Pecharsky V.K., Zavalij P.Y. Fundamentals of Powder Diffraction and Structural Characterization of Materials
Подождите немного. Документ загружается.

652
Chapter 7
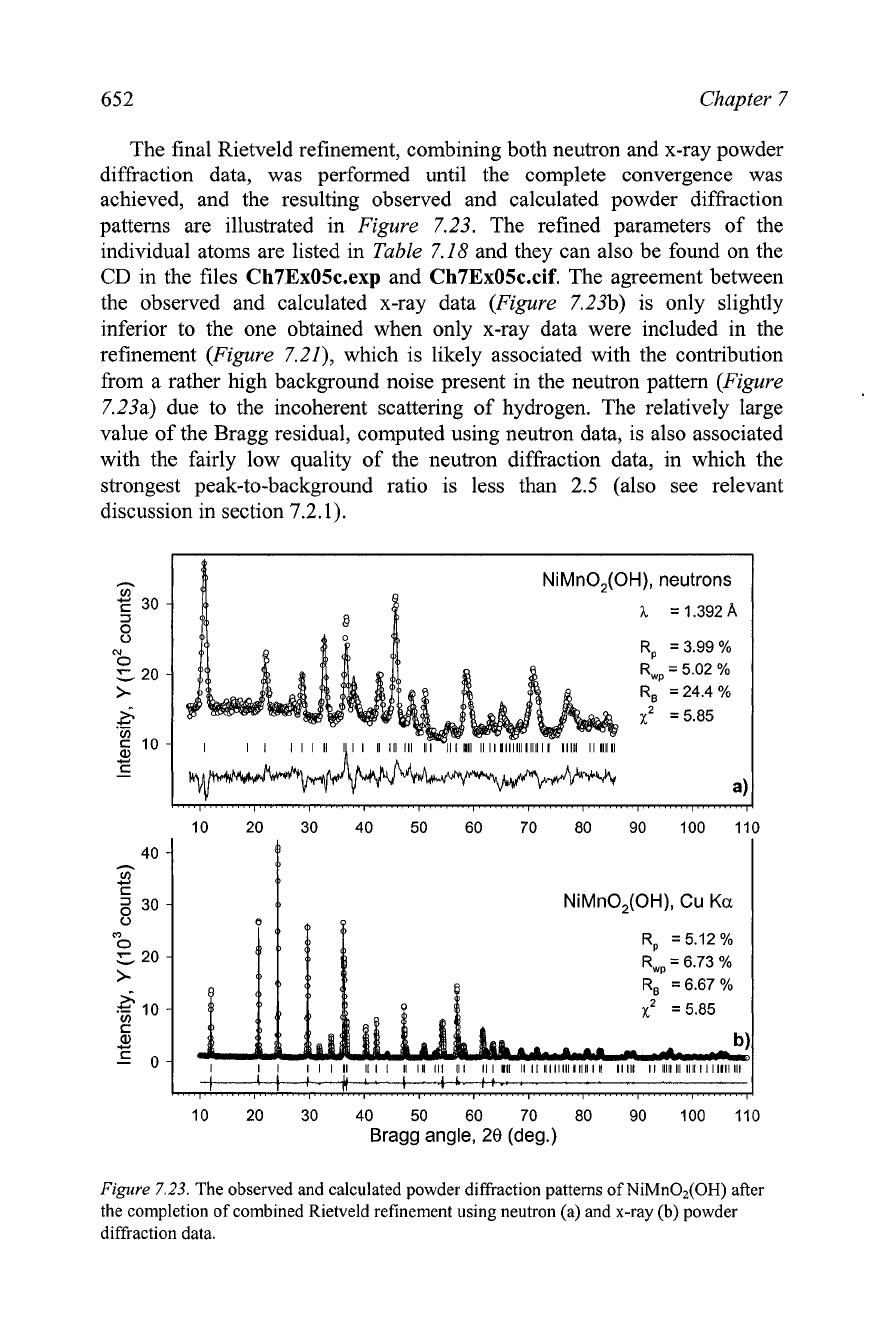
The final Rietveld refinement, combining both neutron and x-ray powder
diffraction data, was performed until the complete convergence was
achieved, and the resulting observed and calculated powder diffraction
patterns are illustrated in Figure 7.23. The refined parameters of the
individual atoms are listed in Table 7.18 and they can also be found on the
CD
in the files
Ch7ExOSc.exp
and
Ch7ExOSc.cif.
The agreement between
the observed and calculated x-ray data (Figure 7.23b) is only slightly
inferior to the one obtained when only x-ray data were included in the
refinement (Figure
7.21), which is likely associated with the contribution
from a rather high background noise present in the neutron pattern (Figure
7.23a) due to the incoherent scattering of hydrogen. The relatively large
value of the Bragg residual, computed using neutron data, is also associated
with the fairly low quality of the neutron diffraction data, in which the
strongest peak-to-background ratio is less than
2.5
(also see relevant
discussion in section
7.2.1).
NiMnO,(OH),
neutrons
h
=
1.392
A
10 20 30 40 50 60 70 80 90 100 110
Bragg angle,
28
(deg.)
Figure
7.23.
The observed and calculated powder diffraction patterns of NiMn02(OH) after
the completion of combined Rietveld refinement using neutron (a) and x-ray (b) powder
diffraction data.
Crystal structure refinement
65
3
The population parameter of hydrogen has been refined to a value of
62(5)
%
and, therefore, the chemical composition of the material is
NiMn03H8, or NiMn03-6(OH)6, where
6
=
0.62(5). Hence, a fraction of the
Mn atoms should be in the 4+ oxidation states. The latter was confirmed by
the magnetic susceptibility measurements. The preferred orientation
parameters, refined for both preferred orientation axes,
i.e. [O 101 and [loo],
are 0.74 and 1.40, respectively, resulting in the texture factors ranging
between 0.52 and 2.10, which corresponds to the preferred orientation
magnitude of about
4.
It appears that all pieces of this crystallographic puzzle are now in place
and they agree both with each other and with all available information.
