Rafferty J.P. (ed.) Minerals
Подождите немного. Документ загружается.

7 Minerals 7
272
must occur for precipitation of the borates, such basin
deposits usually occur in desert regions, as for example
the Kramer district of the Mojave Desert and Death
Valley in California, where enormous beds of stratified
kernite, borax, colemanite, and ulexite are recovered, pri-
marily by stripping away the overburden and mining the
borates by classical open-pit techniques. Other notewor-
thy evaporite deposits occur in the Inderborsky district of
Kazakhstan and in Tuscany, Italy. The sequence of precipi-
tating alkali borates can be duplicated in the laboratory
because the temperatures and pressures of their forma-
tion are low and easily accessible. Solutions of the alkali
borates and the addition of metal ions such as calcium
and magnesium result in the precipitation of yet other
borate compounds. Among the borates commonly found
in evaporite deposits are borax, colemanite, inyoite, kern-
ite, and tincalconite.
The second geologic setting for borate minerals is a
metamorphic carbonate-rich environment, where they are
formed as a result of alteration of the surrounding rocks
by heat and pressure; similar borates also occur as nodules
in some deeply buried sediments. These compounds were
formed at relatively high temperatures and usually consist
of densely packed BO
3
triangles associated with such small
metal ions as magnesium, manganese, aluminum, or iron.
The origin of these borates is not as obvious as that of the
evaporite varieties. Some were produced by the reaction
of boron-bearing vapour derived from hot intruding gran-
ites during metamorphism; others are the recrystallization
products of evaporite borates. Numerous borosilicates
(e.g., dumortierite and tourmaline) were formed under
these conditions. Compounds of this type contain both
BO
3
triangular units and SiO
4
tetrahedral units. Among
the borate minerals associated with metamorphosed envi-
ronments are boracite, ludwigite, sussexite, and kotoite.
273
Arsenate Minerals
An arsenate mineral is a compound of arsenic, oxygen, and
various metals. Most arsenate minerals are rare, having
crystallized under very restricted conditions. At the min-
eralogically famous Långban iron and manganese mines
in central Sweden, more than 50 species of arsenate min-
erals have been described, many peculiar to the locality.
Such compounds occur in open cavities and resulted from
the reaction of arsenic acid (H
3
AsO
4
) upon pyrochroite
[manganese hydroxide; Mn(OH)
2
] at moderate to low
temperature. Arsenates at other localities are often oxi-
dation products of arsenide ores and are deposited at low
temperatures in late-stage veins and open cavities.
Only a few arsenate minerals have economic impor-
tance. Because the transition metals (e.g., cobalt, copper,
nickel) give brilliant colour to some of the arsenates, these
can be used to advantage in prospecting; such arsenate
oxidation products, or blooms, as erythrite (bright pink)
and annabergite (green) are indicators of nickel and cobalt
arsenide ores. Many of the nickel and cobalt deposits at
Sudbury and Cobalt, Ont., were located in this manner.
The arsenate minerals, which are salts of arsenic acid,
contain arsenate groups (AsO
4
) in which four oxygen (O)
atoms are arranged at the corners of a tetrahedron about
a central arsenic (As) atom. Each arsenate tetrahedron has
a net electric charge of -3, which is neutralized by large,
positively charged metal ions (e.g., calcium, manganese,
or ferrous iron) outside the tetrahedron. Unlike the simi-
lar silicate tetrahedra, which link to form chains, sheets,
rings, or frameworks, arsenate tetrahedra are insular.
The crystal structure of the arsenate minerals is very
similar to that of the phosphate and vanadate minerals;
indeed, many arsenate minerals form solid solutions with
both the phosphates and the vanadates.
7 carbonates and other Minerals 7
7 Minerals 7
274
Halide Minerals
Halide minerals make up a group of naturally occurring
inorganic compounds that are salts of the halogen acids
(e.g., hydrochloric acid). Such compounds, with the
notable exceptions of halite (rock salt), sylvite, and fluo-
rite, are rare and of very local occurrence. Compositionally
and structurally, three broad categories of halide minerals
are recognized; these categories, which are also distin-
guishable in their modes of occurrence, include the simple
halides, the halide complexes, and the oxyhydroxy-halides.
