Rafferty J.P. (ed.) Minerals
Подождите немного. Документ загружается.

7 Minerals 7
312
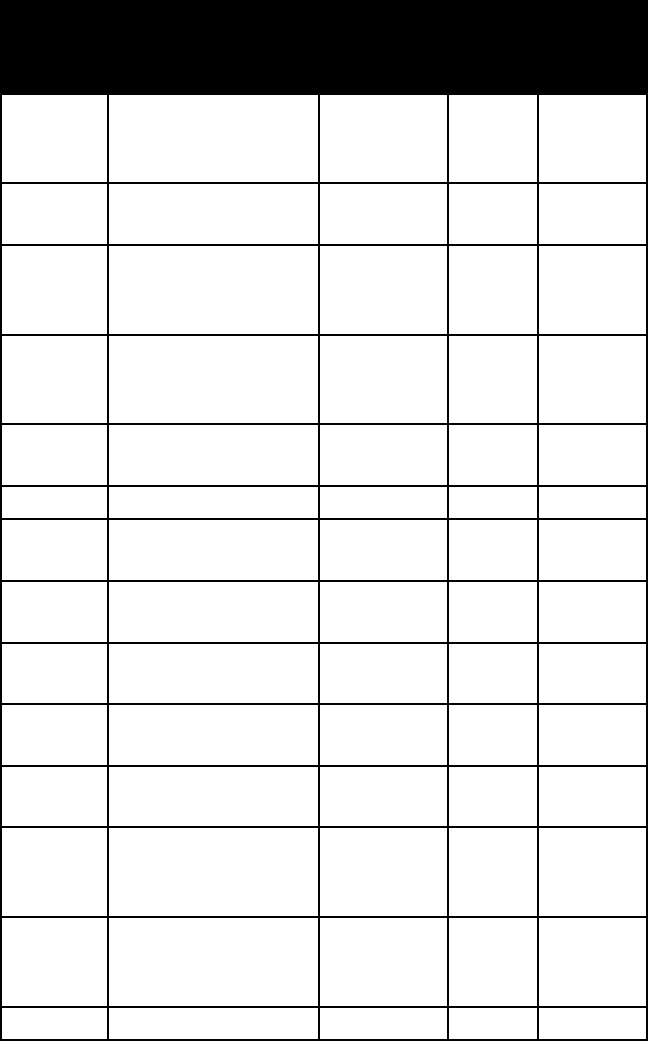
name colour lustre Mohs
hard-
ness
spe-
cific
gravity
orpiment lemon-yellow,
golden-yellow,
brownish yellow
resinous;
pearly on
cleavages
1 1/2–2 3.5
pentland-
ite
light bronze-yellow metallic 3 1/2–4 4.6–5.0
pyrite pale brass-yellow splendent
to glistening
metallic
6–6 1/2 5.0
pyrrhotite bronze-yellow to
pinchbeck-brown,
tarnishing quickly
metallic 3 1/2–4
1/2
4.6–4.7
4.8
(troilite)
realgar aurora-red to
orange-yellow
resinous to
greasy
1 1/2–2 3.5–3.6
rickardite purple-red metallic 3 1/2 7.5
sphalerite brown, black, yellow;
also variable
resinous to
adamantine
3 1/2–4 3.9–4.1
stannite steel-gray to
iron-black
metallic 4 4.3–4.5
stibnite lead- to steel-gray,
tarnishing blackish
metallic 2 4.6
stromey-
erite
dark steel-gray, tar-
nishing blue
metallic 2 1/2–3 6.2–6.3
sylvanite steel-gray to
silver-white
brilliant
metallic
1 1/2–2 8.1–8.2
tetrady-
mite
pale steel-gray,
tarnishing dull or
iridescent
metallic 1 1/2–2 7.1–7.5
umangite dark cherry-red,
tarnishing quickly to
violet-blue
metallic 3 5.6
wurtzite brownish black resinous 3 1/2–4 4.0–4.1
313
7
carbonates and other Minerals 7
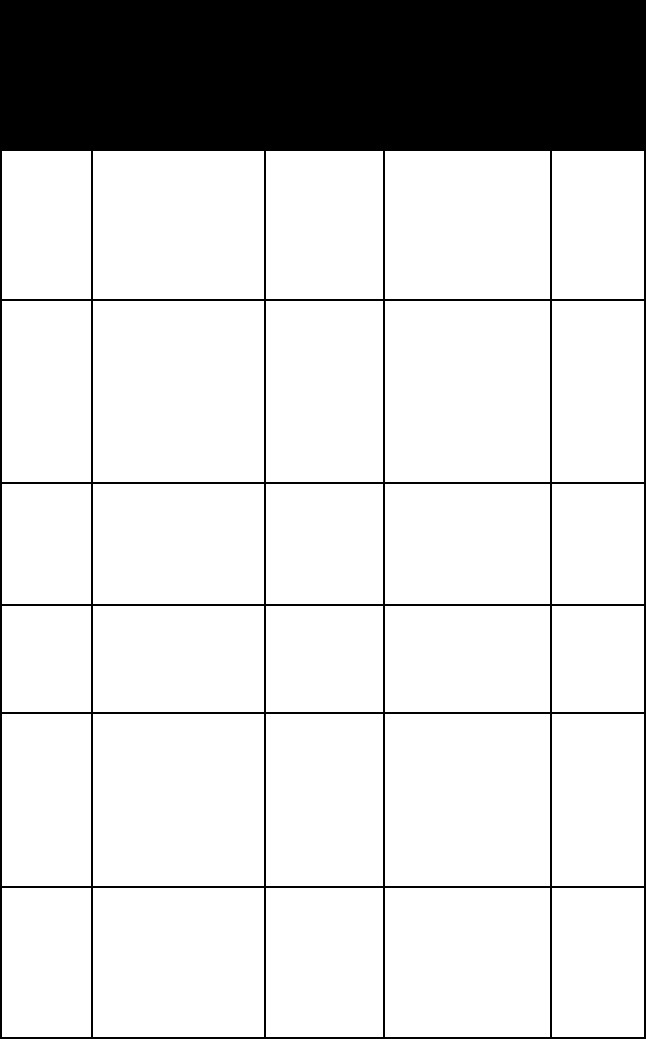
name habit or
form
frac-
ture or
cleavage
refractive
indices or
polished
section
data
crys-
tal
system
argen-
tite
cubic or octa-
hedral crystals,
often in groups;
arborescent or
hairlike massive
subcon-
choidal
fracture
faintly
anisotropic
isomet-
ric
arseno-
pyrite
cubic or dodeca-
hedral crystals
having rough
or curved faces;
granular or com-
pact massive
one
distinct
cleavage
strongly
anisotropic
mono-
clinic
bornite prismatic crys-
tals; columnar,
granular, or com-
