Rafferty J.P. (ed.) Minerals
Подождите немного. Документ загружается.


7 Minerals 7
292
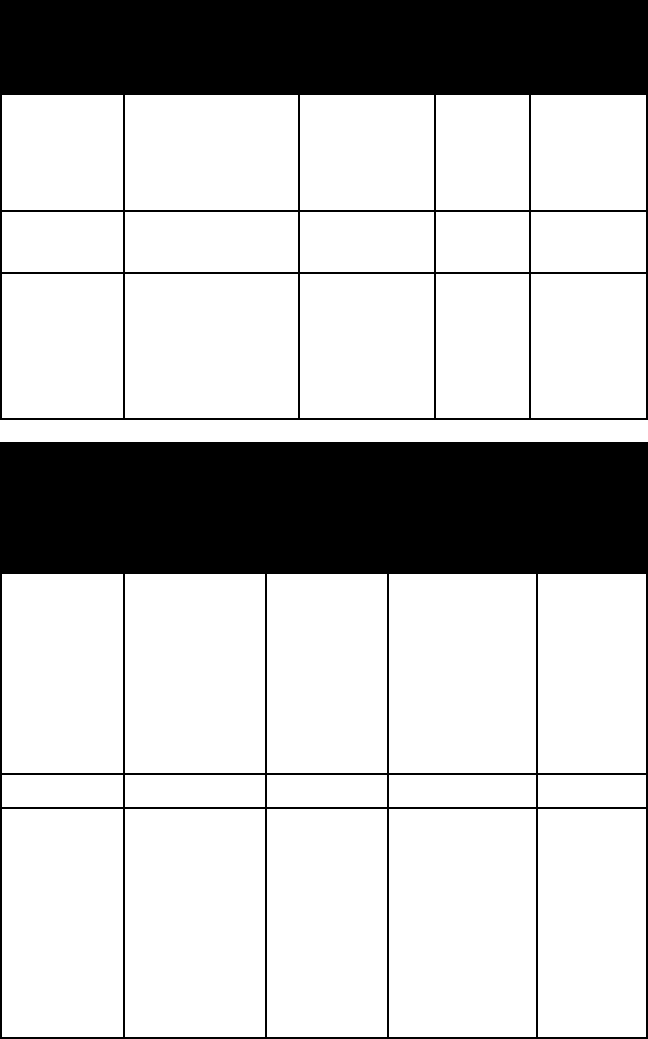
name colour lustre Mohs’
hard-
ness
specific
gravity
monazite yellowish brown
or reddish brown
to brown
usually resin-
ous or waxy;
sometimes
vitreous or
adamantine
5–5½ 4.6–5.4;
usually
5.0–5.2
pyromor-
phite
olive green; yel-
low; gray; brown
to orange
resinous to
subadaman-
tine
3½–4 7.0
torbernite various shades of
green
vitreous to
subadaman-
tine
2–2½ 3.2
triphylite bluish or
greenish gray
(triphylite); clove
brown, honey
yellow, or salmon
(lithiophilite)
vitreous to
subresinous
4–5 3.3–3.6 not
varying
linearly
with com-
position
triplite dark brown;
flesh red; salmon
pink
vitreous to
resinous
5–5½ 3.5–3.9
turquoise blue to various
shades of green;
greenish to yel-
lowish gray
waxy 5–6 2.6–2.8
variscite yellowish green,
pale to emerald
green, bluish
green or colour-
less (variscite);
peach-blossom
red, carmine,
violet (strengite)
vitreous to
faintly waxy
3½–4½ 2.2–2.5
293
7
carbonates and other Minerals 7
name colour lustre Mohs’
hard-
ness
specific
gravity
vivianite colourless when
fresh, darkening
to deep blue or
bluish black
vitreous 1½–2 2.7
wavellite greenish white;
green to yellow
vitreous 3½–4 2.4
xenotime yellowish brown
to reddish brown;
flesh red, grayish
white, pale yel-
low, or greenish
vitreous 4–5 4.4–5.1
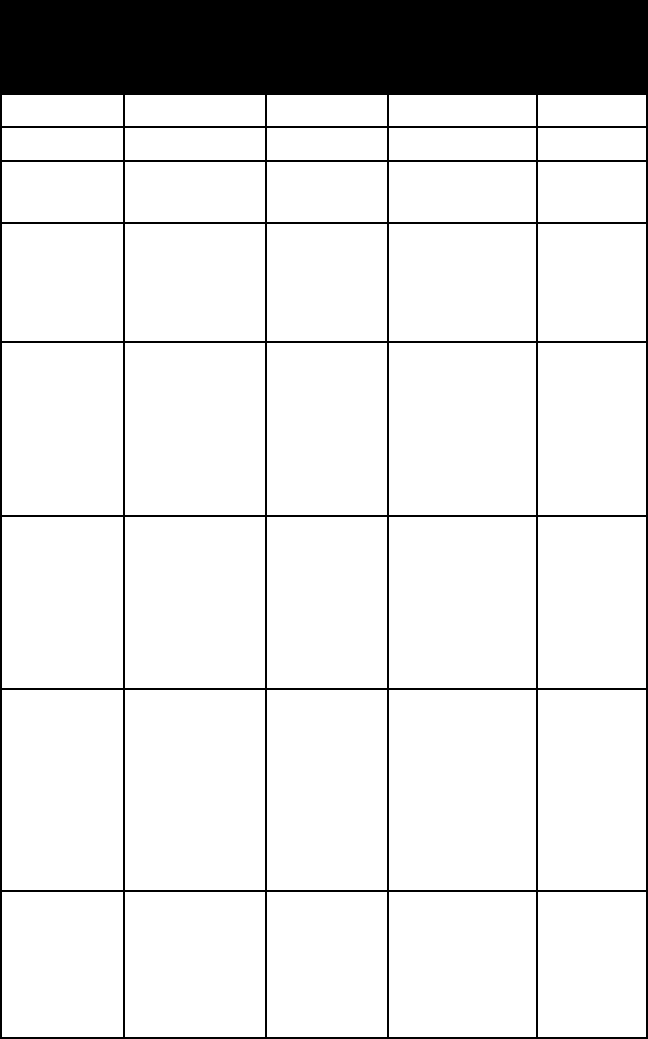
phOSphATe MINerAlS
name habit or
form
frac
-
ture or
cleavage
refrac-
tive
indices
crystal
system
amblygo-
nite
large, trans-
lucent,
cleavable
masses; small
transparent
crystals
one perfect
and one
good
cleavage
ambl mont
alpha =
1.578–1.611
beta =
1.595–1.619
gamma =
1.598–1.633
triclinic
apatite
carbonate-
apatite
prismatic or
thick tabu-
lar crystals;
coarse
granular to
compact mas-
sive; nodular
concretions
conchoidal