The simple halides are salts of the alkali, alkaline
earth, and transition metals. Most are soluble in water;
the transition-metal halides are unstable under exposure
to air. Halite, sodium chloride (NaCl), is the most familiar
example; it often occurs with other evaporite minerals in
enormous beds resulting from the accumulation of brines
and trapped oceanic water in impermeable basins and
their evaporation. Minor amounts of sylvite, potassium
chloride (KCl), also are present in such beds.
Fluorite, or calcium fluoride (CaF
2
), another simple
halide, is found in limestones that have been perme-
ated by aqueous solutions containing the fluoride anion.
Noteworthy deposits of fluorite occur in Mexico;
Cumberland, Eng.; and Illinois, Missouri, Kentucky, and
Colorado in the United States.
Other simple halides such as sal-ammoniac, ammonium
chloride (NH
4
Cl); lawrencite, ferrous chloride (FeCl
2
); and
molysite, ferric chloride (FeCl
3
) occur in fumarolic vents
and are highly unstable in air. A few hydrothermal vein min-
erals in silver deposits, such as chlorargyrite and calomel,
serve as minor and occasional ores of silver and mercury,
respectively. A few double salts (e.g., carnallite and tachy-
hydrite) included among the simple halides have formed
under conditions similar to the formation of halite.
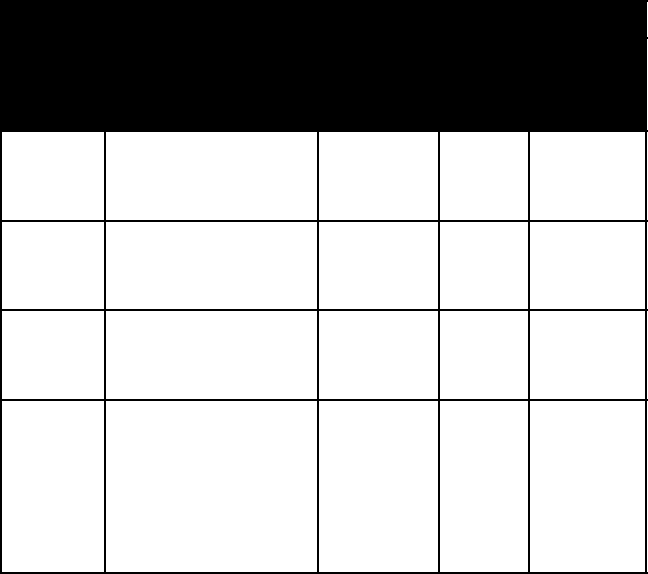
275
hAlIDe MINerAlS
name colour lustre M
ohs
hard-
ness
specific
gravity
atacamite various bright green
shades; dark emerald-
green to blackish
adamantine 3–3½ 3.8
calomel colourless, white,
grayish, yellowish,
brown
adamantine 1½ 7.15
carnallite milk-white; some-
times reddish (from
included hematite)
greasy, dull
to shining
2½ 1.6
cerargy-
rite
colourless when pure
and fresh; usually
gray; becomes purple
or violet-brown on
exposure to light
(cerargyrite)
hornlike 2½ 5.6 (AgCl)
to 6.5
(AgBr)
7 carbonates and other Minerals 7
In the halide complexes, halide anions are tightly
bound to a cation, usually aluminum; the resulting unit
behaves as a single negative ion. The most common exam-
ples are the fluoroaluminates cryolite, cryolithionite,
thomsenolite, and weberite. Enormous quantities of cryo-
lite formerly were mined at Ivigtut, Greenland, to be used
for flux in the recovery of aluminum from bauxite.