pact massive
uneven
fracture
isotropic in
part; pinkish
brown
isomet-
ric
chal-
cocite
short prismatic
or thick tabular
crystals; massive
conchoidal
fracture
weakly
anisotropic
ortho-
rhombic
chalco-
pyrite
compact mas-
sive; tetragonal
crystals
uneven
fracture
weakly anisotro-
pic; often shows
lamellar and
polysynthetic
twinning
tetrago-
nal
cinnabar rhombohedral,
tabular, or pris-
matic crystals;
massive; earthy
coatings
one
perfect
cleavage
omega =
2.756–2.905
epsilon =
3.065–3.256
hexago-
nal

7 Minerals 7
314
name habit or
form
frac-
ture or
cleavage
refractive
indices or
polished
section
data
crys-
tal
system
cobaltite cubic or pyrito-
hedral crystals
with striated
faces
one
perfect
cleavage
isomet-
ric
covellite massive; rarely in
hexagonal plates
one highly
perfect
cleavage
strongly
anisotropic
hexago-
nal
cubanite thick tabular
crystals; massive
conchoidal
fracture
anisotropic ortho-
rhombic
domey-
kite
reniform or bot-
ryoidal masses
uneven
fracture
isotropic isomet-
ric
galena cubic crystals;
cleavable masses
one
perfect
cleavage
isotropic isomet-
ric
gree-
nockite
earthy coating conchoidal
fracture
omega = 2.431–
2.506 epsilon =
2.456–2.529
hexago-
nal
krenne-
rite
short prismatic
crystals
one
perfect
cleavage
strongly aniso-
tropic; creamy
white
ortho-
rhombic
lin-
naeite
octahedral crys-
tals granular to
compact masses
uneven to
subcon-
choidal
fracture
isotropic isomet-
ric
loellin-
gite
prismatic or
pyramidal crys-
tals; massive
uneven
fracture
very strongly
anisotropic
ortho-
rhombic

315
name habit or
form
frac-
ture or
cleavage
refractive
indices or
polished
section
data
crys-
tal
system
marca-
site
tabular or pyra-
midal crystals;
spear-shaped or
cockscomb-like
crystal groups
one
distinct
