Rafferty J.P. (ed.) Minerals
Подождите немного. Документ загружается.

7 Minerals 7
302
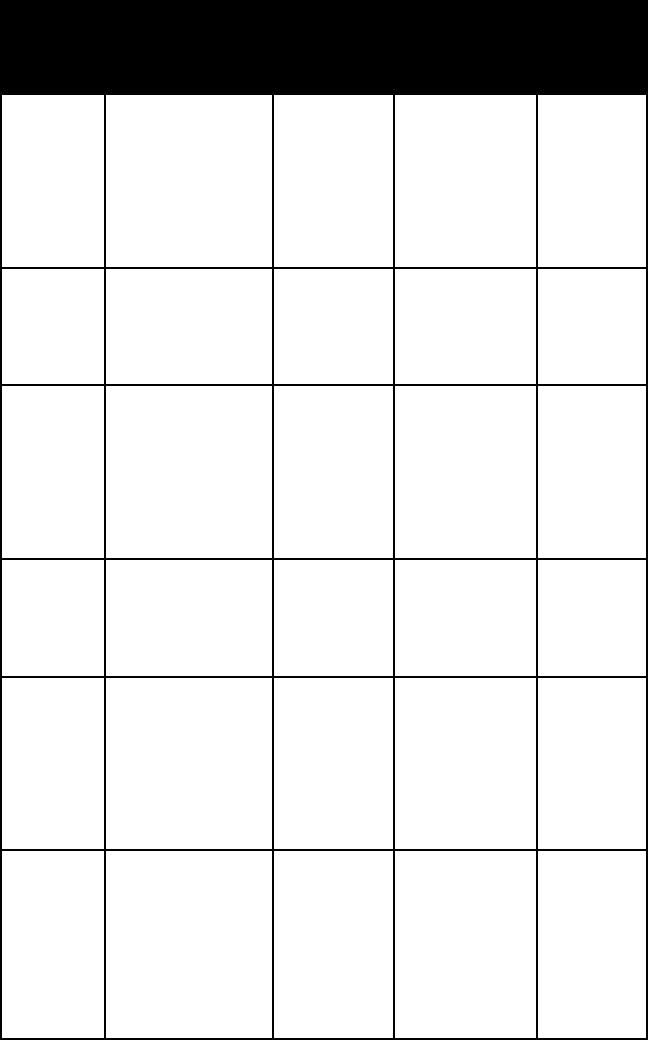
name habit frac-
ture or
cleavage
refrac-
tive
indices
crystal
system
anhydrite granular or
fibrous massive;
concretionary
(tripestone)
two
perfect,
one good
cleavage
alpha =
1.567–1.580
beta =
1.572–1.586
gamma =
1.610–1.625
ortho-
rhombic
antlerite thick tabular
crystals
one perfect
cleavage
alpha = 1.726
beta = 1.738
gamma =
1.789
ortho-
rhombic
barite usually in
tabular crystals;
rosettes (desert
roses); massive
one
perfect,
one good
cleavage
alpha =
1.633–1.648
beta =
1.634–1.649
gamma =
1.645–1.661
ortho-
rhombic
botryo-
gen
reniform,
botryoidal,
or globular
aggregates
one
perfect,
one good
cleavage
alpha = 1.523
beta = 1.530
gamma =
1.582
mono-
clinic
brochan-
tite
prismatic to
hairlike crys-
tal and crystal
aggregates;
granular mas-
sive; crusts
one perfect
cleavage
alpha = 1.728
beta = 1.771
gamma =
1.800
mono-
clinic
cale-
donite
coating of
small elongated
crystals
one perfect
cleavage
alpha =
1.815–1.821
beta =
1.863–1.869
gamma =
1.906–1.912
ortho-
rhombic
303
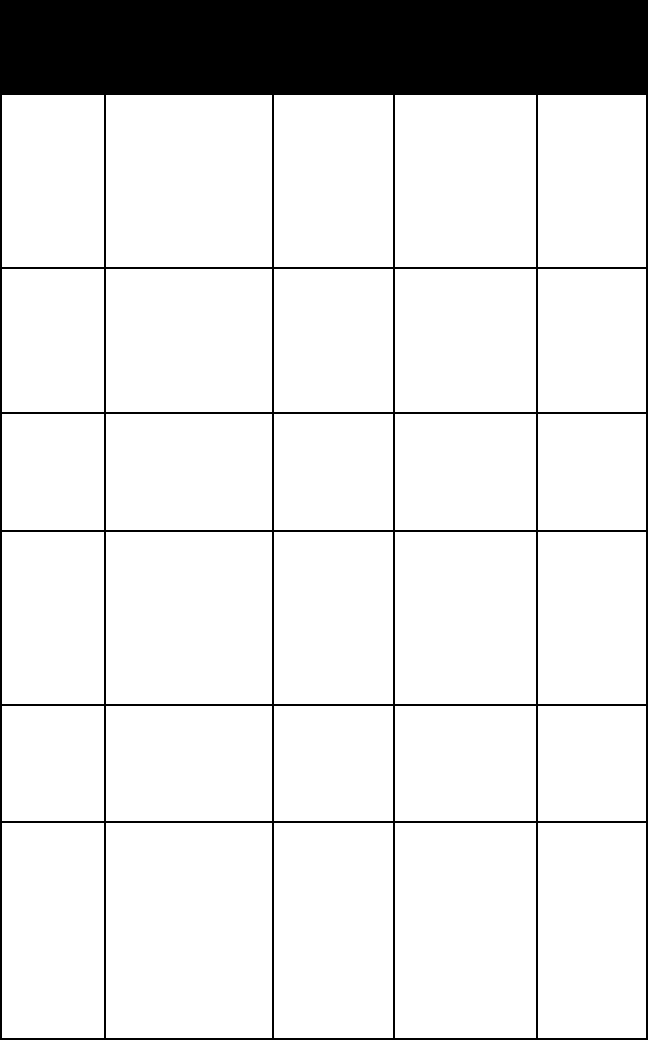
name habit frac-
ture or
cleavage
refrac-
tive
indices
crystal
system
celestite tabular crystals;
fibrous massive
one
perfect,
one good
cleavage
alpha =
1.618–1.632
beta =
1.620–1.634
gamma =
1.627–1.642
ortho-
rhombic