Most oxyhydroxy-halides are rare and highly insoluble
compounds. Many have formed by the action of halide-
bearing waters upon the oxidation products of previously
existing sulfides; atacamite, matlockite, nadorite, and
diaboleite are examples. A few compounds such as a
fiedlerite, laurionite, and penfieldite have formed through
the action of seawater upon ancient lead slags from the
historic deposits at Laurium, Greece.
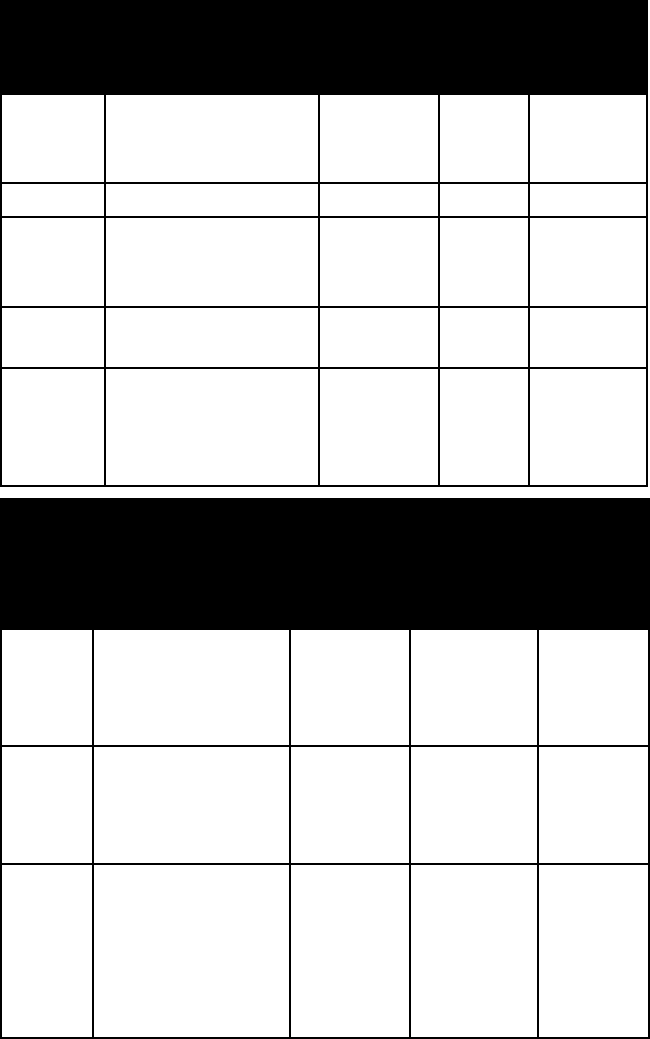
7 Minerals 7
276
hAlIDe MINerAlS
name habit or form
frac-
ture or
cleavage
refrac-
tive
indices
crystal
system
ataca-
mite
brittle, transpar-
ent to translucent
tabular to slender
prismatic crystals
one perfect
cleavage
alpha = 1.831
beta = 1.861
gamma =
1.880
ortho-
rhombic
calomel tabular crystals;
drusy crusts; earthy
masses
one good
cleavage
omega =
1.956–1.991
epsilon =
2.601–2.713
tetragonal
carnall-
ite
granular, massive conchoidal
fracture
alpha =
1.465–1.466
beta =
1.474–1.455
gamma =
1.444–1.446
ortho-
rhombic
name colour lustre Mohs
hard-
ness
specific
gravity
cryolite colourless to white,
brownish, reddish,
brick red
vitreous to
greasy
2½ 3.0
fluorite variable vitreous 4 3.2
halite colourless when
pure, often splotched
blue or purple
vitreous 2 2.2
sal
ammoniac
colourless, white,
grayish, yellow
vitreous 1–2 1.5
sylvite colourless, white,
grayish, bluish, or
red (from included
hematite)
vitreous 2 2.0
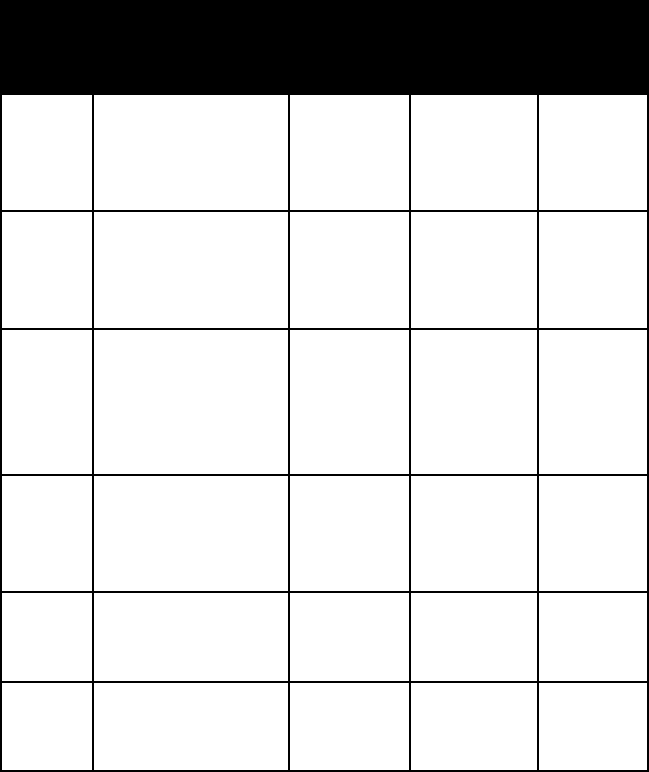
277
nitrate and Iodate Minerals
This small group of naturally occurring inorganic com-
pounds is practically confined to the Atacama Desert
of northern Chile; the principal locality is Antofagasta.
These minerals occur under the loose soil as beds of gray-
ish caliche (a hard cemented mixture of nitrates, sulfates,
halides, and sand) 2–3 m (7–10 feet) thick. The much rarer
name habit or form frac-
ture or
cleavage
refrac-
tive
indices
crystal
system
cerargy-
rite
crusts; waxy coat-