cleavage
strongly aniso-
tropic and
pleochroic;
creamy white,
light yellowish
white, and rosy
white
ortho-
rhombic
mauch-
erite
tabular crystals uneven
fracture
weakly anisotro-
pic; pinkish gray
tetrago-
nal
meta-
cinnabar
tetrahedral crys-
tals; massive
subcon-
choidal
to uneven
fracture
isotropic;
grayish white;
shows lamellar
twinning
isomet-
ric
millerite very slender
to capillary
crystals in
radiating groups,
sometimes
interwoven
two
perfect
cleavages
strongly
anisotropic
hexago-
nal
molyb-
denite
hexagonal
tablets; foliated
massive, in scales
one
perfect
cleavage
very strongly
anisotropic and
pleochroic; white
hexago-
nal
nicco-
lite
reniform
massive; also
branching
no
cleavage
strongly
anisotropic
hexago-
nal
orpi-
ment
foliated, fibrous,
or columnar mas-
sive; reniform
or botryoidal
masses; granular
one
perfect
cleavage
alpha = 2.4
beta = 2.81
gamma = 3.02
mono-
clinic
7 carbonates and other Minerals 7
7 Minerals 7
316
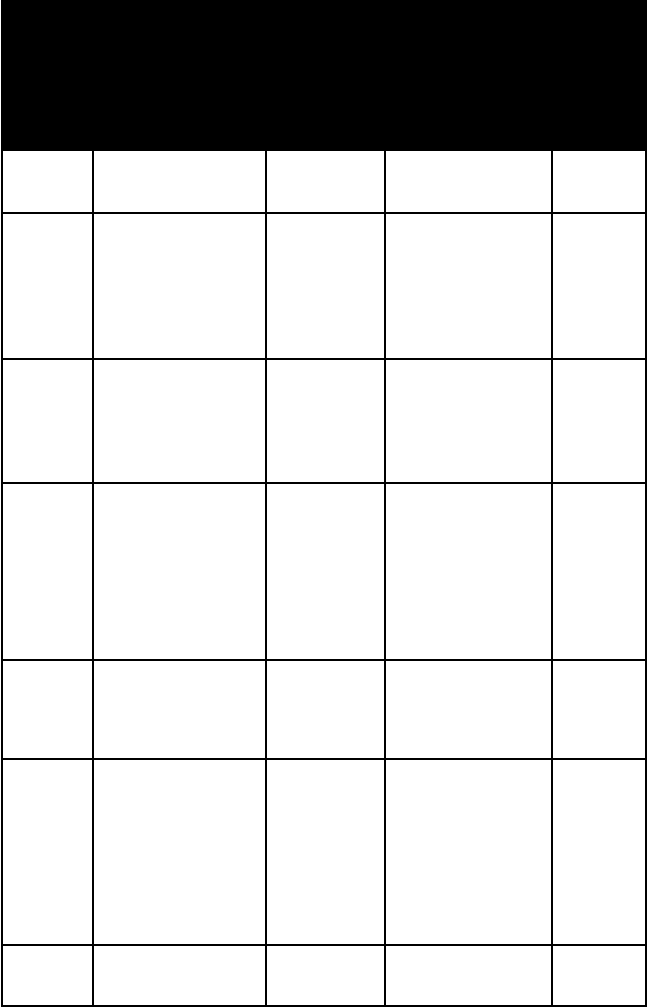
name habit or
form
frac-
ture or
cleavage
refractive
indices or
polished
section
data
crys-
tal
system
pent-
landite
granular
aggregates
conchoidal
fracture
isotropic isomet-
ric
pyrite cubic, pyri-
tohedral, or
octahedral crys-
tals with striated
faces; massive
conchoidal
to uneven
fracture
isotropic;
creamy white
isomet-
ric
pyrrho-
tite
granular mas-
sive; sometimes
platy or tabular
crystals
uneven to
subcon-
choidal
fracture
strongly
anisotropic
hexago-
nal
realgar short, stri-
ated prismatic
crystals; granu-
lar or compact
massive;
incrustations
one good
cleavage,
three less
so
alpha =
2.486–2.538
beta =
2.602–2.684
gamma =
2.620–2.704
mono-
clinic
rickard-
ite
massive irregular
fracture
strongly aniso-
tropic and
pleochroic
ortho-
rhombic