chal-
canthite
short pris-
matic crystals;
granular masses;
stalactites and
reniform masses
conchoidal
fracture
alpha = 1.514
beta = 1.537
gamma =
1.543
triclinic
coquim-
bite
prismatic and
pyramidal crys-
tals; granular
massive
omega = 1.536
epsilon =
1.572
hexagonal
epsomite fibrous or
hairlike
crusts; woolly
efflorescences
one perfect
cleavage
alpha =
1.430–1.440
beta =
1.452–1.462
gamma =
1.457–1.469
ortho-
rhombic
glauberite tabular, dipy-
ramidal, or
prismatic
crystals
one perfect
cleavage
alpha = 1.515
beta = 1.535
gamma =
1.536
mono-
clinic
gypsum elongated
tabular crystals
(some 5 ft long;
others twisted
or bent); granu-
lar or fibrous
masses; rosettes
one perfect
cleavage
alpha =
1.515–1.523
beta =
1.516–1.526
gamma =
1.524–1.532
mono-
clinic
7 carbonates and other Minerals 7
7 Minerals 7
304
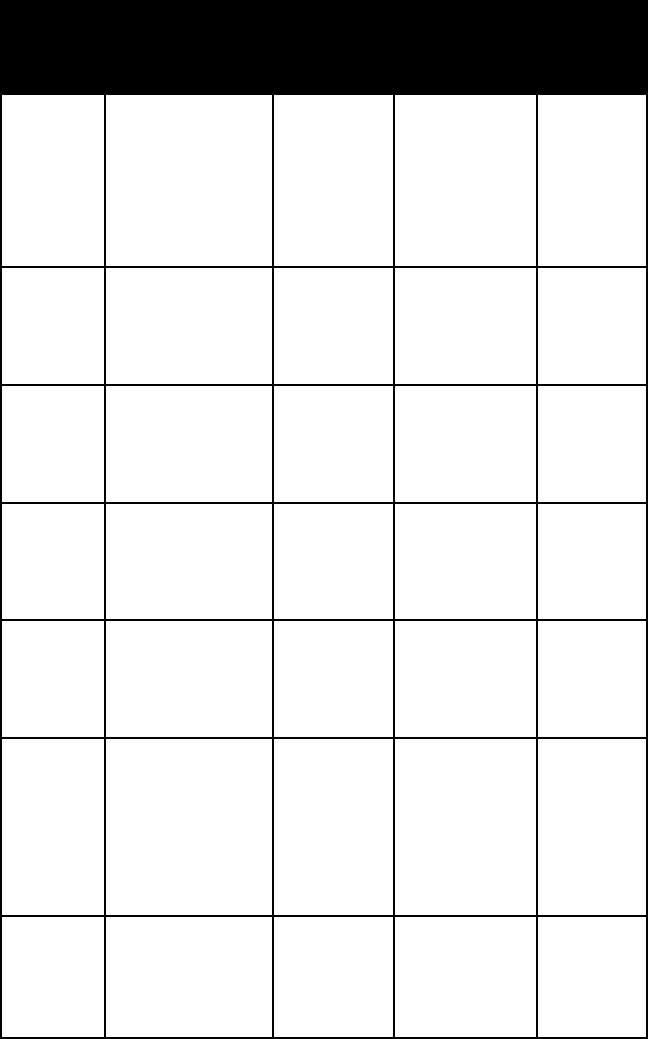
name habit frac-
ture or
cleavage
refrac-
tive
indices
crystal
system
halo-
trichite
aggregates of
hairlike crystals
conchoidal
fracture
alpha =
1.475–1.480
beta =
1.480–1.486
gamma =
1.483–1.490
mono-
clinic
jarosite minute crystals;
crusts; granu-
lar or fibrous
massive
one
distinct
cleavage
omega = 1.82
epsilon = 1.715
hexagonal
kainite granular mas-
sive; crystalline
coatings
one perfect
cleavage
alpha = 1.494
beta = 1.505
gamma =
1.516
mono-
clinic
kieserite granular mas-
sive, intergrown
with other salts