These are: the chemical composition and the oxidation states of the metal
atoms; the crystal structure in general, including the distribution of Mn and
Ni atoms, and the amount and positions of hydrogen atoms; geometry, which
includes bond lengths, coordination polyhedra and hydrogen bonding; and
basic magnetic properties, which confirm a mixture of
Mn3' and Mn4+ in the
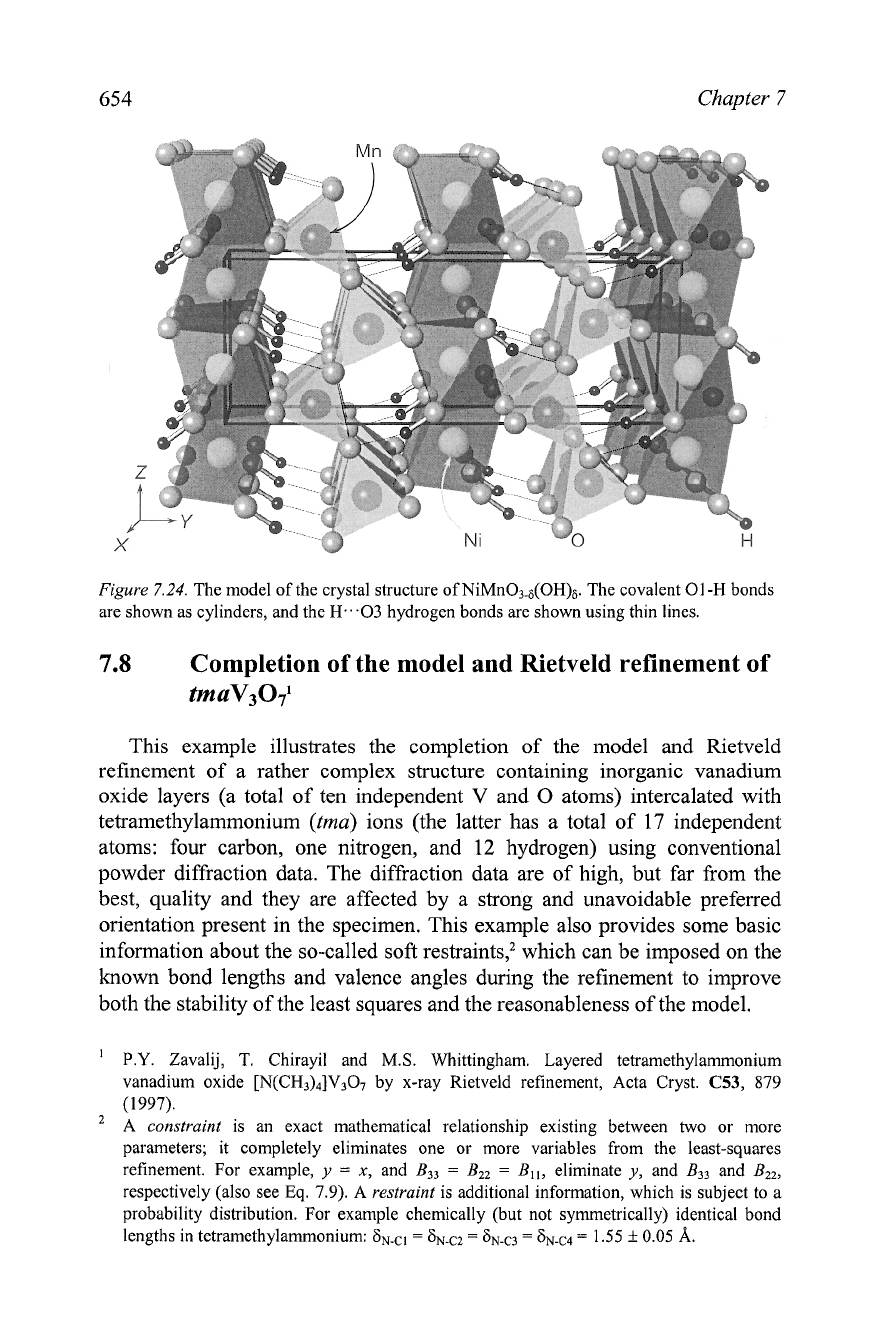
material. Thus, the fully determined and refined crystal structure of
NiMn03-6(OH)6 makes reasonable chemical and physical sense and its
complete model is illustrated in
Figure
7.24.
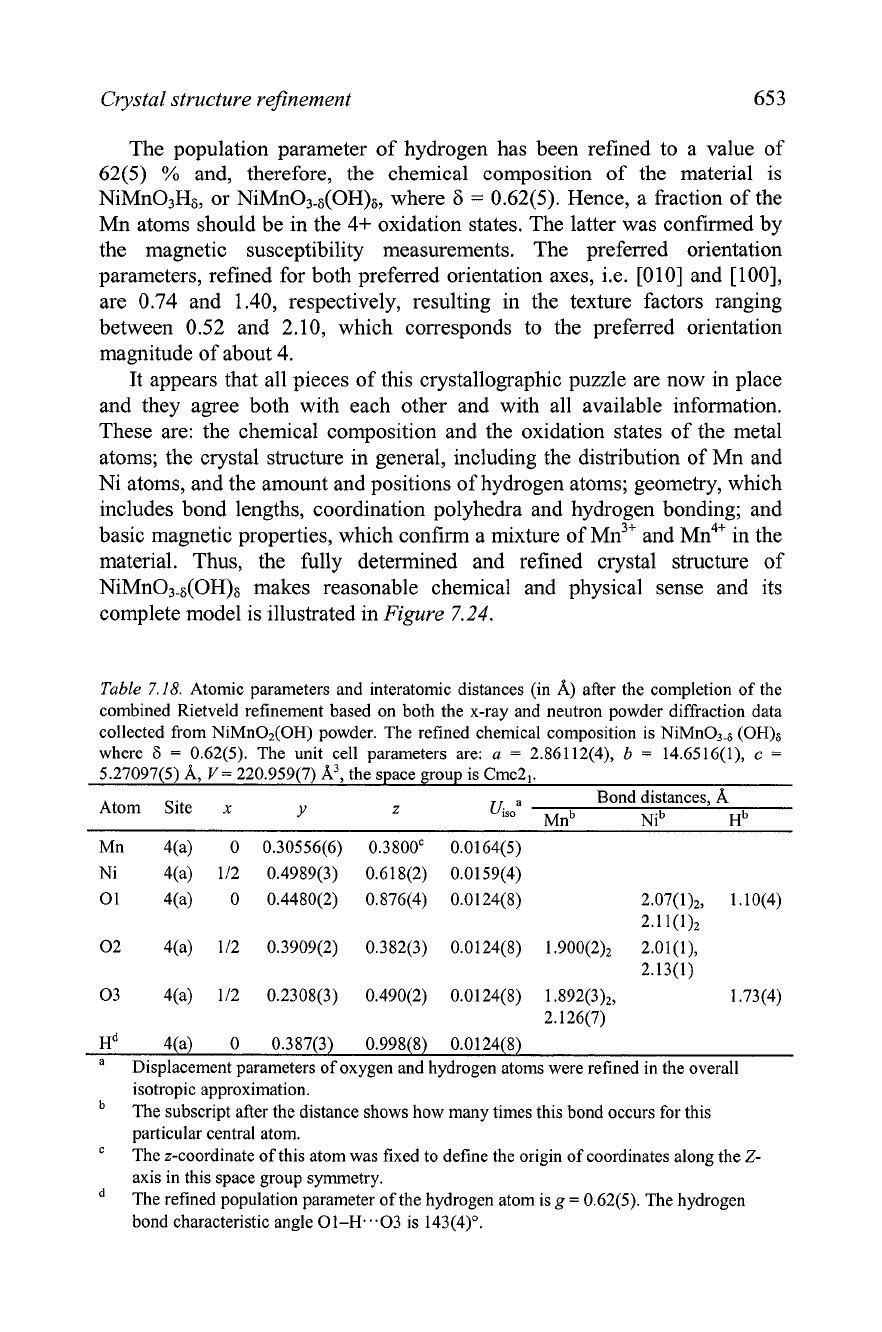
Table
7.18.