sphaler-
ite
tetrahedral or
dodecahedral
crystals, often
with curved
faces; cleavable
masses
one
perfect
cleavage
n = 2.320–2.517 isomet-
ric
stannite granular massive uneven
fracture
anisotropic tetrago-
nal
317
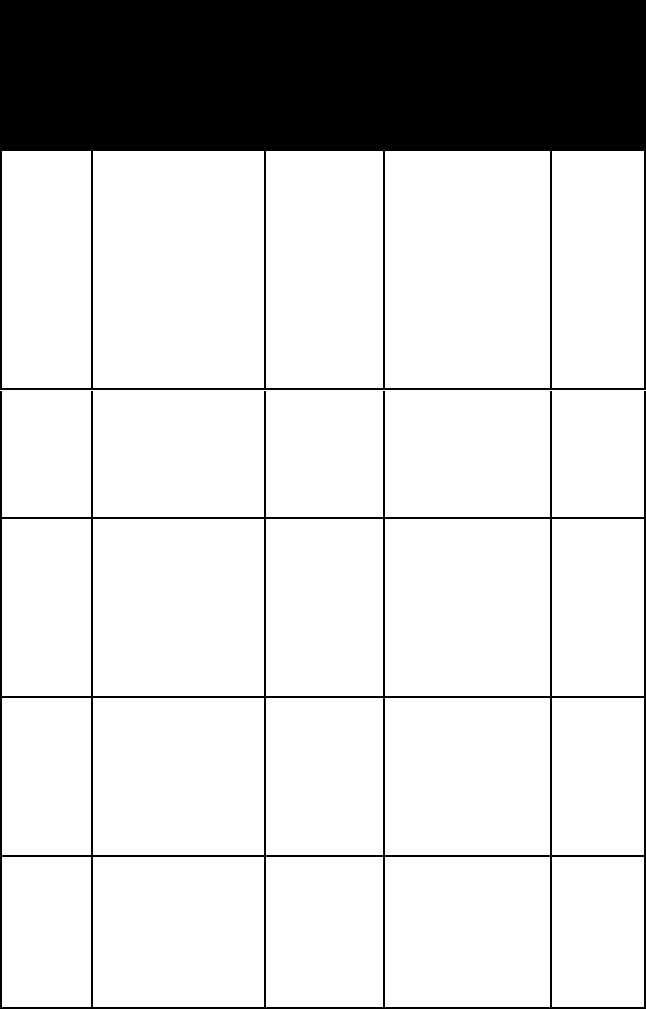
name habit or
form
frac-
ture or
cleavage
refractive
indices or
polished
section
data
crys-
tal
system
stibnite aggregates of
needle-like
crystals; crystals
are easily bent or
twisted
one
perfect
cleavage
alpha =
3.184–3.204
beta =
4.036–4.056
gamma =
4.293–4.313
white; strongly
anisotropic
ortho-
rhombic
stro-
meyer-
ite
pseudohex-
agonal prisms;
compact massive
subcon-
choidal to
conchoidal
fracture
strongly
anisotropic
ortho-
rhombic
sylva-
nite
short prismatic,
thick tabular, or
bladed crystals
one
perfect
cleavage
strongly aniso-
tropic and
pleochroic;
creamy white;
shows polysyn-
thetic twinning
mono-
clinic
tetrady-
mite
foliated to
granular massive;
bladed crystals
one
perfect
cleavage
weakly aniso-
tropic; white;
sometimes
shows a graph-
like intergrowth
hexago-
nal
umang-
ite
small grains;
fine-grained
aggregates
uneven
fracture
strongly aniso-
tropic; dark
red-violet;
apparently
uniaxial
tetrago-
nal
7 carbonates and other Minerals 7
7 Minerals 7
318
Sulfosalts
Sulfosalts constitute an extensive group of minerals, mostly
rare species, marked by some of the most complicated
atomic and crystal structures known to inorganic chemis-
try. They conform to the general composition AmBnXp, in
which m, n, and p are integers; A may be lead, silver, thal-
lium, or copper; B may be antimony, arsenic, bismuth, tin,
or germanium; and X may be sulfur or selenium. Formerly
it was believed that the sulfosalts were salts of complex
hypothetical thioantimonic or thioarsenic acids (e.g.,
