Pecharsky V.K., Zavalij P.Y. Fundamentals of Powder Diffraction and Structural Characterization of Materials
Подождите немного. Документ загружается.


Fundamentals of diffraction
21
1
Yet another level of complexity of vibrational motion is taken into
account by using the so-called anharmonic approximation of atomic
displacement parameters. One of the commonly used approaches is the
cumulant expansion formalism suggested by Johnson,' in which the structure
factor is given by the following general expression:
n
F(h)
=
&fJ
(s) exp(2trih
.
xJ
-
Pll
h, h,
-
j=1
where
-
PJkl
are the anisotropic displacement parameters (see Eq. 2.93);2
-
yJklm are the third order anharmonic displacement parameters;
-
8Jklmn are the fourth order displacement parameters. The expansion in Eq.
2.98 may be continued to include fifth, sixth, and so on order terms. The
sign of the corresponding term is determined from the sign of
iP
where
p
is the order of the anharmonic term;
-
k, 1, m, n (p, q, etc., in the higher order expansions) vary between
1
and
3;
-
hk, hl, h, and h, (h,, h,, etc.) are the corresponding Miller indices (hl
=
h,
h2
=
k,
and h3
=
I,
respectively);
-
other notations are identical to
Eq.
2.87.
Displacement parameters are included in
Eq.
2.97 with all possible
permutations of indices. Thus, for a conventional anisotropic approximation
after considering the diagonal symmetry of the corresponding tensor (Eq.
2.96)
the already known expression for the exponential factor in Eq. 2.93 is easily
obtained. Following the same procedure it is possible to show that in the
case of the third order anharmonic expansion the number of the independent
'
C.K.
Johnson, in: Thermal neutron diffraction: proceedings of the International Summer
School at Harwell, 1-5 July 1968 on the accurate determination of neutron
intensities and structure factors, p. 132-160,
B.T.M.
Willis, Ed., London, Oxford
University Press (1970).
In this treatment, the conventional harmonic anisotropic displacement model may be
considered as a "second order" approximation.

212
Chapter
2
atomic displacement parameters is increased by 10 (yl
yzz2, y333, y1 12, y113,
Y~zz, 7133, y221, y223,
Y233
and y123).' Similarly, the maximum number of
parameters per atom in the fourth order expansion is increased by 1 5 (a1
62222, 83333, 61112, 61113, 61122, 61133, 81123, 61222, 61223, 61233, 61333, 62223, 62233
and 62333), fifth order by
2
1, and so on.
A brief description of the anharmonic approximation is included here for
completeness since rarely, if ever, it is possible to obtain reasonable atomic
displacement parameters of this complexity from powder diffraction data:
the total number of atomic displacement parameters of an atom in the fourth
order anharmonic approximation may reach 31
(6
anisotropic
+
10 third
order
+
15 fourth order). The major culprits preventing their determination in
powder diffraction are uncertainty of the description of Bragg peak shapes,
non-ideal models to account for the presence of preferred orientation, and
the inadequacy of accounting for porosity.
2.1
1.4
Atomic scattering factor
As briefly mentioned above (see section 2.5.2), the ability to scatter
radiation varies depending on the type of an atom and therefore, the general
expression of the structure amplitude contains this factor as a multiplier
(Eq.
2.87). For x-rays, the scattering power of various atoms and ions is
proportional to the number of core electrons. Therefore, it is measured using
a relative scale normalized to the scattering ability of an isolated electron.
The x-ray scattering factors depend on the radial distribution of the electron
density around the nucleus and they are also functions of Bragg angle.
When neutrons are of concern, their coherent scattering by nuclei is
independent of the Bragg angle and the corresponding factors remain
constant for any Bragg reflection. Scattering factors of different isotopes are
represented in terms of coherent scattering lengths of a neutron and are
expressed in femtometers (1 fm
=
lo-'' m).
The best known scattering factors for x-rays and scattering lengths for
neutrons of all chemical elements are listed for common isotopes and their
naturally occurring mixtures (neutrons) and for neutral atoms and common
ions (x-rays) in the International Tables for
Crystall~graphy.~
Just as in the case of the conventional anisotropic approximation, the maximum number of
displacement parameters is only realized for atoms located in the general site position (site
symmetry 1). In special positions some or all of the displacement parameters will be
constrained by symmetry. For example,
y3,,, yl13,
y223
and
y12,
for an atom located in the
mirror plane perpendicular to Z-axis are constrained to
0.