Atomic parameters and interatomic distances (in A) after the completion of the
combined Rietveld refinement based on both the x-ray and neutron powder diffraction data
collected from NiMn02(OH) powder. The refined chemical composition is NiMn03.6 (OH)6
where
6
=
0.62(5). The unit cell parameters are:
a
=
2.86112(4),
b
=
14.6516(1),
c
=
5.27097(5) A,
V=
220.959(7) A3, the space group is Cmc2,.
Atom Site
x
Bond distances, 8,
Y
z
Uisoa
Mnb
~i~
H~
Mn 4(a) 0 0.30556(6) 0.3800' 00.0164(5)
Ni 4(a) 112 0.4989(3) 0.618(2) 0.0159(4)
01 4(a) 0 0.4480(2) 0.876(4) 0.0124(8) 2.07(1),, 1.10(4)
2.1 1(1)2
02 4(a) 112 0.3909(2) 0.382(3) 0.0124(8) 1.900(2)2 2.01(1),
2.13(1)
03 4(a) 112 0.2308(3) 0.490(2) 0.0124(8) 1.892(3),, 1.73(4)
2.126(7)
H~
4(a) 0 0.387(3) 0.998(8) 0.0124(8)
a
Displacement parameters of oxygen and hydrogen atoms were refined in the overall
isotropic approximation.
The subscript after the distance shows how many times this bond occurs for this
particular central atom.
The z-coordinate of this atom was fixed to define the origin of coordinates along the
Z-
axis in this space group symmetry.
The refined population parameter of the hydrogen atom is
g
=
0.62(5). The hydrogen
bond characteristic angle Ol-H...03 is 143(4)O.
654
Chapter
7
Figure
7.24. The model of the crystal structure of NiMn03.8(OH)6. The covalent 01-H bonds
are shown as cylinders, and the H...03 hydrogen bonds are shown using thin lines.
7.8
Completion of the model and Rietveld refinement of
tmaV3071
This example illustrates the completion of the model and Rietveld
refinement of a rather complex structure containing inorganic vanadium
oxide layers (a total of ten independent
V
and
0
atoms) intercalated with
tetramethylammonium (tma) ions (the latter has a total of 17 independent
atoms: four carbon, one nitrogen, and
12
hydrogen) using conventional
powder diffraction data. The diffraction data are of high, but far from the
best, quality and they are affected by a strong and unavoidable preferred
orientation present in the specimen. This example also provides some basic
information about the so-called soft restraints: which can be imposed on the
known bond lengths and valence angles during the refinement to improve
both the stability of the least squares and the reasonableness of the model.
P.Y.
Zavalij,
T.
Chirayil and M.S. Whittingham. Layered tetramethylarnmonium
vanadium oxide [N(CH3)4]V307 by x-ray Rietveld refinement, Acta Cryst.
C53,
879
(1997).
. .
A
constraint
is an exact mathematical relationship existing between two or more
parameters; it completely eliminates one or more variables from the least-squares
refinement. For example,
y
=
x,
and B33
=
BZ2
=
BI1, eliminate
y,
and B33 and B22,
respectively (also see
Eq.
7.9).
A
restraint
is additional information, which is subject to a
probability distribution. For example chemically (but not symmetrically) identical bond
lengths in tetramethylarnmonium: 8N-CI
=
=
6N-C3
=
tiNwC4
=
1.55
*
0.05
A.

Crystal structure
refinement
655
The experimental pattern was collected with a 0.02' step from 7 to 50'
29 and a counting time of 60 seclstep, and from 50 to 69O 29 with a counting
time of 90 seclstep. The high Bragg angle intensities were scaled to the 60
seclstep counting time for all calculations. The following initial parameters
were used in the model completion and refinement:
-
The initial model of the crystal structure was taken from Table
6.40.
-
The default profile parameters were taken from the instrumental
parameter file
Scintag.prm
(see section 7.6).
-
The sample shift parameter
S,
=
8.94 corresponds to the sample
displacement s
=
-0.195 mm, which was determined together with the
unit cell dimensions during the least squares refinement of lattice
parameters.
-
The space group is P2Jn and the unit cell dimensions are a
=
18.482, b
=
6.5526,
c
=
8.4297 8L,
P
=
91.103', as determined earlier.
-
The overall isotropic displacement parameter,
U,,,
=
0.015 8L2.
The starting model is found on the
CD
in the files
Ch7Ex06a.exp
and
Ch7Ex06a.cif
together with all relevant structural parameters, and the
experimental powder pattern is located in the file
Ch7Ex06-CuKa.raw.