HSbS
2
, H
18
As
4
S
15
, H
3
AsS
3
), but X-ray diffraction analyses
indicate that the atomic structures of many sulfosalts are
based on structural fragments of simpler compounds such
as galena (lead sulfide; PbS) blocks and stibnite (antimony
trisulfide; Sb
2
S
3
) sheets. No encompassing theory has been
evolved to rationalize many of these curious compounds.
The complexity of many of the structures evidently results
from their having crystallized at low temperatures and the
consequent high degree of ordering of the metal atoms.
Syntheses of such compositions at higher temperature
usually result in structures simpler than the complicated
low-temperature forms.
Although sulfosalts are much rarer than the sulfide
minerals with which they are often associated, some
name habit or
form
frac-
ture or
cleavage
refractive
indices or
polished
section
data
crys-
tal
system
wurtzite pyramidal crys-
tals; fibrous or
columnar massive;
concentrically
banded crusts
one easy
cleavage
omega =
2.330–2.356
epsilon =
2.350–2.378
hexago-
nal
319
7 carbonates and other Minerals 7
localities are truly remarkable for the variety of species
encountered. At the Lengenbach Mine in Switzerland,
for example, more than 30 distinct species have been
recognized, 15 of which are not found elsewhere. Most sul-
fosalts have formed at low temperature in open cavities,
usually in association with copper–zinc–arsenic sulfide
ores. Very often they occur in cavities of calcite and dolo-
mite, as at the Lengenbach Mine. Most are lead gray in
MOlyBDATE AND
TUNGSTATE MiNERAlS
These naturally occurring inorganic compounds are salts of molybdic
acid, H
2
MoO
4
, and tungstic acid, H
2
WO
4
. Minerals in these groups
often are valuable ores.
