
8Y.
81
(1980).
A.
G.
Wilmhurst.
Aftst.
Oil
Crhrtr
Cllerrl.
Assoc..
p.
12, Novcmbcr 1978.
W. H. Safranek,
Tl~r
Properties
r~fElrctru~r/r/~o.si/r~~l
Metrrls
crud
AIloys,
Amcrican Electroplatcrs
and Surface
Finishers
Socicty. 1986.
S.
A.
Watson.
Nickd. J(1).
8
(September 1988).
P.
A. Schweitzcr,
Ed.,
Corrosiorl
rrrld
Corrosion
Protrctior~
Hrrrlrlhonk.
New York, Dekker,
1983.
(a)
Unlt 27,
An/icwrrosi\v
Brrrrirrs
rrnd
brhihiti\.c.
Prirm)r.s.
Philadelphia: Fcdcration of Societies
for
Coating Technology, 1979. (h)
Unit
26,
Corrosion
cud
//W
Pre/xrrrrtiorl
ofMetcrllic
Srcr-?tcc,s
jbr
Pcriuting,
Philadelphia: FSCT, 1979.
D. Campbell
and
R.
W. Flynn,
Am.
Prtirlt
Cotrrirlgs
J..
p.
55,
March
6.
1978.
A. Poluzzi,
14th
Anmcctl
FATPEC
Corzgres.~
Procredirlgs
1978,
p.
61.
R.
Athcy et al.,
J.
Cotrti~~,gs
Techrlol.
57(726), 71 (July 1985).
C.
H.
Mungcr,
Corrosion
Pretwtion
h!
Protrcti~~
Cotrtir~gs,
National Association
Engineer-
ing, 1984.

Corrosion and Its Control
by
Coatings
1
.O
INTRODUCTORY
1
.l
Energy Transfer
The metallic state is
in
most metals an unstable condition resulting from the smelting
operation. in which energy is imported by the ore as the metal is derived. After extraction,
most metals undergo
a
slow deterioration process during which they shed this energy and
return
to
a
more stable condition in which they are combined with some element of their
environment, such
as
an oxide,
a
sulfide, or some other corrosion product. This energy
conversion process is known as corrosion.
1.2
The Electrochemical Nature
of
Corrosion
Corrosion is most usually driven by some electrochemical inhomogeneity
in
the metal or
its environment.
In
this process, different areas of the metal, having different levels of
free energy and therefore different corrosion potentials, become the electrodes of an elec-
trochemical cell in contact with
a
common electrolyte."' The electrochemical couples
are set up with areas of more active electrochemical potential acting as anodes of the cell,
while more passive areas act
as
cathodes (Fig.
1).
Corrosion takes place at the anodes as
metal dissolves
into
the electrolyte as ions,
so
releasing electrons, which pass through the
metal to the adjacent cathode areas where they react with the environment. This flow of
electricity, the electron passage from anode
to
cathode, and the accompanying charge
transfer back through the electrolyte from cathode to anode, make up the corrosion current.
The rate of the current flow, i.e.. the magnitude
of
the corrosion current
(I)
that develops,
is
a
measure of the amount of degradation and is related to the potential difference
(V)
between the anodic and cathodic sites by Ohm's law:
V
I=-
R
(1)
where
R
is the total resistance
of
the cell.