to uneven
fracture
n = 1.63–1.67 hexagonal
7 Minerals 7
294
name habit or
form
frac-
ture or
cleavage
refrac-
tive
indices
crystal
system
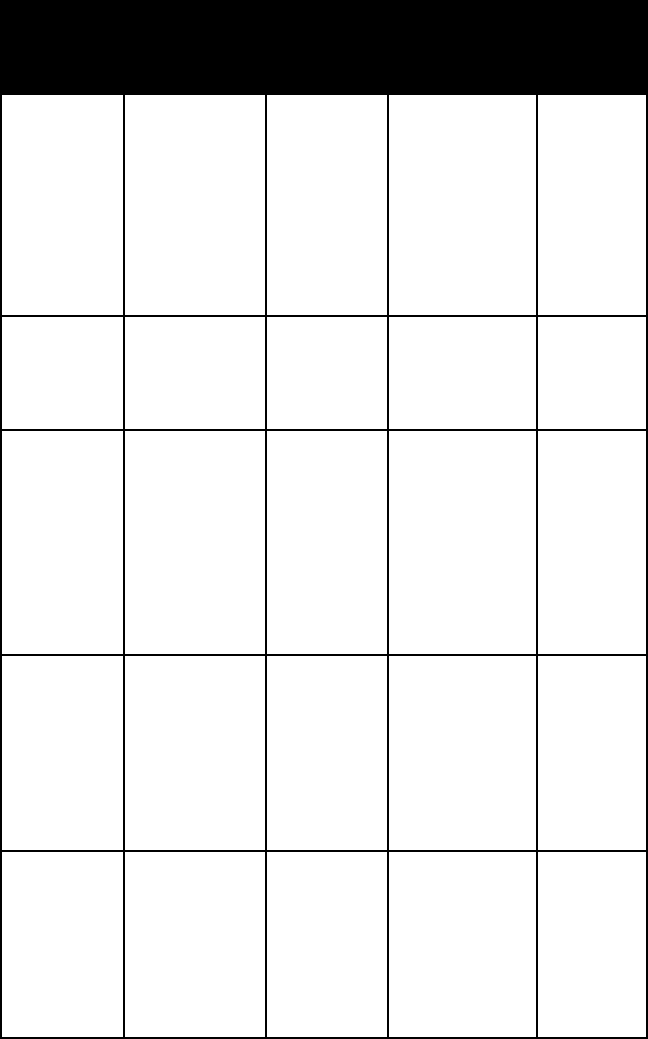
chlorapatite
fluorapatite
hydroxyl-
apatite
autunite thin tabular
crystals; flaky
aggregates;
crusts
one
perfect,
micalike
cleavage
alpha = 1.553
beta = 1.575
gamma = 1.577
tetragonal
brushite transpar-
ent to
translucent
efflorescences
or minute
crystals
two perfect
cleavages
alpha = 1.539
beta = 1.546
gamma = 1.551
mono-
clinic
collophane
(massive
apatite)
crypto-
crystalline
massive;
hornlike con-
cretions and
nodules
n = 1.59–1.61
lazulite crystals;
compact
masses;
grains
two
cleavages;
uneven to
splintery
fracture
lazul scorz
alpha =
1.604–1.639
beta =
1.626–1.670
gamma =
1.637–1.680
mono-
clinic
monazite translu-
cent, small
flattened
crystals
one
distinct
cleavage
alpha =
1.79–1.80
beta = 1.79–1.80
gamma =
1.84–1.85
mono-
clinic
295
name habit or
form
frac-
ture or
cleavage
refrac-
tive
indices
crystal
system
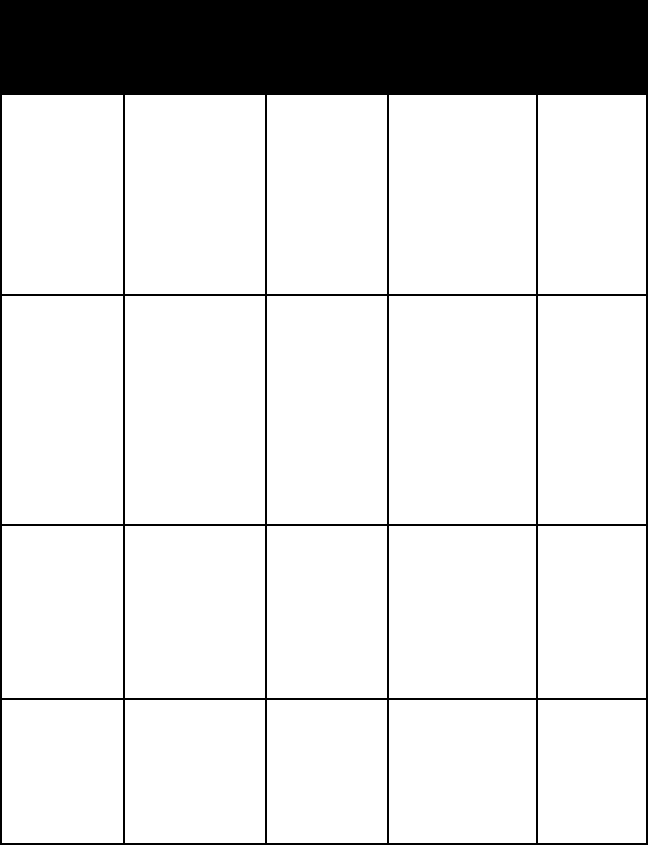
pyromor-
phite
barrel-
shaped
prisms;
globular,
kidney-shaped,
or grape-like
masses
uneven to
subcon-
choidal
fracture
epsilon =
2.030–2.031
omega =
2.041–2.144
hexagonal
torbernite tabular crys-
tals; micalike
masses
one per-
fect, platy
cleavage
epsilon = 1.582
omega = 1.592
tetragonal
triphylite transpar-
ent to
translucent
cleavable
or compact
massive
one perfect
cleavage
triph lith
alpha =
1.694–1.669
beta =
1.695–1.673
gamma =
1.700–1.682
ortho-
rhombic
triplite massive one good
cleavage
alpha =
1.643–1.696
beta =
1.647–1.704
gamma =
1.668–1.713
mono-
clinic
turquoise opaque,
dense, cryp-
tocrystalline
to fine granu-
lar massive
one perfect
and one
good
cleavage
alpha = 1.61
beta = 1.62
gamma = 1.65
triclinic
7 carbonates and other Minerals 7
7 Minerals 7
296
name habit or
form