ings; hornlike
masses
uneven to
subcon-
choidal
fracture
n =
2.071–2.253
isometric
cryolite coarsely granular
masses
no
cleavage
alpha = 1.338
beta = 1.338
gamma =
1.339
mono-
clinic
fluorite brittle, transpar-
ent or translucent
cubes and two-
cube penetration
twins
perfect
octahedral
cleavage
n =
1.432–1.437
isometric
halite transparent cubic
(often cavernous or
stepped) crystals;
granular masses
perfect
cubic
cleavage
n = 1.544 isometric
sal
ammo-
niac
skeletal aggregates conchoidal
fracture
n = 1.639 isometric
sylvite transparent cubes
or granular masses
perfect
cubic
cleavage
n = 1.490 isometric
7 carbonates and other Minerals 7
278
7
Minerals 7
iodate minerals occur sporadically, intermixed with the
nitrates, and are distinguished from the former by their
yellow colour. The caliche has accumulated as a result of
drainage. Because most of these compounds are soluble
and unstable, they are practically confined to arid regions
and soils, which possess a paucity of microorganisms.
Before World War I, Chile possessed a near monop-
oly on nitrates, with as many as 100 plants in operation.
The introduction of practical methods for the fixation of
nitrogen early in the 20th century resulted in a decline of
the marketing of natural nitrates.
The nitrate and iodate minerals are structurally related
to the carbonate minerals. The most important nitrates
are soda nitre, nitre (saltpetre), darapskite, and humber-
stonite. Among the iodates are lautarite and dietzeite.
oxide Minerals
Any naturally occurring inorganic compound with a struc-
ture based on close-packed oxygen atoms in which smaller,
positively charged metal or other ions occur in interstices
is considered an oxide. Oxides are distinguished from
other oxygen-bearing compounds such as the silicates,
borates, and carbonates, which have a readily definable
group containing oxygen atoms covalently bonded to an
atom of another element.