795
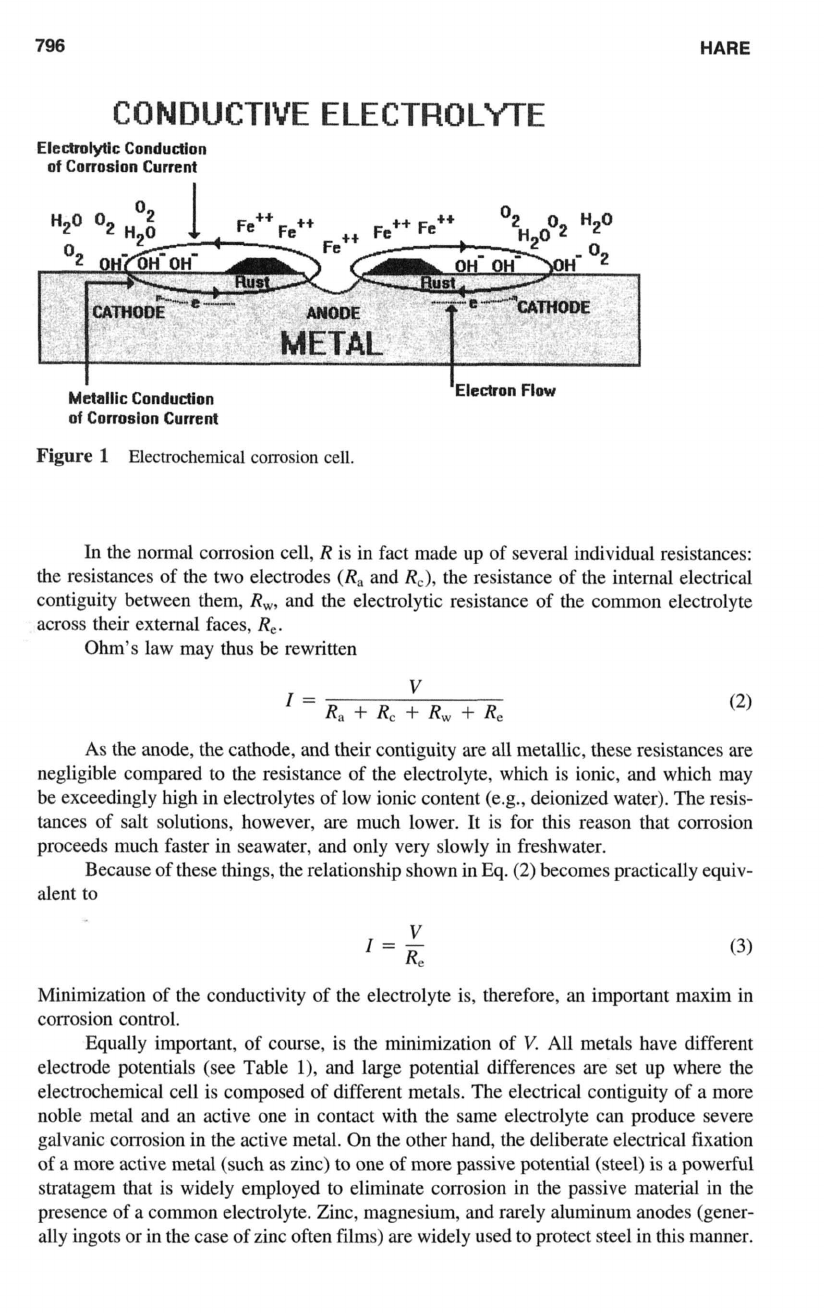
796
HARE
CONDUCTIVE ELECTROLYTE
Elcctrolytlc
Condudion
of
Corrosion Current
Metallic
Condudion
of
Corrosion Current
Figure
1
Electrochemical corrosion cell.
In the normal corrosion cell,
R
is in fact made up of several individual resistances:
the resistances of the two electrodes
(R,
and
RC),
the resistance of the internal electrical
contiguity between them,
R,,
and the electrolytic resistance of the common electrolyte
across their external faces,
R,.
Ohm’s law may thus
be
rewritten
V
R,
-l-
R,
+
R,
+
R,
I=
As the anode, the cathode, and their contiguity are all metallic, these resistances are
negligible compared to the resistance of the electrolyte, which is ionic, and which may
be exceedingly high in electrolytes of low ionic content (e.g., deionized water). The resis-
tances of salt solutions, however, are much lower. It is for this reason that corrosion
proceeds much faster in seawater, and only very slowly in freshwater.
Because of these things, the relationship shown in Eq.
(2)
becomes practically equiv-
alent to
V
I=-
Re
Minimization of the conductivity of the electrolyte is, therefore, an important maxim in
corrosion control.
Equally important, of course, is the minimization of
V.
All metals have different
electrode potentials (see Table
l),
and large potential differences are set up where the
electrochemical cell is composed of different metals. The electrical contiguity of a more
noble metal and an active one in contact with the same electrolyte can produce severe
galvanic corrosion in the active metal. On the other hand, the deliberate electrical fixation
of
a more active metal (such as zinc) to one of more passive potential (steel) is a powerful
stratagem that is widely employed to eliminate corrosion in the passive material in the
presence of a common electrolyte. Zinc, magnesium, and rarely aluminum anodes (gener-
ally ingots
or
in the case of zinc often films) are widely used to protect steel in this manner.