two perfect
cleavages
alpha = 1.520
beta = 1.533
gamma =
1.584
mono-
clinic
linarite elongated
tabular crystals,
either singly or
in groups
one perfect
cleavage;
conchoidal
fracture
alpha = 1.809
beta = 1.839
gamma =
1.859
mono-
clinic
mirabilite short prisms;
lathlike or
tabular crystals;
crusts or fibrous
masses; granular
massive
one perfect
cleavage
alpha =
1.391–1.397
beta =
1.393–1.410
gamma =
1.395–1.411
mono-
clinic
plumbo-
jarosite
crusts, lumps,
compact masses
of microscopic
hexagonal plates
one fair
cleavage
omega = 1.875
epsilon =
1.786
hexagonal
305
7 carbonates and other Minerals 7
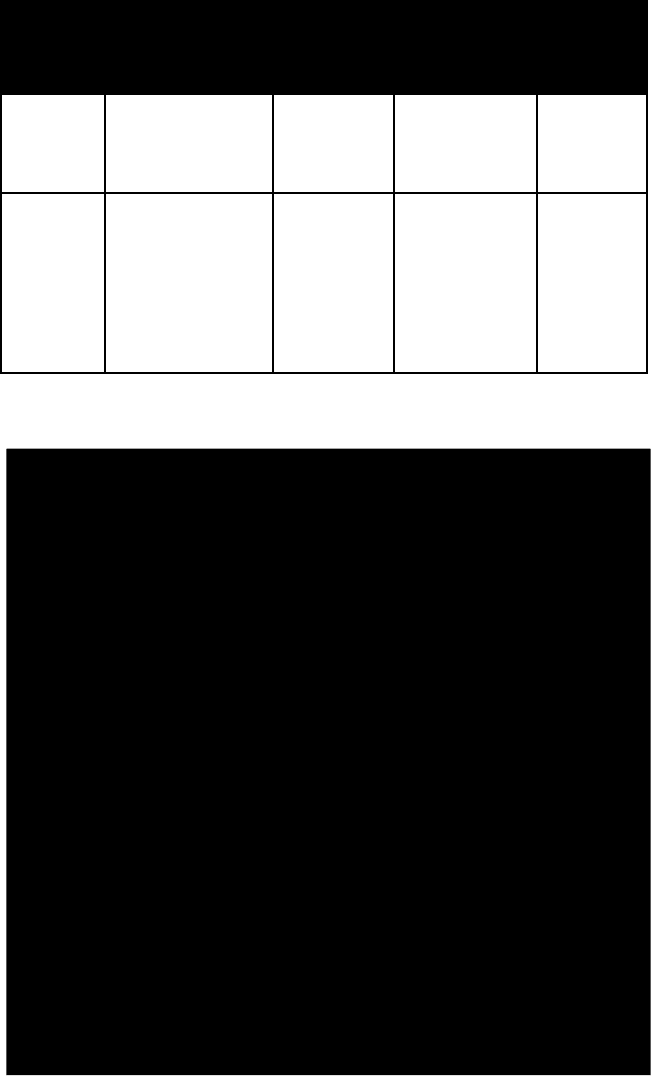
name habit frac-
ture or
cleavage
refrac-
tive
indices
crystal
system
polyhalite fibrous to foli-
ated massive
one perfect
cleavage
alpha = 1.547
beta = 1.560
gamma = 1.567
triclinic
thenard-
ite
rather large
crystals; crusts,
efflorescences
one
perfect,
one fair
cleavage
alpha =
1.464–1.471
beta =
1.473–1.477
gamma =
1.481–1.485
ortho-
rhombic
VANADATE MiNERAlS
Vanadate minerals are made up of naturally occurring compounds
of vanadium (V), oxygen (O), and various metals. Most of these
minerals are rare, having crystallized under very restricted condi-
tions. Although vanadinite occasionally is mined as a vanadium ore
and carnotite as a uranium ore, most vanadates have no economic
importance; they are prized by mineral collectors, however, for their
brilliant colours.
The structures of the vanadate minerals are complex. Some
vanadate minerals contain vanadate tetrahedra (VO
4
), in which
four oxygen atoms occupy the corners of a tetrahedron surround-
ing a central vanadium atom. Each vanadate tetrahedron has a net
charge of -3, which is neutralized by large, positively charged metal
ions (e.g., calcium, manganese, or ferrous iron) outside the tetra-
hedron. Unlike the similar silicate tetrahedra, which link to form
chains, sheets, rings, or frameworks, vanadate tetrahedra are insu-
lar. The vanadates containing these tetrahedra are structurally and
chemically similar to the phosphate and arsenate minerals; indeed,
some vanadium in many of these vanadates often is replaced by
phosphorus or arsenic, forming solid-solution series with both the
phosphates and the arsenates. Like the phosphate and sulfate miner-
als, many vanadates are complexes of transition metals, particularly
of ferrous iron, manganese, and copper.

306
7
Minerals 7
Other vanadates, particularly those that contain uranium, con-
tain V
2
O
8
6-
ions, in which two atoms of vanadium are surrounded by
eight atoms of oxygen arranged in two square pyramids that share one
edge. Very complex clusters also exist but are usually classed with the
complex oxide minerals rather than with the vanadate minerals.