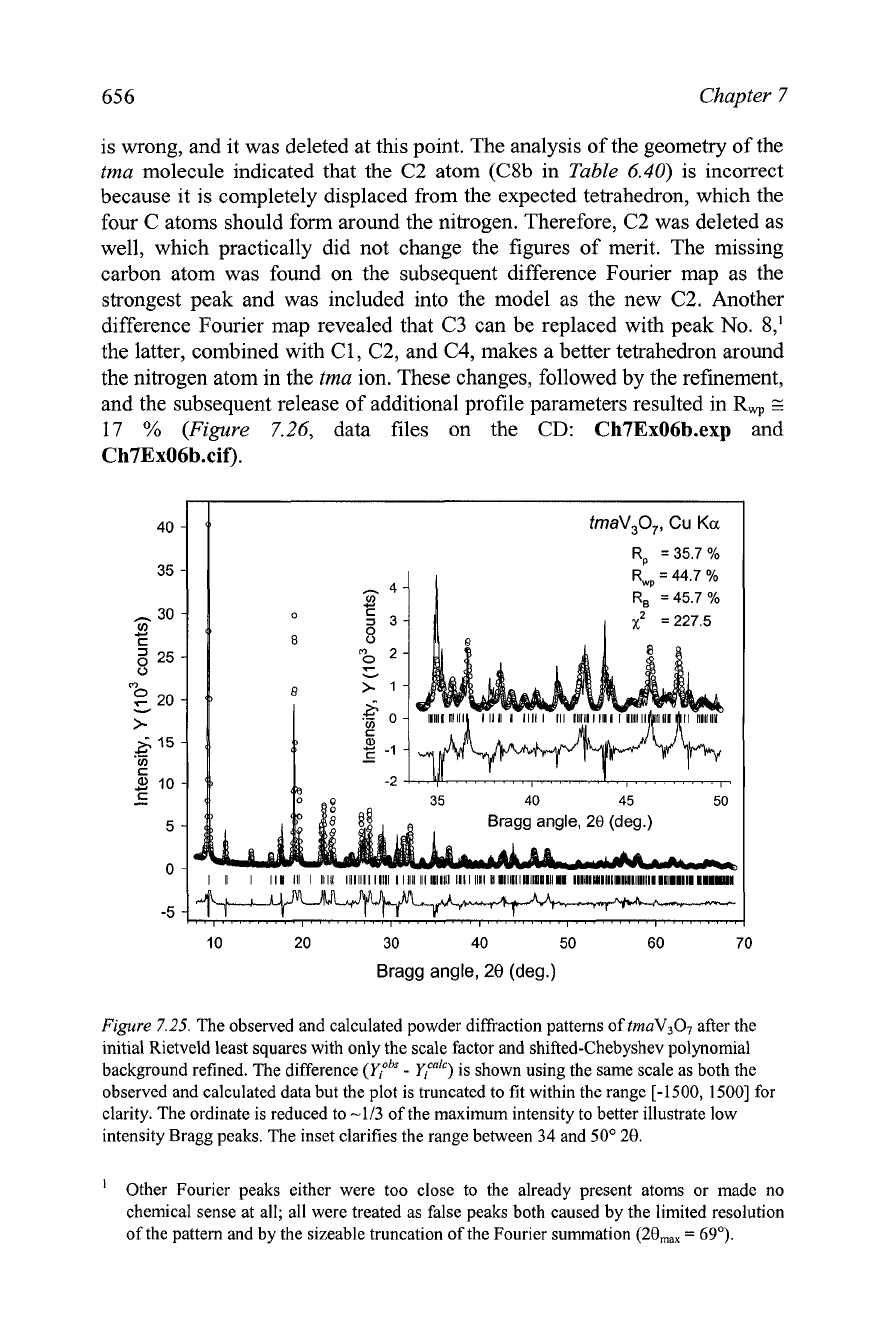
Initially, only the scale factor and 6 coefficients for a shifted-Chebyshev
polynomial approximation of the background were refined. The resulting
residuals, which are shown in the second row of Table 7.19, are higher than
one could expect for a nearly complete model (all non-hydrogen atoms were
thought to be located). As can be seen from the inset in Figure 7.25, both the
calculated intensities and peak shapes are far off from their observed values.
The intensity mismatch may be associated, to a certain extent, with a
considerable preferred orientation, which is expected from the highly
anisotropic shapes of the crystallites (see the inset in Figure 6.26) and nearly
guaranteed easy cleaving of the particles along the planes parallel to the
vanadate layers expanded by the tetramethylammonium. Therefore, the
subsequent refinement included the grain size broadening parameter
(X)
and
the preferred orientation along the [loo] axis, resulting in some improvement
of the fit.
The next refinement step included the unit cell dimensions and peak
asymmetry, noticeably lowering the residuals. At this point, the difference
Fourier map was calculated, which revealed a potential missing atom. The
coordinates of this peak were incorporated into the model as 08. This
addition, together with the refinement of the coordinates and individual
Ui,,
of all atoms, performed in order to perhaps detect erroneously positioned
atoms (one of the eight oxygen atoms is obviously false), substantially
improves the fit, lowering
R,
from -30 to -20
%.
The analysis of individual
Ui,,
(Table 7.20) shows that one of them is extremely high:
U,,,
E
0.6
8L2
for
07. This atom has a short distance to V1 (0.78
A)
suggesting that its location
656
Chapter 7
is wrong, and it was deleted at this point. The analysis of the geometry of the
tma molecule indicated that the C2 atom (C8b in Table
6.40)
is incorrect
because it is completely displaced from the expected tetrahedron, which the
four C atoms should form around the nitrogen. Therefore, C2 was deleted as
well, which practically did not change the figures of merit. The missing
carbon atom was found on the subsequent difference Fourier map as the
strongest peak and was included into the model as the new C2. Another
difference Fourier map revealed that C3 can be replaced with peak No.
8,'
the latter, combined with C1, C2, and C4, makes a better tetrahedron around
the nitrogen atom in the tma ion. These changes, followed by the refinement,
and the subsequent release of additional profile parameters resulted in
R,
z
17
%
(Figure 7.26, data files on the CD:
Ch7Ex06b.e~~
Ch7Ex06b.cif).
and
10 20 30 40 50 60 70
Bragg angle,
20
(deg.)
Figure
7.25.
The observed and calculated powder diffraction patterns of tmaV307 after the
initial Rietveld least squares with only the scale factor and shifted-Chebyshev polynomial
background refined. The difference
(Y:~"
-
yicnlc)
is shown using the same scale as both the
observed and calculated data but the plot is truncated to fit within the range [-1500, 15001 for
clarity. The ordinate is reduced to -113 of the maximum intensity to better illustrate low
intensity Bragg peaks. The inset clarifies the range between 34 and 50"
20.