Furthermore, if an atom is
located in the center of inversion, all parameters of the odd order anharmonic tensors
(3,
5,
etc.) are reduced to
0.
International Tables for Crystallography, vol. C,
2nd
edition, A.J.C. Wilson and
E.
Prince,
Eds., Second edition, Kluwer Academic Publishers, Boston/Dordrecht/London (1 999).
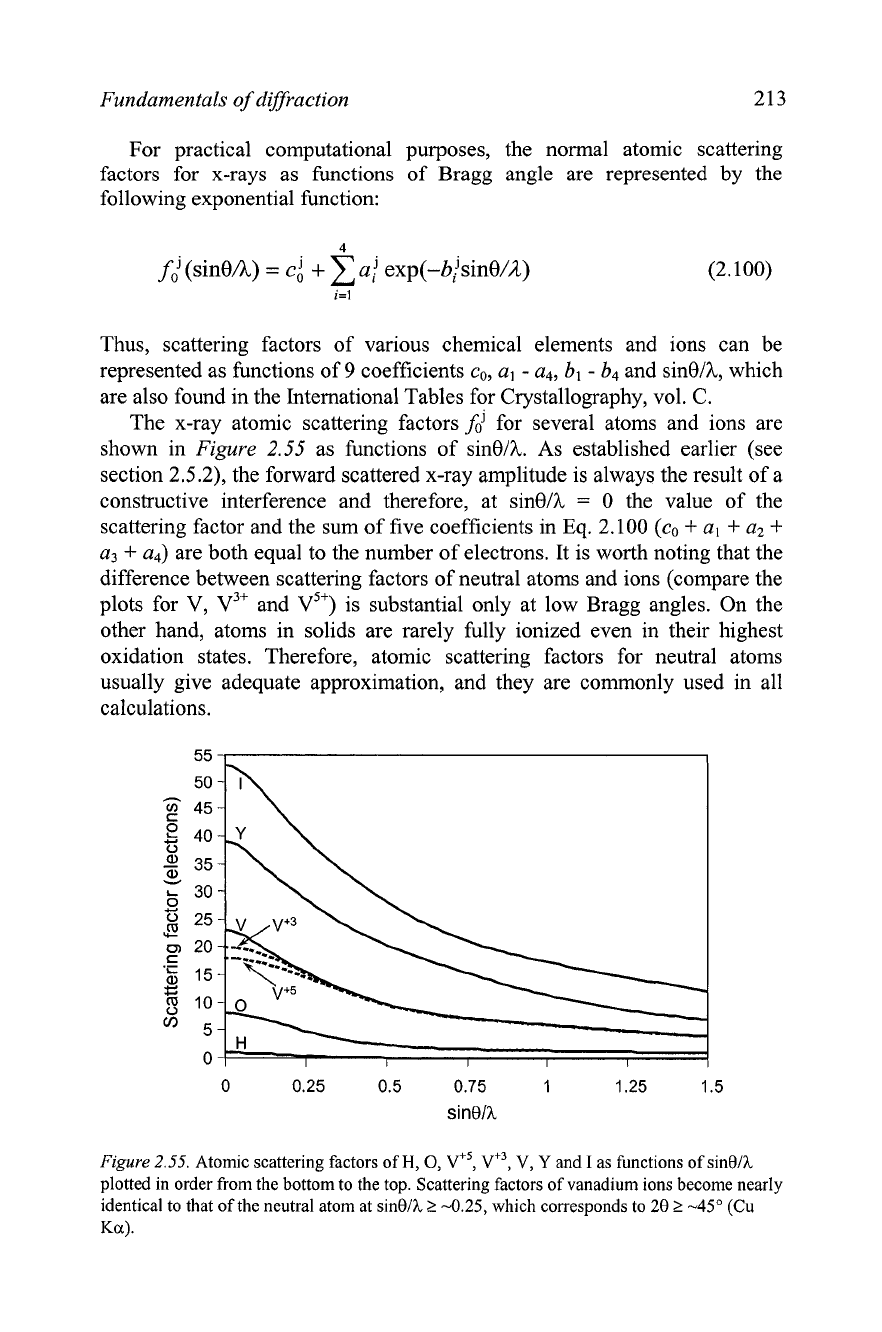
Fundamentals of diffraction 213
For practical computational purposes, the normal atomic scattering
factors
for x-rays as functions of Bragg angle are represented by the
following exponential function:
Thus, scattering factors of various chemical elements and ions can be
represented as functions of
9
coefficients
co,
a,
-
a4, bl
-
b4 and sinelh, which
are also found in the International Tables for Crystallography, vol.