frac-
ture or
cleavage
refrac-
tive
indices
crystal
system
variscite fine-grained,
round or
grapelike
aggregates,
nodules,
veins, or
crusts
one good
cleavage
varis stren
alpha =
1.563–1.707
beta =
1.588–1.719
gamma =
1.594–1.741
ortho-
rhombic
vivianite rounded
prismatic
crystals;
kidney-shaped,
tubelike,
or globu-
lar masses;
concretions
one perfect
cleavage
alpha =
1.579–1.616
beta =
1.602–1.656
gamma =
1.629–1.675
mono-
clinic
wavellite translucent,
hemispherical,
or globular
aggregates
one perfect
and one
good
cleavage
alpha =
1.520–1.535
beta =
1.526–1.543
gamma =
1.545–1.561
ortho-
rhombic
xenotime small pris-
matic crystals;
coarse radial
aggregates;
rosettes
uneven to
splintery
fracture
epsilon =
1.816–1.827
omega =
1.721–1.720
tetragonal
Sulfate Minerals
These minerals are naturally occurring salts of sulfuric
acid. About 200 distinct kinds of sulfates are recorded in
mineralogical literature, but most of them are of rare and
local occurrence. Abundant deposits of sulfate minerals,
297
7
carbonates and other Minerals 7
such as barite and celestite, are exploited for the prepa-
ration of metal salts. Many beds of sulfate minerals are
mined for fertilizer and salt preparations, and beds of pure
gypsum are mined for the preparation of plaster of paris.
All sulfates possess an atomic structure based on dis-
crete insular sulfate (SO
4
2-
) tetrahedra, i.e., ions in which
four oxygen atoms are symmetrically distributed at the
corners of a tetrahedron with the sulfur atom in the cen-
tre. These tetrahedral groups do not polymerize, and the
sulfate group behaves as a single negatively charged mol-
ecule, or complex. Thus, sulfates are distinct from the
silicates and borates, which link together into chains,
rings, sheets, or frameworks.
Sulfate minerals can be found in at least four kinds:
as late oxidation products of preexisting sulfide ores, as
evaporite deposits, in circulatory solutions, and in depos-
its formed by hot water or volcanic gases. Many sulfate
minerals occur as basic hydrates of iron, cobalt, nickel,
zinc, and copper at or near the source of preexisting pri-
mary sulfides. The sulfide minerals, through exposure
to weathering and circulating water, have undergone
oxidation in which the sulfide ion is converted to sul-
fate and the metal ion also is changed to some higher
valence state. Noteworthy beds of such oxidation prod-
ucts occur in desert regions, such as Chuquicamata,
Chile, where brightly coloured basic copper and ferric
iron sulfates have accumulated. The sulfate anions gener-
ated by oxidation processes may also react with calcium
carbonate rocks to form gypsum, CaSO
4
∙2H
2
O. Sulfates
formed by the oxidation of primary sulfides include ant-
lerite [Cu
3
(SO
4
)(OH)
4
], brochantite [Cu
4
(SO
4
)(OH)
6
],
chalcanthite [Cu
2+
(SO
4
)∙5H
2
O], anglesite (PbSO
4
), and
plumbojarosite [PbFe
3+
6
(SO
4
)
4
(OH)
12
].
Soluble alkali and alkaline-earth sulfates crystal-
lize upon evaporation of sulfate-rich brines and trapped
7 Minerals 7
298
oceanic salt solutions. Such brines can form economically
important deposits of sulfate, halide, and borate minerals
in thick parallel beds, as the potash deposits at Stassfurt,
Ger., and the southwestern United States. Many of the
sulfate minerals are salts of more than one metal, such as
polyhalite, which is a combination of potassium, calcium,
and magnesium sulfates.
Sulfate minerals common in evaporite deposits
include anhydrite, gypsum, thenardite (Na
2
SO
4
), epso-
mite (MgSO
4
∙7H
2
O), glauberite [Na
2
Ca(SO
4
)
2
], kainite
(MgSO
4
∙KCl∙3H
2
O), kieserite (MgSO
4
∙H
2
O), mirabilite
(Na
2
SO
4
∙10H
2
O), and polyhalite [K
2
Ca
2
Mg(SO
4
)
4
∙2H
2
O].