'
Other Fourier peaks either were too close to the already present atoms or made no
chemical sense at all; all were treated as false peaks both caused by the limited resolution
of the pattern and by the sizeable truncation of the Fourier summation
(20,,,
=
69").
Crystal structure refinement
657
Table 7.19. The progress of Rietveld refinement of the crystal structure of tmaV307 from x-
ray powder diffraction data.
Refined parameters RD RWD
RB
xZ
Initial (CD:
Ch7Ex06-CuKa.raw, Ch7Ex06a.exp,
and 60.7 68.6 99.4 535
-
Ch7Ex06a.cif)
Scale, shifted-Chebyshev polynomial background (Figure 7.25)
Plus grain broadening
(X)
and preferred orientation, PO, with
texture axis
[I
001
Plus unit cell dimensions and asymmetry,
a
Added 08; coordinates and Uindi, included, Table 7.20
Deleted 07 and C2
Added C2 and replaced C3 from a difference Fourier map
(Figure 7.26) (CD:
Ch7Ex06b.e~~
and
Ch7Ex06b.cif)
Strain broadening
(Y),
asymmetry
(a),
and sample shift
Scale, background, and unit cell dimensions: first with porosity
(absorption)
a,
and a2; and then with PO [loo],
X,
sample shift
and overall Uiso
Excluded 8 to 12" 29; PO [Ol 01, then
Y,
a,
X,,
Ya
Plus coordinates of V and
0
Plus coordinates of N and C
Plus PO axes ratio, individual Uiso(V), overall Uis,(0) and Uiso
(N, C) (Figure 7.27) (CD:
Ch7Ex06c.e~~
and
Ch7Ex06c.cif)
Plus soft restraints on C-N distances and C-N-C angles (Table
7.21) (CD:
Ch7ExO6d.e~~
and
Ch7ExO6d.cif)
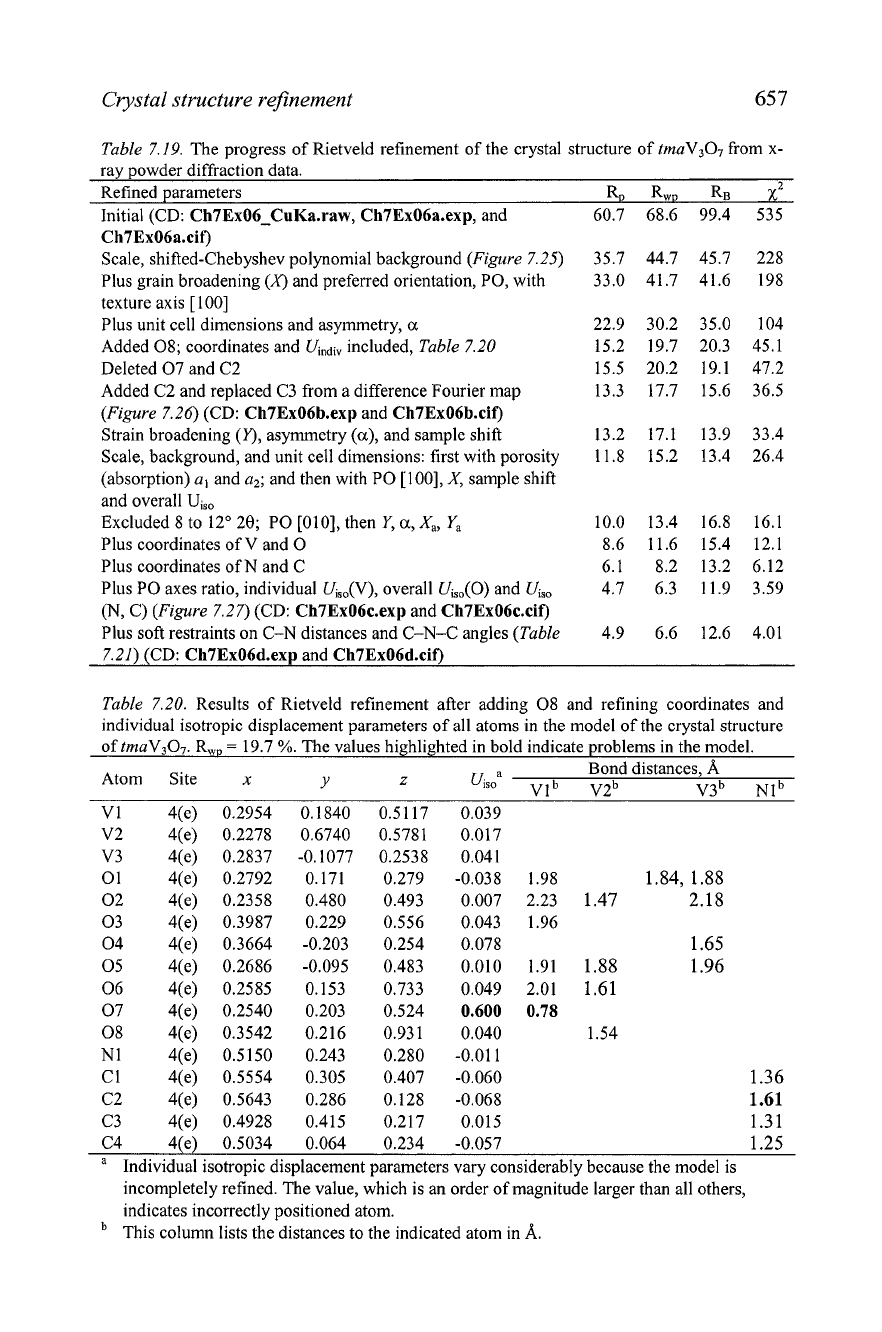
Table 7.20. Results of Rietveld refinement after adding 08 and refining coordinates and
individual isotropic displacement parameters of all atoms in the model of the crystal structure
of tmaV3O7. R,,,,
=
19.7
%.
The values highlighted in bold indicate problems in the model.
Atom Site
x
Bond distances,
A
Y
Z
Ui~~a
Vl
b
V2b
~3' ~1~
VI 4(e) 0.2954 0.1840 0.5117 0.039
C4 4(e) 0.5034 0.064 0.234 -0.057 1.25
a
Individual isotropic displacement parameters vary considerably because the model is
incompletely refined. The value, which is an order of magnitude larger than all others,
indicates incorrectly positioned atom.