C.
The x-ray atomic scattering factors
fd
for several atoms and ions are
shown in Figure
2.55
as functions of sinelh. As established earlier (see
section 2.5.2), the forward scattered x-ray amplitude is always the result of a
constructive interference and therefore, at sinell,
=
0 the value of the
scattering factor and the sum of five coefficients in Eq. 2.100 (co
+
a,
+
a2
+
a3
+
a4) are both equal to the number of electrons. It is worth noting that the
difference between scattering factors of neutral atoms and ions (compare the
plots for V,
v3+
and v5+) is substantial only at low Bragg angles. On the
other hand, atoms in solids are rarely fully ionized even in their highest
oxidation states. Therefore, atomic scattering factors for neutral atoms
usually give adequate approximation, and they are commonly used in all
calculations.
Figure
2.55.
Atomic scattering factors of
H,
0,
vf5,
v+~,
V,
Y
and
I
as functions of sin01h
plotted in order from the bottom to the top. Scattering factors of vanadium ions become nearly
identical to that of the neutral atom at
sinO/h
2
-0.25, which corresponds to
28
t
-45"
(Cu
Ka)
.

214
Chapter
2
Considering
Figure
2.53 and
Figure
2.55, both the temperature and
scattering factors decrease exponentially with sin01A. Hence, when the
displacement parameter of an individual atom becomes unphysical during a
least squares refinement, this usually means that the improper type of an
atom has been placed in the suspicious site. If the chemical element placed
in a certain position has fewer electrons than it should, then during the
refinement its displacement
parameter(s) becomes negative or much lower
than those of the correctly placed atoms. This reduction in the displacement
parameters(s) (Eq. 2.91 to 2.95) offsets the inadequately low scattering
factor (Eq. 2.100) by increasing the temperature factor. Similarly, when the
site has too many electrons than it really does, displacement parameter(s) of
the corresponding atom will be unusually high during the refinement.
Similar effects are observed in neutron diffraction.
Anomalous displacement
parameter(s) will be also observed when an
atom is placed in a position that is empty or only partially occupied
(i.e. the
site has sizeable defects).
In
this case, displacement parameter(s) become
extremely large, effectively reducing the contribution from this atom to the
structure factor (see
Figure
2.53). All sites, which have unusually low or
unusually high displacement parameter(s), should be tested by refining
population
parameter(s), while setting the displacement parameters at some
reasonable values to avoid possible and sometimes severe correlations. This
refinement may reveal the nature of the chemical element located in a
specific position by analyzing the number of electrons (or scattering length
in neutron diffraction).
For example, assume that a Ni atom has been placed in a certain position
and a least squares refinement results in a large and positive isotropic
displacement parameter for this site but displacement parameters of other
atoms are in the "normal" range. After the population parameter of Ni has
been refined while keeping its displacement parameter fixed at the average
value of other "normal" atoms, the resulting
gNi
=
0.5. Considering
Figure
Ni Ni
-
2.55, the approximate number of electrons in this position is equal to
Z
g
-
28~0.5
=
14
e.
Hence, the refinement result points to one of the following: i)
the site is occupied by Ni atoms at 50
%
population (50
%
of the unit cells
have Ni in this position and in the remaining 50
%,
the same position is
vacant), or ii) the site is occupied by the element that has approximately 14
electrons,
i.e. Si. Depending on many other factors (e.g. precision of the
experiment) the same position may be also occupied by
A1
(13
electrons),
P
(1
5
electrons) or by other neighboring atoms.
The final decision about the population of this "Ni" site can be made only
when all available data about the material are carefully considered: i) which
chemical elements may be present in this sample or were used during its
preparation, if known? ii) what are the results of the chemical analysis? iii)

Fundamentals of diffraction 215
what is the gravimetric density of the material? and iv) what is the
environment of this particular position? For example, an octahedral
coordination usually points to
A1 but not Si or
P.