CORROSION AND ITS CONTROL BY COATINGS
797
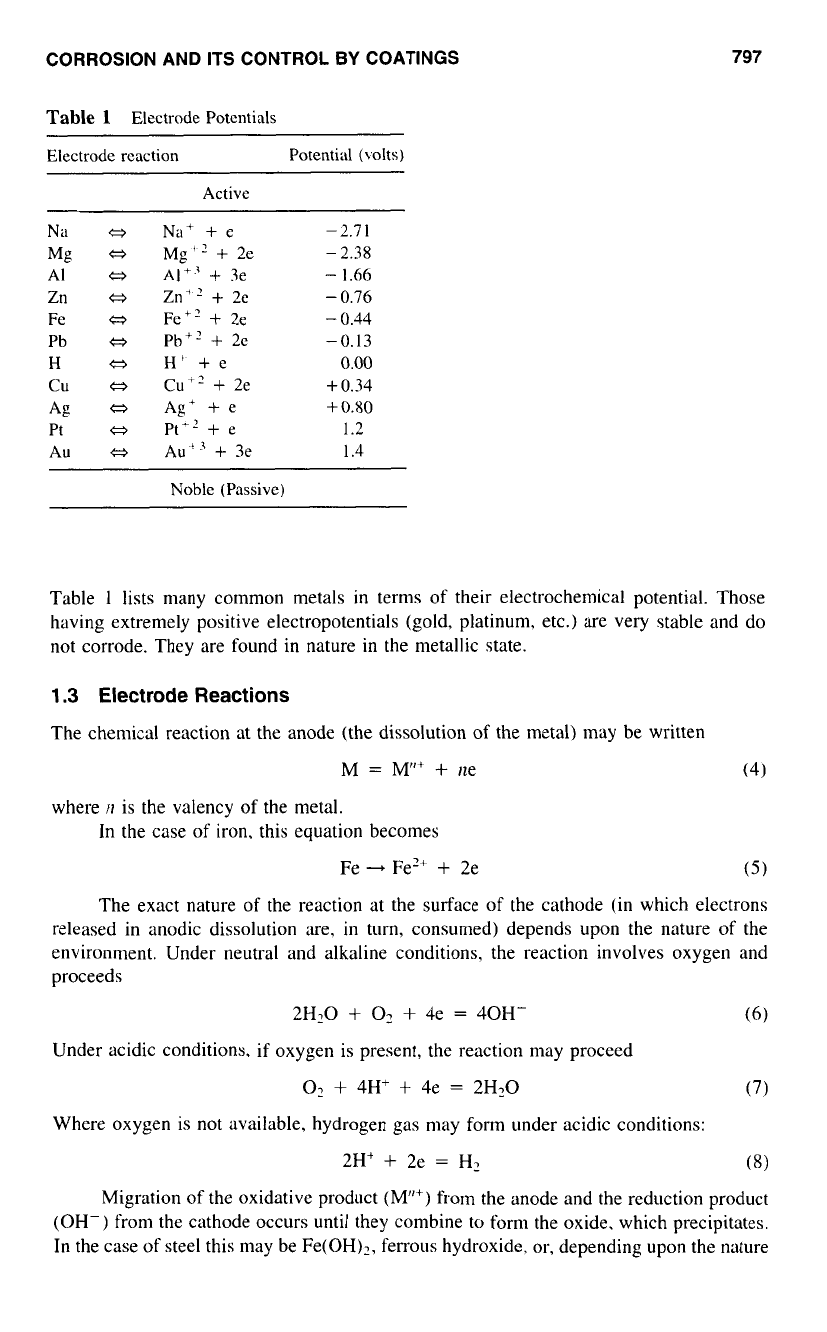
Table
l
Electrode Potentials
Electrode rcaction Potential (volts)
Active
Na
Q
AI
tj
Zn
a
Fe
Q
Pb
Q
H
Q
cu
Q
Mg
Q
Ag
Pt
Q
Au
Q
Na+
+
e
Mg"
+
2e
AI+.3
+
3e
Zn+'
+
2e
Fe+?
+
2e
Phi'
+
2e
H'
+e
Cu"
+
2e
Ag'
+
e
Pt"
+
e
Au'
+
3e
-
2.7
1
-
2.38
-
1.66
-
0.76
-
0.44
-0.13
0.00
+
0.34
+
0.80
1.2
1.4
Noble (Passive)
Table
1
lists many common metals in terms of their electrochemical potential. Those
having extremely positive electropotentials (gold, platinum, etc.) are very stable and do
not corrode. They are found in nature in the metallic state.