This column lists the distances to the indicated atom in
A.
Chapter
7
tmaV,O,,
Cu
Ka
R,
=13.3%
R,,
=
17.7
%
10 20 30 40 50 60 70
Bragg angle,
28
(deg.)
Figure
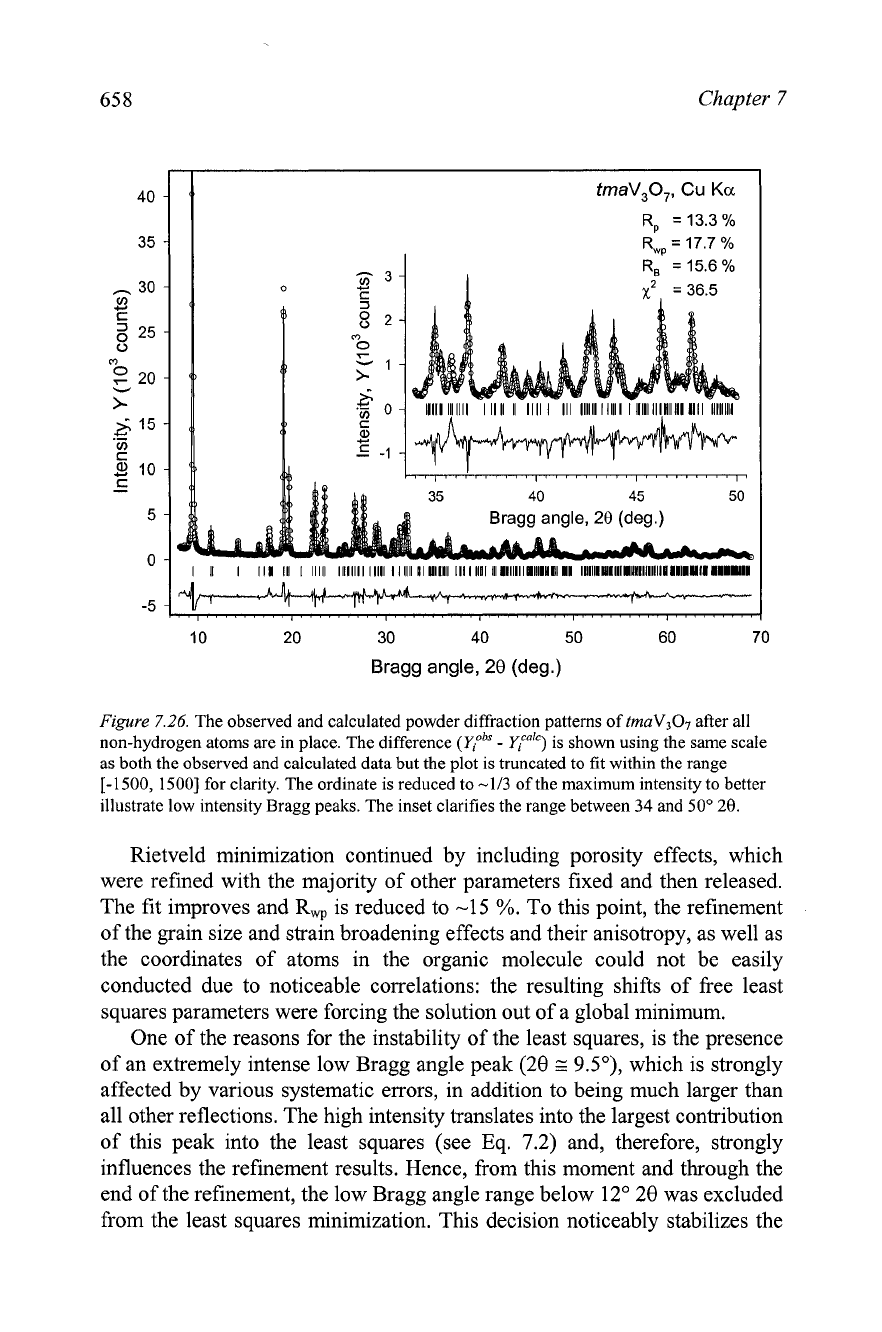
7.26.
The observed and calculated powder diffraction patterns of
tmaV307
after all
non-hydrogen atoms are in place. The difference
(qobs
-
ylCaIC)
is shown using the same scale
as both the observed and calculated data but the plot is truncated to fit within the range
1-1500,
15001 for clarity. The ordinate is reduced to
-113
of the maximum intensity to better
illustrate low intensity Bragg peaks. The inset clarifies the range between
34
and
SO0
20.
Rietveld minimization continued by including porosity effects, which
were refined with the majority of other parameters fixed and then released.
The fit improves and R, is reduced to -15
%.
To this point, the refinement
of the grain size and strain broadening effects and their anisotropy, as well as
the coordinates of atoms in the organic molecule could not be easily
conducted due to noticeable correlations: the resulting shifts of free least
squares parameters were forcing the solution out of a global minimum.
One of the reasons for the instability of the least squares, is the presence
of an extremely intense low Bragg angle peak (28
G
9.5"), which is strongly
affected by various systematic errors, in addition to being much larger than
all other reflections. The high intensity translates into the largest contribution
of this peak into the least squares (see Eq. 7.2) and, therefore, strongly
influences the refinement results. Hence, from this moment and through the
end of the refinement, the low Bragg angle range below 12" 28 was excluded
from the least squares minimization. This decision noticeably stabilizes the
Crystal structure refinement
65
9
least squares, although it has little influence on all figures of merit, and the
latter is actually quite unexpected.'
Next, a second preferred orientation axis,
[OlO],
was included into the
minimization, which was based both on the model of the crystal structure
(the orientation of chains in the
vanadate layers) and on the observed shapes
of the crystallites (the plates are elongated in one direction). The refinement
was completed by subsequently releasing the coordinates of atoms forming
an inorganic framework (the
V
and
0
atoms) and then organic molecule (the
N
and
C
atoms), after which the individual Ui,, for the metal atoms (Vl-V3)
and overall
U,,,
for the oxygen atoms and the organic molecule (two
different overall parameters) together with all profile parameters except for
U,
V,
Wand
P.