The structural unit of these minerals is a tetrahedral group
formed by four oxygen atoms at the corners of a tetrahedron sur-
rounding a molybdenum or tungsten atom. Each MoO
4
or WO
4
tetrahedron has a net charge of -2, which is neutralized by metal ions
outside the tetrahedron. Unlike the silicate or borate minerals, which
form chains, rings, sheets, or framework structures by sharing oxy-
gen atoms between adjacent tetrahedra, the molybdate and tungstate
minerals share none; they are similar in this respect to the phosphate,
vanadate, arsenate, and chromate minerals. Because the molybdenum
ion and the tungsten ion have similar radii, they may substitute for
one another within the structure of any naturally occurring example;
thus, they tend to form solid solution series.
Among the molybdate and tungstate minerals, only the powellite-
scheelite series (calcium-bearing molybdate/tungstates) and wulfenite
(lead molybdate) are noteworthy. Scheelite is a valuable tungsten ore;
wulfenite is a minor ore of lead.
One other series of tungstates is important. Wolframite, another
name for the hübnerite-ferberite series of manganese/iron tung-
states, is perhaps the most important ore of tungsten. These minerals
have a structure, unlike that of the other tungstates, based on WO
6
octahedra—i.e., each tungsten atom is surrounded by six oxygen atoms
arranged at the corners of an octahedron. These minerals are classed
with the complex oxides and are related to the niobates and tantalates.
7 Minerals 7
320
SulfOSAlTS
name colour lustre M
ohs
hardness
specific
gravity
argyrodite bluish to pur-
plish black; steel
gray when fresh
metallic 2½ 6.1–6.3
bourno-
nite
steel gray to
iron black
metallic 2½–3 5.8–5.9
enargite gray black to
iron black
metallic 3 4.4–4.5
polybasite iron black metallic 2–3 6.0–6.2
proustite scarlet
vermillion
adaman-
tine
2–2½ 5.6
pyrargyrite deep red adaman-
tine
2½ 5.8
stephanite iron black metallic 2–2½ 6.2–6.3
tetrahe-
drite
flint gray to iron
or dull black
metallic 3–4½ 4.6–5.1
colour with a metallic lustre, brittle (rarely malleable),
crystalline, and difficult to tell apart without recourse to
X-ray diffraction and electron microprobe analyses. The
thallium-bearing sulfosalts often are deep red and trans-
parent, as sometimes are the sulfosalts of silver.
Although under exceptional circumstances some
sulfosalts may constitute silver ores (i.e., proustite, pyrar-
gyrite, and stephanite), and other species have constituted
ores of silver (in minor amounts), mercury, arsenic, and
antimony (i.e., boulangerite, livingstonite, enargite, and
tennantite-tetrahedrite), their economic importance is
trivial. Aside from mineralogical curiosities, the sulfo-
salts are of interest because their electronic properties are
related to those of semiconductors.
321
7
carbonates and other Minerals 7
SulfOSAlTS
name habit frac
-
ture or
cleavage
refractive
indices or
polished
section
data
crystal
system
argy-
rodite
crystals and
crystal aggre-
gates; crusts;
compact
masses
conchoidal
to uneven
fracture
violet gray
white; isotro-
pic (canfieldite)
or weakly pleo-
chroic
(argyrodite)
isometric
bour-
nonite
prismatic to
tabular crystals;
crystal aggre-
gates; granular
to compact
masses
subcon-
choidal
to uneven
fracture
white; weakly
anisotropic and
very weakly
pleochroic
ortho-
rhombic
enar-
gite
tabular crys-
tals; granular
masses
one perfect
cleavage
gray to light
rose brown;
strongly aniso-
tropic; weakly
pleochroic
ortho-
rhombic
poly-
basite
tabular crys-
tals; massive
uneven
fracture
gray white;
moderately
anisotro-
pic; weakly
pleochroic
monoclinic
proust-
ite
prismatic crys-
tals; compact
masses
one distinct
cleavage
omega =
2.979–3.088
epsilon =
2.711–2.792
hexagonal
pyrar-
gyrite
prismatic crys-
tals; compact
masses
one distinct
cleavage
omega = 3.084
epsilon = 2.881
hexagonal