The oxide minerals can be grouped as simple oxides and
multiple oxides. Simple oxides are a combination of one
metal or semimetal and oxygen, whereas multiple oxides
have two nonequivalent metal sites. The oxide structures
are usually based on cubic or hexagonal close-packing of
oxygen atoms with the octahedral or tetrahedral sites (or
both) occupied by metal ions; symmetry is typically iso-
metric, hexagonal, tetragonal, or orthorhombic.

279
A sample of the oxide mineral cuprite from Morenci, Ariz. U.S. Geological
Survey (Bureau of Mines, Mineral Specimens C\01786)
7 carbonates and other Minerals 7
The simple oxides can be subdivided on the basis
of the ratio of the numbers of atoms of metal (or other
elements) and oxygen, giving general formulas of the
A
x
O
y
type. In such formulas A represents a metal atom,
and x and y represent integers. Chemical compositions
then fall into categories such as those designated AO,
A
2
O, A
2
O
3
, AO
2
. Specific simple oxide minerals include
periclase (MgO), cuprite (Cu
2
O), hematite (Fe
2
O
3
), and
uraninite (UO
2
).
Complex oxides show a more varied chemistry, often
with extensive solid solution. Most common is the spi-
nel group, with the general formula AB
2
O
4
, in which A
and B are ions of different metals, the same metal with
different oxidation states, or a combination of the two;
A (with oxidation state +2), B (with oxidation state +3) is
the commonest, as, for example, in spinel itself, MgAl
2
O
4
.
Frequently occurring doubly charged ions include
7 Minerals 7
280
magnesium, iron, zinc, and manganese, while common
triply charged ions are aluminum, iron, manganese, and
chromium.
Oxide minerals occur as decomposition products of
sulfide minerals, in pegmatites, early crystallizing miner-