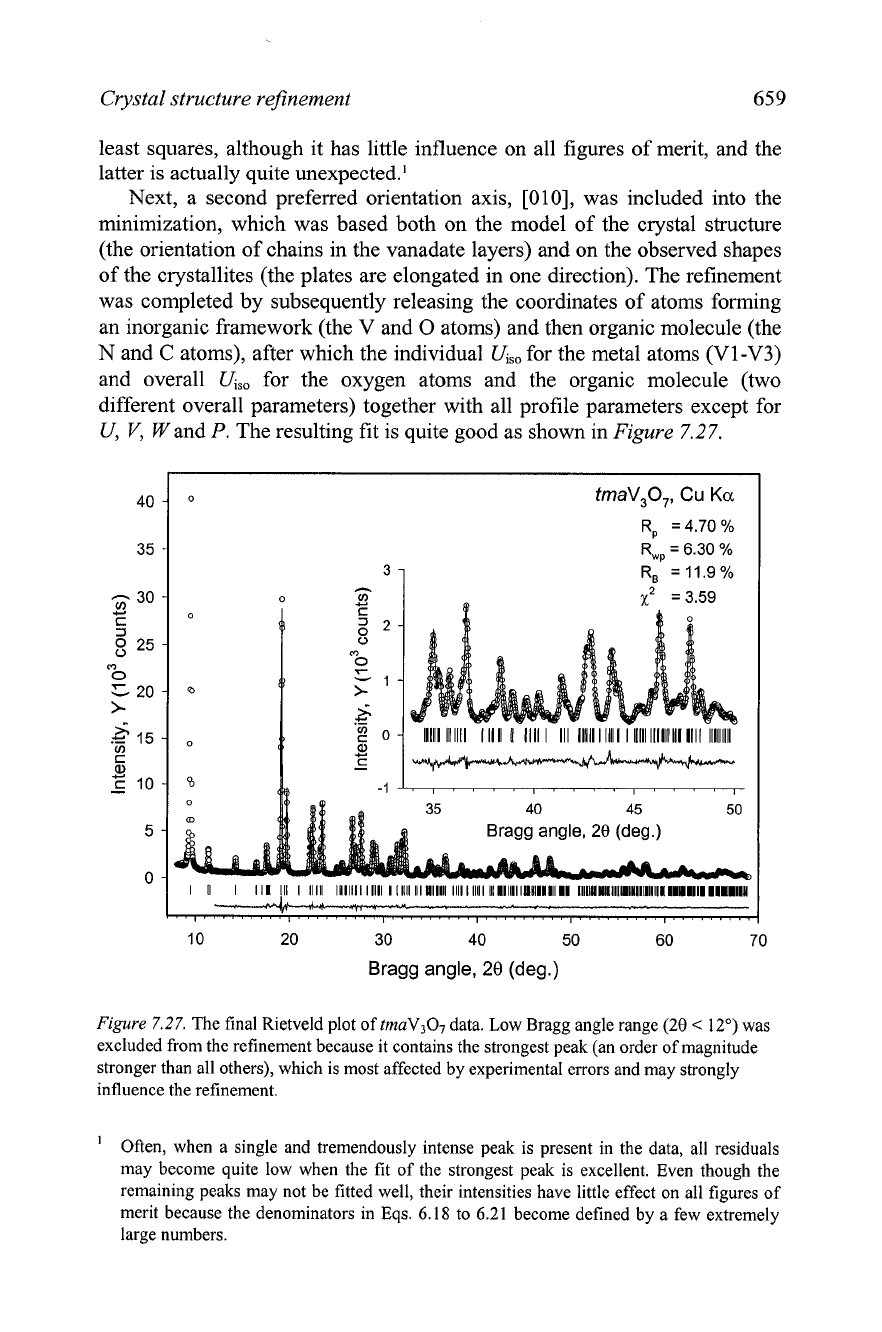
The resulting fit is quite good as shown in
Figure
7.27.
R,
=
4.70
%
R,,
=
6.30
%
R,
=
11.9%
Bragg angle,
20
(deg.)
I
II I
1
I1
111
1
11111
1111111 11111
l
11111
111
111111 1111 111111 IU IIIIIIIIIIIIIIIII
II
lllllUlllllUlYllllllllllllNllll
IltlllU
10 20 30 40 50 60 70
Bragg angle,
20
(deg.)
Figure 7.27.
The final Rietveld plot of tmaV307 data. Low Bragg angle range
(20
<
12")
was
excluded from the refinement because it contains the strongest peak (an order of magnitude
stronger than all others), which is most affected by experimental errors and may strongly
influence the refinement.
'
Often, when a single and tremendously intense peak is present in the data, all residuals
may become quite low when the fit of the strongest peak is excellent. Even though the
remaining peaks may not be fitted well, their intensities have little effect on all figures of
merit because the denominators in Eqs.
6.18
to
6.21
become defined by a few extremely
large numbers.

660
Chapter
7
Despite the reasonable fit between the observed and calculated
intensities, the analysis of the interatomic distances and bond angles reveals
that the geometry of the tetramethylammonium ion, though acceptable
considering data quality, quantity,' and complexity, is slightly out of the
expected range. The geometry was improved by using the so-called soft
restraint^,^
which are realized in GSAS. The limits on the C-N bond lengths
were set to 1.500
+
0.015
A
and the values of C-N-C angles were restrained
to 109.5
+
1.5". The weights for bonds and angles were manually selected as
4
and 6, respectively, after testing their influence on the least squares.
Application of soft restraints slightly increases all residuals, as seen from a
comparison of the two last rows in
Table
7.19 but it substantially improves
the geometry of the organic molecule
(Table
7.21). Before the soft-restrained
refinement, the
C-N
bond lengths were in the 1.48 to 1.67
A
range with the
average bond length 1.56
A.
The C-N-C angles varied from 100 to 127O,
and the spread from the ideal tetrahedral angle was quite large. After the
refinement with the soft restraints, the C-N bond lengths fall within the 1.50
to 1.55
A
range and bond angles are between 106 and 1 14O, both of which
are acceptable. It is worth noting that instead of soft restraints, a rigid-body
refinement of the
tma
molecule, whose geometry is well known, could also
be employed (the rigid body approach is yet another method of geometrical
restrictions, which is realized in GSAS).