It should be emphasized that the calculation of the number of electrons
described above is not exact since x-ray scattering factors are only directly
proportional to the number of electrons at 20
=
0" and the proportionality
becomes approximate at sinelk
>
0. The population parameter(s) refinement
results are usually more reliable in neutron diffraction because neutron
scattering lengths are independent of the Bragg angle.
Normal x-ray scattering factor,
fo
(Eq.
2.100) describes the scattering
ability of different atoms as a function of sinell. (or interplanar distance, d,
since
sinelk
=
112d) and, therefore, is wavelength independent. This is only
true for light chemical elements and relatively short wavelengths. Most
atoms scatter x-rays anomalously and their scattering factors become
functions of the wavelength.' Anomalous scattering is taken into account by
including two additional parameters into the overall scattering factor of each
chemical element in the following form:
where:
-
s
is sinelk;
-
fbj
is the normal atomic scattering factor that depends only on the type of
the scattering atom (number of electrons) and is a function of sine/h;
-
~fj'
and
~f
j''
are the real and imaginary components, respectively, of the
anomalous scattering factor and they depend on both the atom type and
the wavelength.
The anomalous scattering coefficients are also listed in the International
Tables for Crystallography for all chemical elements and commonly used
wavelengths of laboratory x-rays. They can be measured or calculated for
any wavelength, which is important when using synchrotron
radiati~n.~ The
anomalous scattering is usually at least an order of magnitude lower than
normal scattering. Generally, the magnitude of the anomalous scattering
coefficients is proportional to the wavelength and inversely proportional to
the number of electrons in an atom. Anomalous scattering is at its maximum
when the wavelength is near the corresponding absorption edge of an atom.
Some isotopes also scatter neutrons anomalously.
The most precise values are obtained from experimental measurements. When one of the
two anomalous scattering coefficients is measured, the second can be relatively precisely
calculated using Kramers
-
Kronig equation. More details about the anomalous scattering
can be found in a special literature, e.g. see
J.
Als-Nielsen and
D.
McMorrow, Elements of
modern x-ray physics, John Wiley
&
Sons, Ltd., New York
(2001).

216
Chapter
2
2.11.5
Phase
angle
The structure amplitude, expressed earlier as a sum of exponents, can be
also represented in a different format. Thus, by applying Euler's formula
ek
=
cosx
+
isinx
(2.102)
Eq. 2.89 becomes
The sums of cosines and sines in Eq. 2.103 signify the real (A) and
imaginary (B) components of a complex number, respectively, which the
structure amplitude indeed is. Hence, considering the notations introduced in
Eq. 2.87, Eq. 2.103 can be rewritten as:
Complex numbers can be represented as vectors in two dimensions with
two mutually perpendicular axes: real and imaginary. Accordingly, the
complex structure amplitude (Eq. 2.104) may be imagined as a vector
P
and
its real and imaginary components are the projections of this vector on the
real and imaginary axes, respectively, as shown schematically in
Figure
2.56,
left. From simple geometry, the following relationships are true:
where IF(h)l, IA(h)l and IB(h)l are the lengths of the corresponding vectors
(or their absolute values), and a(h) is the angle that vector F(h) makes with
the positive direction of the real axis, also known as the phase angle (or the
phase) of the structure amplitude. It is worth noting that since
A(h) and B(h)
are mutually perpendicular, the phase shift between the imaginary and real
components of the structure amplitude is always +n/2.