1.3
Electrode Reactions
The chemical reaction at the anode (the dissolution
of
the metal) may be written
M
=
M"'
+
ne (4)
where
tI
is the valency of the metal.
In
the case
of
iron, this equation becomes
Fe
-
Fe''
+
2e
The exact nature of the reaction at the surface
of
the cathode (in which electrons
released in anodic dissolution are, in turn, consumed) depends upon the nature
of
the
environment. Under neutral and alkaline conditions. the reaction involves oxygen and
proceeds
2H20
+
O2
+
4e
=
40H-
(6)
Under acidic conditions, if oxygen is present, the reaction may proceed
O?
+
4H'
+
4e
=
2H.0
(7)
Where oxygen is not available, hydrogen gas may form under acidic conditions:
2H+
+
2e
=
H2
(8)
Migration of the oxidative product
(M")
from the anode and the reduction product
(OH-) from the cathode occurs until they combine
to
form the oxide, which precipitates.
In the case of steel this may be Fe(OH),, ferrous hydroxide. or, depending upon the nature

798
HARE
of the environment, one
of
several precursor products, such as ferrous hydroxy chloride
in salt water. Ferrous products are readily soluble, and this favors migration,
so
that oxide
formations are not intimately associated with the anode but are loosely adherent and
porous. Given sufficient oxygen, a second oxidation reaction will occur in steel corrosion,
which converts the divalent ion to the trivalent ferric state,
Fe”
-
Fe”
+
e
The solubility
of
the trivalent corrosion product is much less than that
of
the ferrous
product. Under normal circumstances, however. where the secondary oxidative process
occurs gradually after the ferrous ions have migrated away from the anode, the corrosion
product is no more tightly adherent than is the ferrous product from which
it
is formed,
and films of rust, hydrated ferric oxide (Fe203.H10). are usually loose and crumbly.
1.4
Polarization
The accumulation of ions at the anode tends
to
insulate the metal from the electrolyte and
reduce the amount of ions going into solution. In effect, the anode deposit or film increases
the resistance
of
the electrochemical cell. and the cell is said to be anodically “polarized.”
Similarly at the cathode, cathodic films are possible. In oxygen-deprived systems, for
example, hydrogen gas may accumulate at the cathode, preventing further access of hydro-
gen ions and the consumption
of
electrons. The cathode reaction is therefore stifled, and
the metal is said
to
be cathodically polarized. (Where oxygen levels are high enough. the
cathode reaction occurs irrespective of the amount of H+ ions, and in this case, the oxygen
is said to have depolarized the cathode. allowing increased corrosion).
The presence of these electrode films has a great effect on the rate of corrosion
current transfer (i.e., the rate at which corrosion occurs). This can be illustrated in the
resultant modification
of
the Ohm’s law
Eq.
(3).
Thus
where
R;,,
and
RC(
represent the resistances of the anode and cathode films. respectively.
These resistances may be very high, and as such polarizing films build up. the rate of
corrosion is greatly diminished (see Fig.
2).
1.5
Electrode Film Breakdown and Depolarization
The permanence of such films has great significance
to
the control of corrosion
of
a
specific metal. On aluminum, for example. under near neutral conditions, the naturally
formed anode oxide film is very dense and insoluble (ifthin) and quite resistant to mechani-
cal removal.
R;,,
becomes very high, and
I
becomes negligible. Aluminum, therefore, is
quite resistant
to
corrosion under neutral conditions.
Where the environment is sufficiently acidic or alkaline. however, the oxide film
on aluminum is chemically dissolved. Thus the metal is stripped
of
polarizing film (depo-
larized) and corrodes rapidly. i.e., the cell reaction resumes its linear relationship between
corrosion and time.