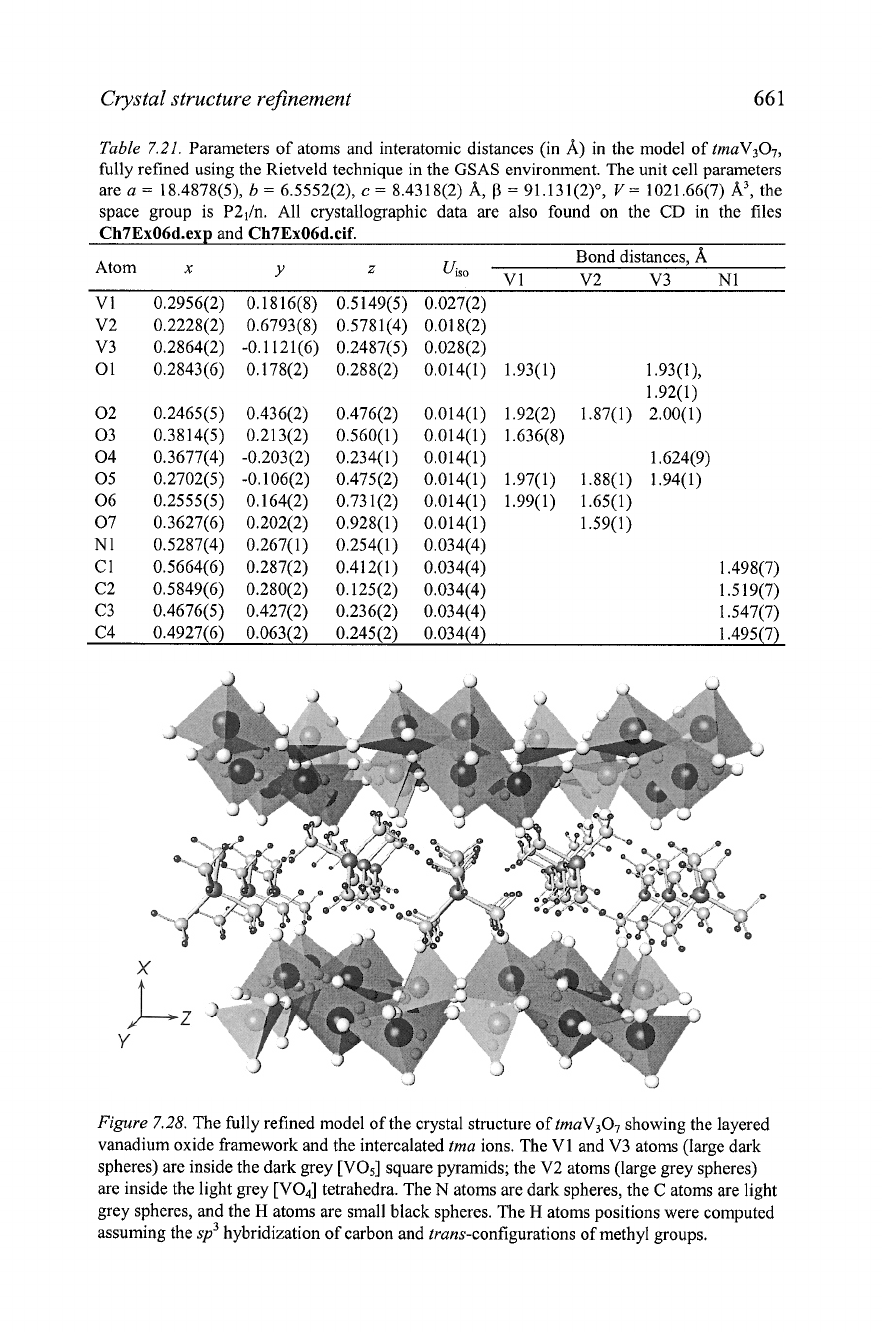
The final Rietveld refinement yields a reasonable structural model
(Figure
7.28), which fits nicely as a new member in the series of V307-based
structures with vanadium oxide layers differing only by the orientations of
the square pyramids and
tet~ahedra.~
A
specific feature of this refinement is a
strong preferred orientation along two axes, [loo] and [OlO], with
parameters
T~~~
=
0.76 and
T~~~
=
1.38 in a
2
to 1 ratio. The preferred
orientation multipliers range from 0.58 to 2.08, which results in a total
magnitude of about 4, similar to the case described in the previous section.
Due to the presence of the large number of lightly scattering atoms, intensity diffracted by
this powder specimen becomes extremely low above
20
=
70'
(sinO/h
<
0.37
A-').
In a
typical powder diffraction experiment, it is necessary to collect the data to sinO/h
z
0.5
A-I,
preferably to even higher values.
Soft restraints set limits on the bond distances andlor angles, which are known and are
desired to reach or keep during the least squares minimization. Soft restraints are not exact
(see the footnote on page
654),
and the specified geometry may never be achieved when
distances and angles are set to non-realistic values. The influence of soft restraints, when
compared to a straightforward profile fitting, is regulated by varying the so-called weight,
which can be increased to force the geometry closer to the desired values. The latter
generally considerably worsens the fit if actual bond angles and distances contradict
diffraction data.
P.Y.
Zavalij,
F.
Zhang, and MS. Whittingham, Crystal structure of layered
bis(ethy1enediamine)nickel
hexavanadate as a new representative of the
V6Ol4
series. Acta
Cryst.
B55,953 (1999).
Crystal structure re$nement
66
1
Table
7.21.
Parameters of atoms and interatomic distances (in A) in the model of tmaV307,
fully refined using the Rietveld technique in the GSAS environment. The unit cell parameters
are
a
=
18.4878(5), b
=
6.5552(2),
c
=
8.4318(2) A,
P
=
91.131(2)O,
V
=
1021.66(7) A', the
space group is P2,In. All crystallographic data are also found on the
CD
in the files
Ch7Ex06d.e~~
and
Ch7Ex06d.cif.
Atom
x
Bond distances,
A
Y
z
90
V1 V2 V3 N1
Vl 0.2956(2) 0.1816(8) 0.5149(5) 0.027(2)
Figure
7.28.
The fully refined model of the crystal structure of tmaV307 showing the layered
vanadium oxide framework and the intercalated tma ions. The V1 and V3 atoms (large dark
spheres) are inside the dark grey [V05] square pyramids; the V2 atoms (large grey spheres)
are inside the light grey [VOJ tetrahedra. The
N
atoms are dark spheres, the C atoms are light
grey spheres, and the
H
atoms are small black spheres. The
H
atoms positions were computed
assuming the
sp3
hybridization of carbon and trans-configurations of methyl groups.