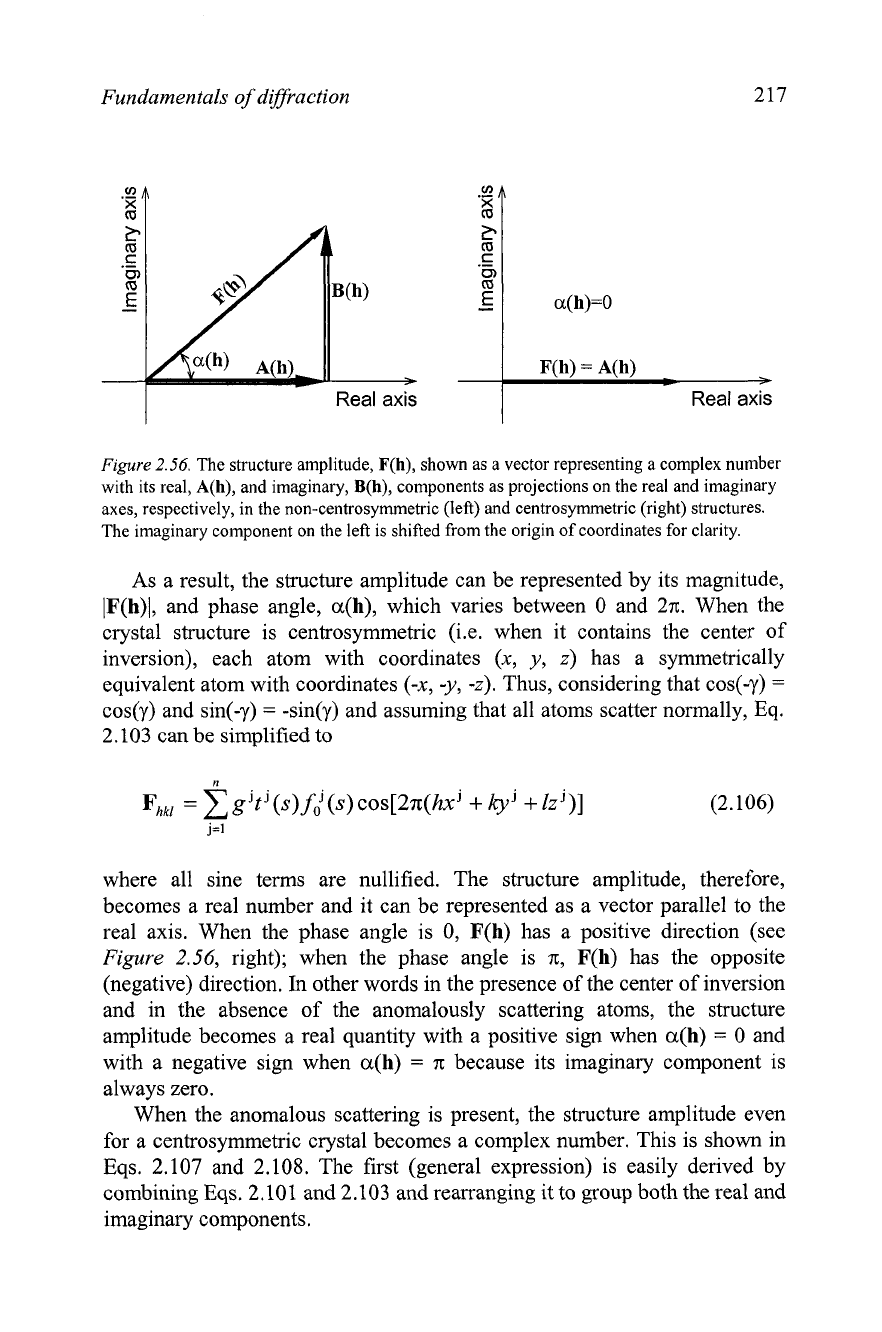
Fundamentals
of
diffraction
Real axis
I
Real axis
Figure
2.56.
The structure amplitude, F(h), shown as a vector representing a complex number
with its real, A(h), and imaginary,
B(h),
components as projections on the real and imaginary
axes, respectively, in the non-cenhosyrnmetric (left) and centrosymrnetric (right) structures.
The imaginary component on the left is shifted from the origin of coordinates for clarity.
As a result, the structure amplitude can be represented by its magnitude,
IF(h)l, and phase angle, a(h), which varies between 0 and 2n. When the
crystal structure is centrosymmetric (i.e. when it contains the center of
inversion), each atom with coordinates (x, y,
z)
has a symmetrically
equivalent atom with coordinates (-x, -y,
-z).
Thus, considering that cos(-y)
=
cos(y) and sin(-y)
=
-sin(y) and assuming that all atoms scatter normally, Eq.
2.103 can be simplified to
where all sine terms are nullified. The structure amplitude, therefore,
becomes a real number and it can be represented as a vector parallel to the
real axis. When the phase angle is 0, F(h) has a positive direction (see
Figure
2.56,
right); when the phase angle is n, F(h) has the opposite
(negative) direction.
In
other words in the presence of the center of inversion
and in the absence of the anomalously scattering atoms, the structure
amplitude becomes a real quantity with a positive sign when a(h)
=
0 and
with a negative sign when a(h)
=
7~
because its imaginary component is
always zero.