On other metals such as steel, the oxide film is normally less adherent and easily
dislodged mechanically. Such physical removal
of
the oxide film has the same result as
chemical removal. the corrosion rate becoming more linear
until
additional corrosion
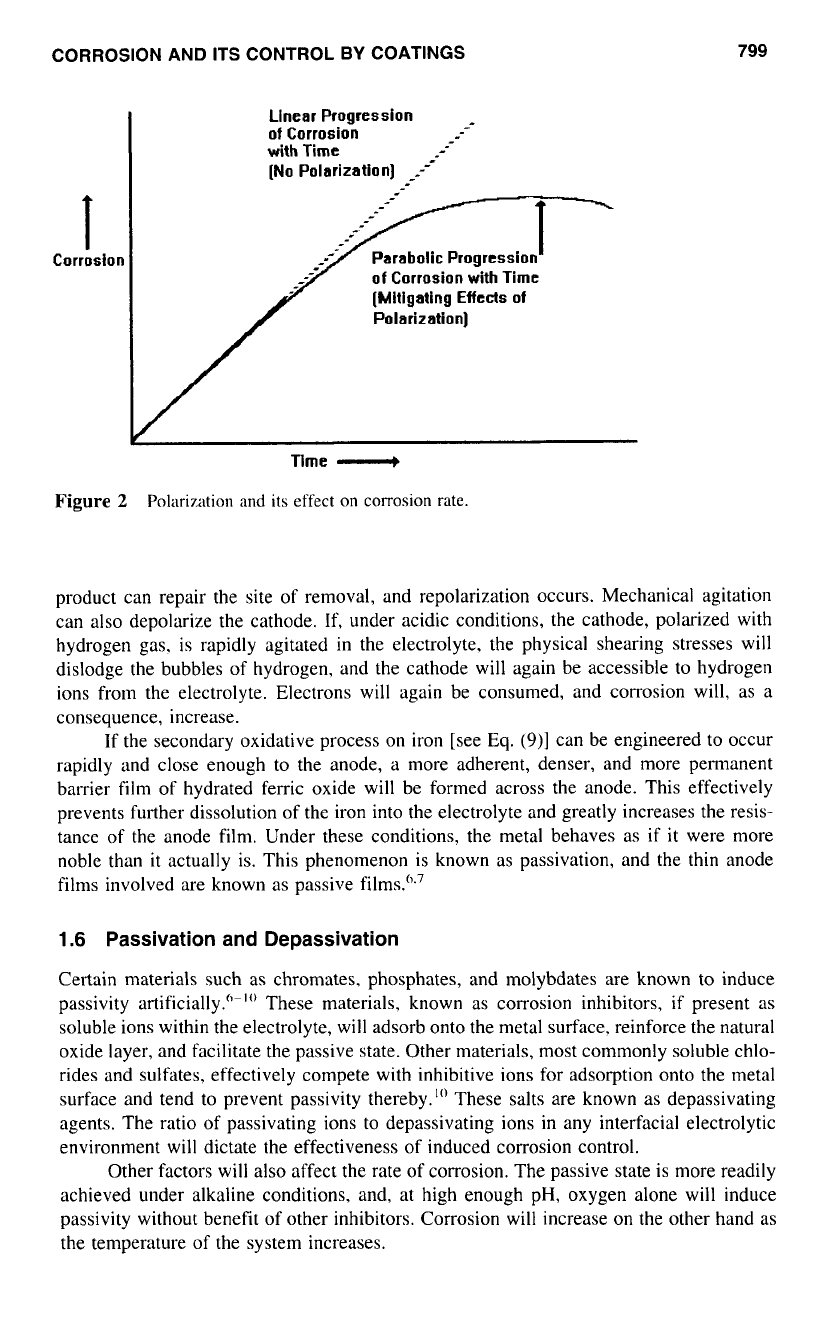
CORROSION AND
ITS
CONTROL
BY
COATINGS
799
Time
II)
Figure
2
Polarization
and
its
effect
on
corrosion rate.
product can repair the site of removal, and repolarization occurs. Mechanical agitation
can
also
depolarize the cathode. If, under acidic conditions, the cathode, polarized with
hydrogen gas, is rapidly agitated in the electrolyte, the physical shearing stresses will
dislodge the bubbles
of
hydrogen, and the cathode will again be accessible to hydrogen
ions from the electrolyte. Electrons will again be consumed, and corrosion will,
as
a
consequence, increase.
If the secondary oxidative process
on
iron [see
Eq.
(9)]
can be engineered to occur
rapidly and close enough
to
the anode,
a
more adherent, denser, and more permanent
barrier film of hydrated ferric oxide will be formed across the anode. This effectively
prevents further dissolution of the iron into the electrolyte and greatly increases the resis-
tance of the anode film. Under these conditions, the metal behaves as
if
it
were more
noble than it actually is. This phenomenon is known as passivation, and the thin anode
films involved are known
as
passive films.".'