name colour lustre Mohs
hard-
ness
specific
gravity
carnotite bright yellow to
lemon or greenish
yellow
dull or
earthy
soft 4–5
descloizite brownish red to
blackish brown;
various shades from
orange-red to black
and green
greasy 3–3½ 5.9–6.2
tyuyamu-
nite
canary yellow;
lemon to greenish
yellow
waxy; also
pearly
about 2 variable
with water
content
vanadinite various shades of
yellow, orange, red,
and brown
subresinous
to subada-
mantine
about 3 6.5–7.1
name habit or
form
frac-
ture or
cleavage
refrac-
tive
indices
crystal
system
carnotite powder of micro-
scopic platy or
lathlike crystals
one perfect
cleavage
alpha =
1.750
beta = 1.925
gamma =
1.950
monoclinic

307
7
carbonates and other Minerals 7
name habit or
form
frac-
ture or
cleavage
refrac-
tive
indices
crystal
system
des-
cloizite
crusts of inter-
grown crystals;
rounded fibrous
masses
no cleav-
age; uneven
fracture
desc mott
alpha =
2.18–2.21
beta =
2.25–2.31
gamma =
2.34–2.33
ortho-
rhombic
tyuy-
amunite
compact to crypto-
crystalline massive;
scales and lathlike
crystals; radiating
crystal aggregates
one perfect,
micalike
cleavage
ortho-
rhombic
vanadi-
nite
hairlike or barrel-
shaped (frequently
hollow) prismatic
crystals
uneven to
conchoidal
fracture
omega =
2.628–2.370
epsilon =
2.505–2.313
hexagonal
Sulfide Minerals
Sulfide minerals make up a group of compounds that join sul-
fur with one or more metals. Most of the sulfides are simple
structurally, exhibit high symmetry in their crystal forms,
and have many of the properties of metals, including metal-
lic lustre and electrical conductivity. They often are strikingly
coloured and have a low hardness and a high specific gravity.
The composition of the sulfide minerals can be
expressed with the general chemical formula AmSn, in
which A is a metal, S is sulfur, and m and n are integers, giv-
ing A
2
S, AS, A
3
S
4
and AS
2
stoichiometries. The metals that
occur most commonly in sulfides are iron, copper, nickel,
7 Minerals 7
308
lead, cobalt, silver, and zinc, though about fifteen others
enter sulfide structures.
Almost all sulfide minerals have structural arrange-
ments that belong to six basic types, four of which are
important. These arrangements are close-packing combina-
tions of metal and sulfur, governed by ionic size and charge.
The simplest and most symmetrical of the four impor-
tant structural types is the sodium chloride structure, in
which each ion occupies a position within an octahedron
consisting of six oppositely charged neighbours. The most
common sulfide crystalling in this manner is galena (PbS),
the ore mineral of lead. A type of packing that involves
two sulfide ions in each of the octahedral positions in the
sodium chloride structure is the pyrite structure. This is a
high-symmetry structure characteristic of the iron sulfide,
pyrite (FeS
2
O). The second distinct structural type is that
of sphalerite (ZnS), in which each metal ion is surrounded
by six oppositely charged ions arranged tetrahedrally. The
third significant structural type is that of fluorite, in which
the metal cation is surrounded by eight anions; each anion,
in turn, is surrounded by four metal cations. The reverse of
this structure—the metal cation surrounded by four anions
and each anion surrounded by eight metal cations—is called
the antifluorite structure. It is the arrangement of some of
the more valuable precious metal tellurides and selenides
among which is hessite (Ag
2
Te), the ore mineral of silver.
In virtually all of the sulfides, bonding is covalent, but
some have metallic properties. The covalent property of
sulfur allows sulfur-sulfur bonds and the incorporation of
S
2
pairs in some sulfides such as pyrite. Several sulfides,
including molybdenite (MoS
2
) and covellite (CuS), have
layer structures. Several rare sulfide varieties have the
spinel (q.v.) structure.
Phase relations of sulfides are particularly complex,
and many solid state reactions occur at relatively low
309
temperatures (100–300° C [212–572° F]), producing com-
plex intergrowths. Particular emphasis has been placed on
the experimental investigation of the iron-nickel-copper
sulfides because they are by far the most common. They
also are important geologic indicators for locating pos-
sible ore bodies and provide low-temperature reactions
for geothermometry.