When the anomalous scattering is present, the structure amplitude even
for a centrosymmetric crystal becomes a complex number. This is shown in
Eqs. 2.107 and 2.108. The first (general expression) is easily derived by
combining Eqs. 2.10 1 and 2.10
3
and rearranging it to group both the real and
imaginary components.
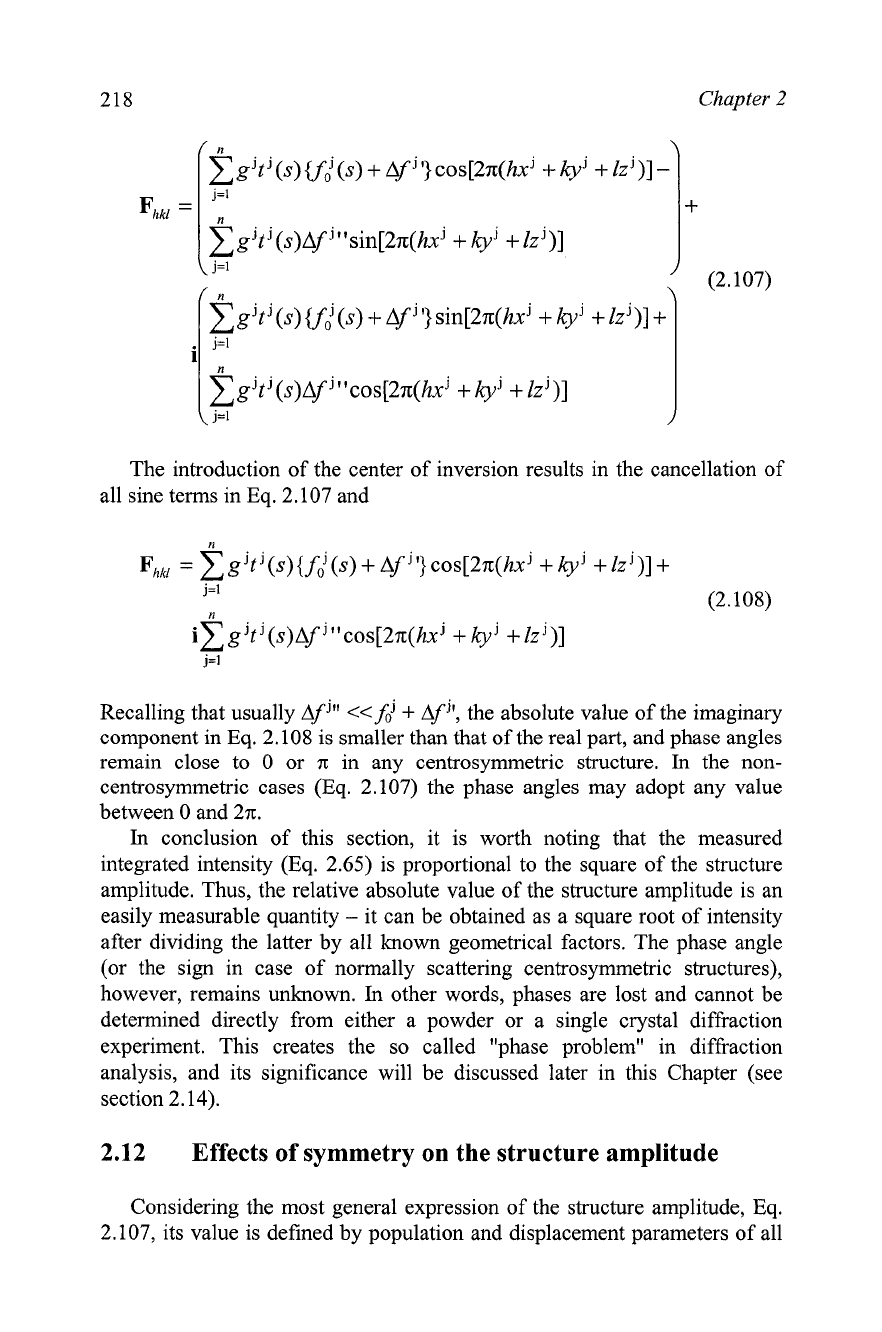
g't
J
(s)
~fi
(s)
+
Af
j
jl
cos[2n(hxJ
+
kyj
+
lzj)]
-
j=1
1
I
(2.107)
g't' (s)
~f,'
(s)
+
Af
'
I}
sin[2n(hx'
+
ky
j
+
b'
)]
+
Zg'tJ (s)Af
J
"cos[2n(hx'
+
kyJ
+
lzJ)]
j=1
Chapter
2
+
The introduction of the center of inversion results in the cancellation of
all sine terms in Eq. 2.107 and
Recalling that usually
Af
j"
<<fdj
+
Af
'I, the absolute value of the imaginary
component in Eq. 2.108 is smaller than that of the real part, and phase angles
remain close to
0 or
n:
in any centrosymmetric structure.