1.6
Passivation and Depassivation
Certain materials such
as
chromates, phosphates, and molybdates are known to induce
passivity artificially.""" These materials, known
as
corrosion inhibitors,
if
present
as
soluble ions within the electrolyte, will adsorb onto the metal surface, reinforce the natural
oxide layer, and facilitate the passive state. Other materials, most commonly soluble chlo-
rides and sulfates, effectively compete with inhibitive ions for adsorption onto the metal
surface and tend to prevent passivity thereby."' These salts are known
as
depassivating
agents. The ratio
of
passivating ions to depassivating ions in any interfacial electrolytic
environment will dictate the effectiveness
of
induced corrosion control.
Other factors will also affect the rate of corrosion. The passive state is more readily
achieved under alkaline conditions. and, at high enough pH, oxygen alone will induce
passivity without benefit of other inhibitors. Corrosion will increase on the other hand
as
the temperature of the system increases.
800
HARE
a.]
Small
Large
cathode develops
Anode
current which falls on small
anode, producing Intense
localized corrosion in the
form of dangerous pitting.
Cathode
Anode
Area
Are Smaller cathode develops
less current whlch falls on
large anode area, corrosion
is spread
out
wer
a
larger
area.
Attack
is
less scvere
and non-locallzed.
on
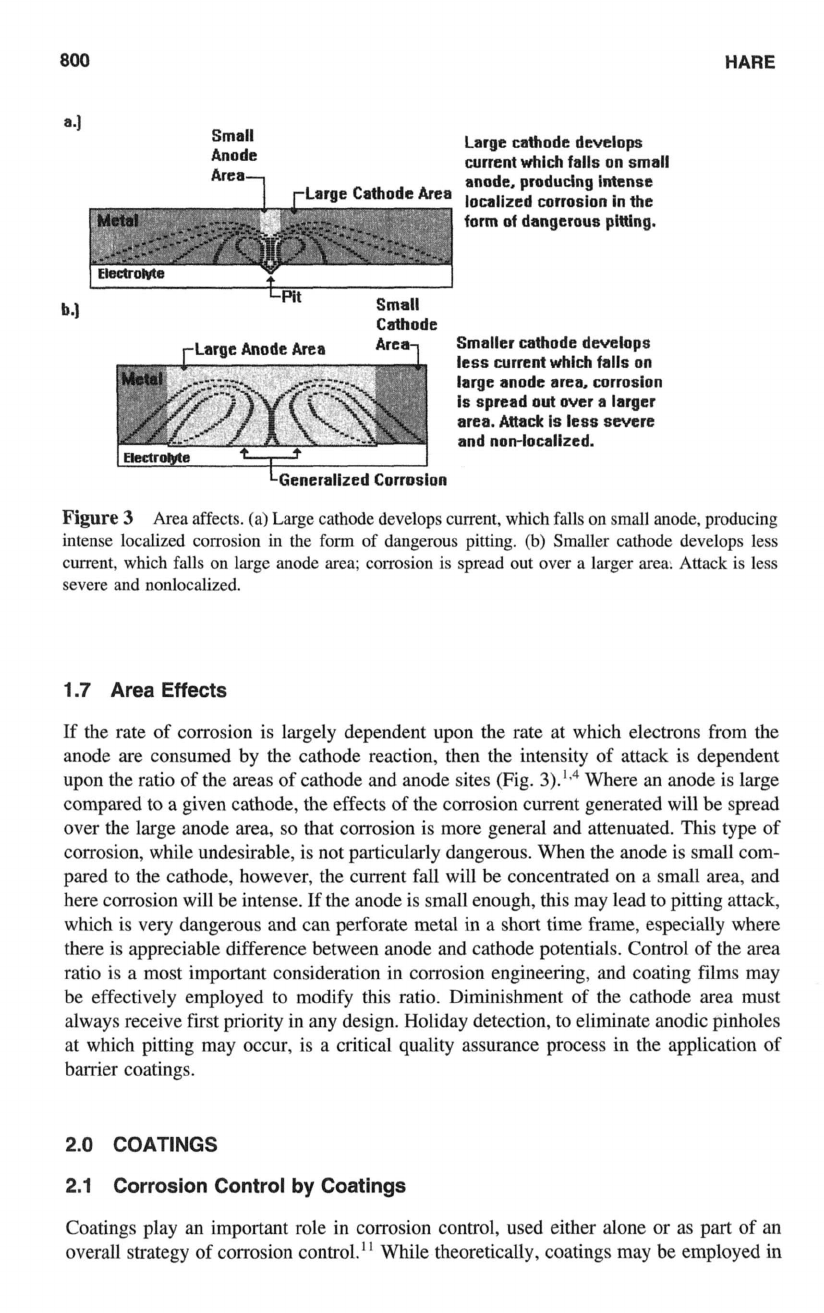
Figure
3
Area affects. (a) Large cathode develops current, which falls on small anode, producing
intense localized corrosion in the form
of
dangerous pitting.