als in ultrabasic rocks, and as accessory minerals in many
igneous rocks.
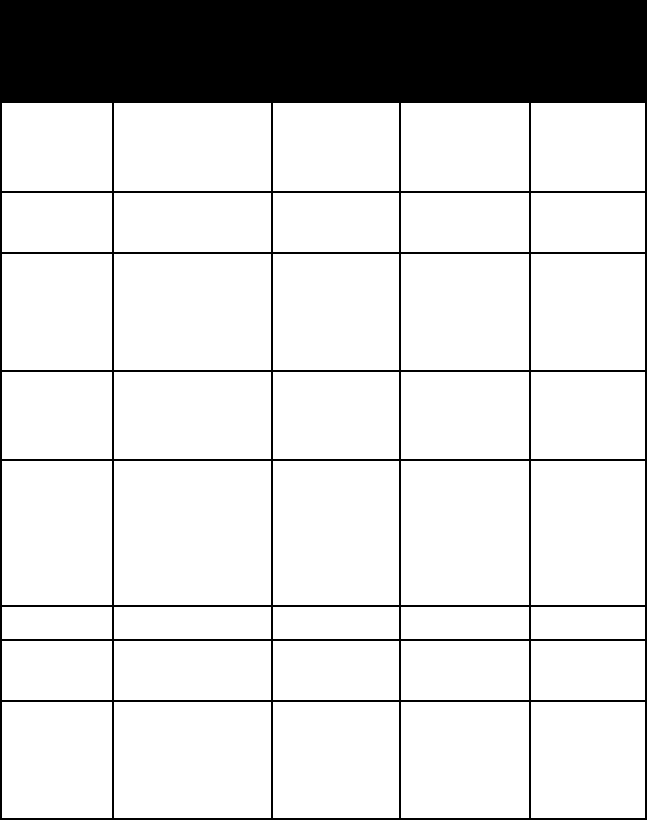
OxIDe MINerAlS
name colour lustre mohs
h
ardness
specific
gravity
anatase brown to indigo
blue and black;
also variable
adamantine
to metallic
adamantine
5½–6 3.8–4.0
boehmite white, when
pure
3 3.0–3.1
brookite various browns metallic
adaman-
tine to
submetallic
5½–6 4.1–4.2
brucite white to pale
green, gray, or
blue
waxy to
vitreous
2½ 2.4
cassiterite reddish or yel-
lowish brown
to brownish
black
adamantine
to metallic
adaman-
tine, usually
splendent
6–7 7.0
chromite black metallic 5½ 4.5–4.8
chryso-
beryl
variable vitreous 8½ 3.6–3.8
columbite iron black to
brownish black;
often with iri-
descent tarnish
6–6½ 5.2 (colum-
bite) to 8.0
(tantalite)
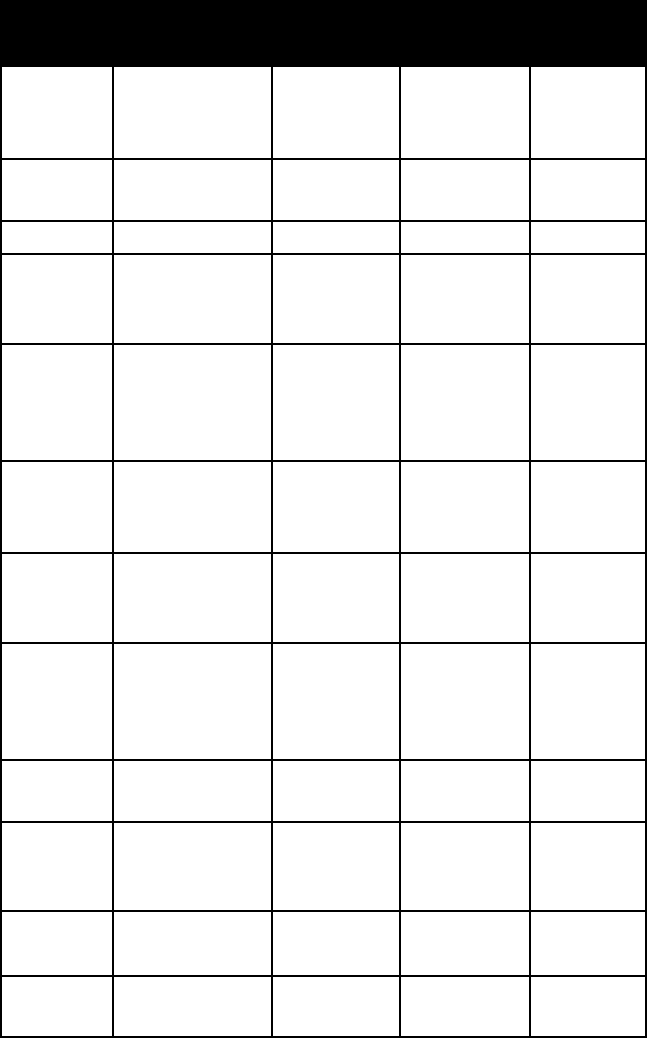
281
name colour lustre mohs
hardness
specific
gravity
corundum red (ruby); blue
(sapphire); also
variable
adamantine
to vitreous
9 (a hard-
ness
standard)
4.0–4.1
cuprite various shades
of red
adamantine
to earthy
3½–4 6.1
delafossite black metallic 5½ 5.4–5.5
diaspore white, grayish
white, colour-
less; variable
brilliant
vitreous
6½–7 3.2–3.5
euxenite black brilliant
submetallic
to greasy or
vitreous
5½–6½ 5.3–5.9
franklinite brownish black
to black
metallic to
semimetal-
lic
5½–6½ 5.1–5.2
gibbsite white; grayish,
greenish, red-
dish white
vitreous 2½–3½ 2.3–2.4
goethite blackish brown
(crystals);
yellowish or
reddish brown
adaman-
tine-metal-
lic
5–5½ 3.3–4.3
hausman-
nite
brownish black submetallic 5½ 4.8
hematite steel gray; dull
to bright red
metallic or
submetallic
to dull
5–6 5.3
ilmenite iron black metallic to
submetallic
5–6 4.7–4.8
lepido-
crocite
ruby red to
reddish brown
submetallic 5 4.0–4.1
7 carbonates and other Minerals 7