In
the
non-
centrosymmetric cases (Eq. 2.107) the phase angles may adopt any value
between 0 and 2n.
In
conclusion of this section, it is worth noting that the measured
integrated intensity (Eq. 2.65) is proportional to the square of the structure
amplitude. Thus, the relative absolute value of the structure amplitude is an
easily measurable quantity
-
it can be obtained as a square root of intensity
after dividing the latter by all known geometrical factors. The phase angle
(or the sign in case of normally scattering centrosymmetric structures),
however, remains unknown.
In
other words, phases are lost and cannot be
determined directly from either a powder or a single crystal diffraction
experiment. This creates the so called "phase problem" in diffraction
analysis, and its significance will be discussed later in this Chapter (see
section 2.14).
2.12
Effects of symmetry on the structure amplitude
Considering the most general expression of the structure amplitude, Eq.
2.107, its value is defined by population and displacement parameters of all
Fundamentals of diffraction
219
atoms present in the unit cell, their scattering ability and coordinates. We
know that coordinates and other parameters of atoms in the unit cell are
related by symmetry. As was shown in Chapter 1 (Eqs. 1.39 and
1.48), the
coordinates of two symmetrically equivalent atoms (x and xl) are related as
where
x
=
(x,
y,
z, 1) x'
=
(x',
y',
z', 1) and
A
is an augmented matrix
representing a symmetry operation. By expanding Eq. 2.109, substituting the
result to express the identical arguments of sine and cosine parts in Eq. 2.107
and regrouping it as shown in Eq. 2.1 10
27th.
x'=
27t
~(~~11
+
k~l
+Ir31)
+
~(~~12
+
kr22
+Ir,,)
+
I
(2.1 10)
z(hr13
+
4,
+
Ir3,)
+
(ht,
+
kt2 +It3)
it is easy to see that the symmetry of the direct space (crystal structure) is
carried over into the reciprocal space by modifying structure amplitudes.
Thus, the contribution of two symmetrically equivalent atoms
x
and x' into
the corresponding reciprocal lattice points, h and h', may be expressed as
where
h
and
h'
are augmented reciprocal lattice vectors, and
is obtained
from
A
by transposing the rotational part of the augmented matrix:
I
and
=
As a result of symmetry transformation (Eqs. 2.1 11 and 2.1 12), both the
magnitude of the structure amplitude and its phase may change. Finite
symmetry operations
(tl, t2
and
t3
are all 0) usually affect the phase angle,
while infinite operations, i.e. those which have a non-zero translational
component, affect both the magnitude and the phase.