(b)
Smaller cathode develops less
current, which falls on large anode area; corrosion is spread out
over
a larger area. Attack is less
severe and nonlocalized.
1.7 Area Effects
If the rate of corrosion is largely dependent upon the rate at which electrons from the
anode are consumed by the cathode reaction, then the intensity of attack is dependent
upon the ratio of the areas of cathode and anode sites (Fig.
3).Iq4
Where an anode is large
compared to a given cathode, the effects
of
the corrosion current generated will be spread
over the large anode area,
so
that corrosion is more general and attenuated. This type of
corrosion, while undesirable, is not particularly dangerous. When the anode is small com-
pared to the cathode, however, the current fall will be concentrated on a small area, and
here corrosion will be intense. If the anode is small enough, this may lead to pitting attack,
which is very dangerous and can perforate metal in a short time frame, especially where
there is appreciable difference between anode and cathode potentials. Control of the area
ratio is a most important consideration in corrosion engineering, and coating films may
be effectively employed
to
modify this ratio. Diminishment of the cathode area must
always receive first priority in any design. Holiday detection, to eliminate anodic pinholes
at which pitting may occur, is a critical quality assurance process in the application of
barrier coatings.
2.0 COATINGS
2.1 Corrosion Control
by
Coatings
Coatings play an important role in corrosion control, used either alone
or
as part of an
overall strategy of corrosion control." While theoretically, coatings may be employed in

CORROSION AND
ITS
CONTROL
BY
COATINGS
801
Table
2
Basic Tcchniqucs Employcd
in
Corrosion Control By Coatings
Barrier coatings Resistance inhibition
Inhibitive pigments Modification
of
interfacial environment (establishmcnt
of
passive
films)
Zinc-rich coatings Prevention
of
current
discharge
from
metal
to
environment
by
affixation
Oxygen deprivation
of
anode
film
a variety of ways to modify, hinder, or thwart the corrosion reaction, three or four devices
find practical application (see Table
2).
These devices are utilized
in
the three types of coatings employed
in
corrosion
control."." There are barrier coatings, functioning via resistance
inhibition".'^.".'"
and/
or oxygen deprivation,14." inhibitive coatings, functioning by modifying the interfacial
chemical environment against the metal s~rface,~~~~"~' and zinc-rich coatings, which pre-
vent current discharge from the steel
to
the environment by employing a more anodically
active metal (almost exclusively zinc) as
a
pigment at loadings high enough
to
ensure
electrical contiguity with the substrate and low electrical resistance across the filn~.'~
2.2
Barrier Coatings
It is a practical impossibility to prevent enough moisture transmission through even the
densest films
to
control the cathode reaction by moisture deprivation.".'x.'"
It
is, however,
possible by the use
of
impermeable bamer coatings
to
suppress both oxygen transmission
and the transmission of ionic solutions
to
a
sufficient extent that corrosion may be pre-
The prevention of oxygen access to the metal deprives the cathode reaction
of
critical fuel, and the film's resistance
to
ionic transfer renders any moisture that does
access the substrate
so
high in resistivity that current transfer between the anodic and
cathodic areas is frustrated (Fig.
4).
cluded,l?.~J.~~.l(>
Barrier film allows
H-0
09
NaCl
penetratlon of water hut
restrlcts the access
of
ionic material and
i
il
ENVIRONMENT
possibly oxygen.
-------------
L
.""""""_
""""""
.""""""
"-
""
""""
""""""".
H20
02
NaCl
H20
02
iUaC!
RESISTANCE
INHIBITION
Ionic concentration
""""""
H,,O
0
-
"""""_
H20
02
PAINT
""""""_
H20
02
.""""""_
FILM
electrical resistance OXYGEN DEPRIVATION
and no corrosion.
Prohibition of oxygen transfer
to
interface deprives cathode reaction
of
necessary fuel.
Figure
4
Colrosion
control
by
barricr coatings.