Sulfides occur in all rock types. Except for dissemina-
tion in certain sedimentary rocks, these minerals tend to
occur in isolated concentrations which make up mineral
bodies such as veins and fracture fillings or which comprise
replacements of preexisting rocks in the shape of blan-
kets. Sulfide mineral deposits originate in two principal
processes, both of which have reducing conditions: (1) sep-
aration of an immiscible sulfide melt during the early stages
of crystallization of basic magmas; and (2) deposition from
aqueous brine solutions at temperatures in the 300–600 °C
(572–1,112 °F) range and at relatively high pressure, such as at
the seafloor or several kilometres beneath Earth’s surface.
The sulfide deposits formed as a result of the first process
include mainly pyrrhotites, pyrites, pentlandites, and chal-
copyrites. Most others occur because of the latter process.
Weathering may act to concentrate dispersed sulfides.
Pyrite (FeS
2
) and pyrrhotite (Fe
1 -
xS) are the most com-
mon sulfide minerals. Brassy yellow pyrite, often called
“fool’s gold,” occurs variously as an accessory mineral in
many rocks, in veins, and even as a chief component of
some fossils. Pyrrhotite, which typically has a bronze-
like appearance and is slightly magnetic, is a common
accessory mineral in mafic igneous rocks. Both of these
minerals, which are associated with each other in some
deposits, have yielded large quantities of sulfur recovered
for uses such as the production of sulfuric acid.
Sulfide minerals are the source of various precious
metals, most notably gold, silver, and platinum. They also
7 carbonates and other Minerals 7
7 Minerals 7
310
are the ore minerals of most metals used by industry, as for
example antimony, bismuth, copper, lead, nickel, and zinc.
Other industrially important metals such as cadmium and
selenium occur in trace amounts in numerous common
sulfides and are recovered in refining processes.
SulfIDe MINerAlS
name colour lustre M
ohs
hard-
ness
spe-
cific
gravity
argentite blackish lead-gray metallic 2–2 1/2 7.2–7.4
arsenopy-
rite
silver-white to
steel-gray
metallic 5 1/2–6 6.1
bornite copper-red to
pinchbeck-brown,
tarnishing quickly to
iridescent purple
metallic 3 5.1
chalcocite blackish lead-gray metallic 2 1/2–3 5.5–5.8
chalcopy-
rite
brass-yellow, often
tarnished and
iridescent
metallic 3 1/2–4 4.1–4.3
cinnabar cochineal-red to
brownish or lead-gray
adamantine
to metallic
2–2 1/2 8.1
cobaltite silver-white to red;
steel-gray or grayish
black
metallic 5 1/2 6.3
covellite indigo-blue; highly
iridescent; brass-
yellow or deep red
submetallic
to resinous
(crystals);
subresin-
ous to dull
(massive)
1 1/2–2 4.6–4.8
cubanite brass- to
bronze-yellow
metallic 3 1/2 4.0–4.2
311
name colour lustre Mohs
hard-
ness
spe-
cific
gravity
domey-
kite
tin-white to steel-
gray; tarnishes
yellowish brown,
becoming iridescent
metallic 3–3 1/2 7.2–7.9
galena lead-gray metallic 2 1/2–3 7.6
gree-
nockite
various shades of yel-
low and orange
adamantine
to resinous
3–3 1/2 4.9
krenne-
rite
silver-white to light
brass-yellow
metallic 2–3 8.6
linnaeite light gray to steel- or
violet-gray, tarnish-
ing to copper-red or
violet-gray
brilliant
metal-
lic (when
fresh)
4 1/2–5
1/2
4.5–4.8
loellingite silver-white to
steel-gray
metallic 5–5 1/2 7.4–7.5
marcasite tin-white, deepen-
ing with exposure to
bronze-yellow
metallic 6–6 1/2 4.9
mauch-
erite
reddish platinum-
gray, tarnishing
copper-red
metallic 5 8.0
metacin-
nabar
grayish black metallic 3 7.65
millerite pale brass-yellow,
tarnishing iridescent
gray
metallic 3–3 1/2 5.3–5.7
molybde-
nite
lead-gray metallic 1–1 1/2 4.6–4.7
niccolite pale copper-red,
tarnishing gray to
blackish
metallic 5–5 1/2 7.8
7 carbonates and other Minerals 7