Groundwater carrying sulfate anions reacts with cal-
cium ions in muds, clays, and limestones to form beds of
gypsum. The massive material is called alabaster or plaster
of paris (originally found in the clays and muds of the Paris
basin). If such beds become deeply buried or metamor-
phosed (altered by heat and pressure), anhydrite may form
by dehydration of the gypsum.
Numerous sulfates, usually simple, are formed directly
from hot aqueous solutions associated with fumarolic
(volcanic gas) vents and late-stage fissure systems in ore
deposits. Noteworthy examples include anhydrite, barite,
and celestine.
SulfATe MINerAlS
name colour lustre M
ohs
hard-
ness
specific
gravity
alum colourless; white vitreous 2–2½ 1.8
alunite white; grayish,
yellowish, reddish,
reddish brown
vitreous 3½–4 2.6–2.9
299
name colour lustre Mohs
hard-
ness
specific
gravity
alunogen white; yellowish
or reddish
vitreous to
silky
1½–2 1.8
anglesite colourless to
white; often
tinted gray, yellow,
green, or blue
adamantine
to resinous
or vitreous
2½–3 6.4
anhydrite colourless to blu-
ish or violet
vitreous to
pearly
3½ 3.0
antlerite emerald to black-
ish green; light
green
vitreous 3½ 3.9
barite colourless to
white; also
variable
vitreous to
resinous
3–3½ 4.5
botryogen light to dark
orange red
vitreous 2–2½ 2.1
brochan-
tite
emerald to black-
ish green; light
green
vitreous 3½–4 4.0
caledonite deep verdigris
green or bluish
green
resinous 2½–3 5.8
celestite pale blue; white,
reddish, greenish,
brownish
vitreous 3–3½ 4.0
chal-
canthite
various shades of
blue
vitreous 2½ 2.3
coquim-
bite
pale violet to deep
purple
vitreous 2½ 2.1
7 carbonates and other Minerals 7
7 Minerals 7
300
name colour lustre Mohs
hard-
ness
specific
gravity
epsomite colourless; aggre-
gates are white
vitre-
ous; silky
to earthy
(fibrous)
2–2½ 1.7
glauberite gray; yellowish vitreous to
slightly waxy
2½–3 2.75–2.85
gypsum colourless; white,
gray, brown-
ish, yellowish
(massive)
subvitreous 2 (a
hard-
ness
stan-
dard)
2.3
halotrich-
ite
colourless to
white
vitreous 1.5 1.7 (pick) to
1.9 (halo)
jarosite ochre yellow to
dark brown
suba-
damantine
to vitreous;
resinous on
fracture
2½–3½ 2.9–3.3
kainite colourless; gray,
blue, violet, yel-
lowish, reddish
vitreous 2½–3 2.2
kieserite colourless; grayish
white, yellowish
vitreous 3.5 2.6
linarite deep azure blue vitreous to
subadaman-
tine
2.5 5.3
mirabilite colourless to
white
vitreous 1½–2 1.5
plumbo-
jarosite
golden brown to
dark brown
dull to glis-
tening or silky
soft 3.7
301
7
carbonates and other Minerals 7
name colour lustre Mohs
hard-
ness
specific
gravity
polyhalite colourless; white or
gray; often salmon
pink from included
iron oxide
vitreous to
resinous
3.5 2.8
thenardite colourless; red-
dish, grayish,
yellowish, or yel-
low brown
vitreous to
resinous
2½–3 2.7
SulfATe MINerAlS
name habit frac
-
ture or
cleavage
refrac-
tive
indices
crystal
system
alum columnar or
granular massive
conchoidal
fracture
n = 1.453–1.466 isometric
alunite granular to
dense massive
conchoidal
fracture
omega = 1.572
epsilon = 1.592
hexagonal
alunogen fibrous masses
and crusts
one perfect
cleavage
alpha =
1.459–1.475
beta =
1.461–1.478
gamma =
1.884–1.931
triclinic
anglesite granular to com-
pact massive;
tabular or pris-
matic crystals
one good,
one
distinct
cleavage
alpha =
1.868–1.913
beta =
1.873–1.918
gamma =
1.884–1.931
ortho-
rhombic