2.12.1
Friedel pairs and Friedel's law
We begin with considering the relationships between the structure
-
--
amplitudes of two centrosyrnmetric reciprocal lattice points: (hkl) and (h kl),
220
Chapter
2
the so-called Friedel pair. The analysis is relatively simple and is based on
the known relationships between the three trigonometric functions:
cos(-x)
=
cos(x),
but
(2.1 13)
sin(-x)
=
-
sin(x) and tan(-x)
=
-
tan(x)
Regardless of whether the crystal structure is centrosymmetric or not, in
the absence of the anomalous scattering it directly follows from Eqs. 2.103,
2.104, 2.105 and2.113 that
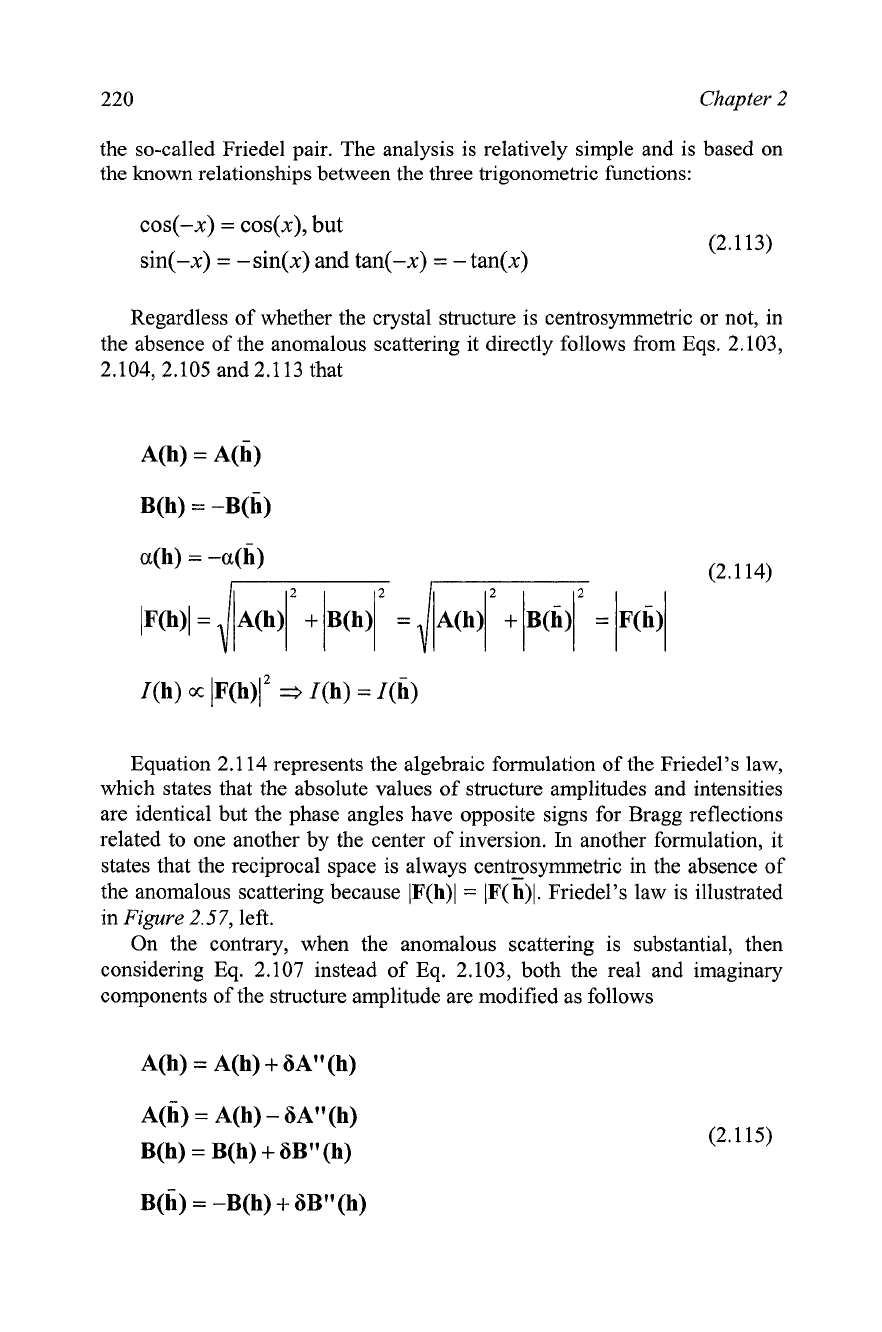
Equation 2.1 14 represents the algebraic formulation of the Friedel's law,
which states that the absolute values of structure amplitudes and intensities
are identical but the phase angles have opposite signs for Bragg reflections
related to one another by the center of inversion.
In
another formulation, it
states that the reciprocal space is always centrosymmetric in the absence of
the anomalous scattering because IF(h)l
=
lF(h)l. Friedel's law is illustrated
in
Figure
2.57,
left.
On the contrary, when the anomalous scattering is substantial, then
considering Eq. 2.107 instead of Eq. 2.103, both the real and imaginary
components of the structure amplitude are modified as follows
A(h)
=
A(h)
+
6A" (h)
~(h)
=
A(h)
-
6A" (h)
B(h)
=
B(h)
+
6B
"
(h)
~(h)
=
-B(h)
+
6B" (h)
