Pecharsky V.K., Zavalij P.Y. Fundamentals of Powder Diffraction and Structural Characterization of Materials
Подождите немного. Документ загружается.


Fundamentals of diffraction
waves behave as particles (e.g. photons), and particles (e.g. neutrons and
electrons) behave as waves with wavelength
h
determined by the de Broglie
equation:
where
h
is Planck's constant
(h
=
6.626~10"~ Jas),
m
is the particle's rest
mass, and v is the particle's velocity (mv
=
p,
particle momentum).
For example, a neutron (rest mass,
m
=
1.6749~10-~~ kg) moving at a
constant velocity v
=
3000 m/s will also behave as a wave with
h
=
1.3
19
A.
Furthermore, charged particles, e.g. electrons, can be focused using magnetic
lenses. Thus, modem high-resolution electron microscopes allow direct
imaging of atomic structures (for the most part in two dimensions on a
surface) with the resolution sufficient to distinguish individual atoms. Direct
imaging methods, however, require sophisticated equipment and the
accuracy in determining atomic positions is substantially lower than that
possible by means of diffraction techniques.' Hence, direct visualization of a
structure with atomic resolution is invaluable in certain applications but the
three-dimensional crystal structures are determined exclusively from
diffraction data.
The process of transforming diffraction patterns in order to reinstate the
underlying crystal structures in the three-dimensional direct space is
governed by the theory of diffraction. The latter rests on several basic
assumptions, yet it is accurate and practical. We have no intent to cover the
comprehensive derivation of the x-ray diffraction theory since it is mainly of
interest to experts, and can be found in many excellent books and
review^.^
Therefore, in this chapter we will discuss the nature and sources of radiation
that are in common use today and consider the principles and fundamental
laws of diffraction in general. We will also consider diffraction from
a
crystalline matter
-
specifically from polycrystalline materials
-
and describe
diffraction pattern as a function of crystal symmetry, atomic structure and
conditions of the experiment.
Despite recent progress in the three-dimensional x-ray holography [e.g. see M. Tegze,
G.
Faigel, S. Marchesini,
M.
Belakhovsky, and A. I. Chumakov, Three-dimensional
imaging of atoms with isotropic 0.5
A
resolution, Phys. Rev. Lett.
82,4847
(1999)], which
in principle enables visualization of the atomic structure in three dimensions, its accuracy
in determining coordinates of atoms and interatomic distances is much lower than possible
by employing conventional diffraction methods.
For example, see the International Tables for Crystallography, vol.
B,
Second edition,
U.
Shmueli, Ed. (2001) and vol. C, Second edition, A.J.C. Wilson and E. Prince, Eds. (1999),
Published for the International Union for Crystallography by Kluwer Academic
Publishers,
Boston/Dordrecht/London,
and references therein.

Chapter
2
2.2
Properties and sources of radiation
Nearly immediately after their discovery, x-rays were put to use to study
the internal
structure of objects that are opaque to visible light but
transparent to x-rays,
e.g. parts of a human body using radiography, which
takes advantage of varying absorption: bones absorb x-rays stronger than
surrounding tissues. It is interesting to note that the lack of understanding of
their nature, which did not occur until 19 12, did not prevent the introduction
of x-rays into medicine and engineering. Today the nature and the properties
of x-rays and other types of radiation are well understood and they are
briefly considered in this section.
2.2.1
Nature and properties of x-rays
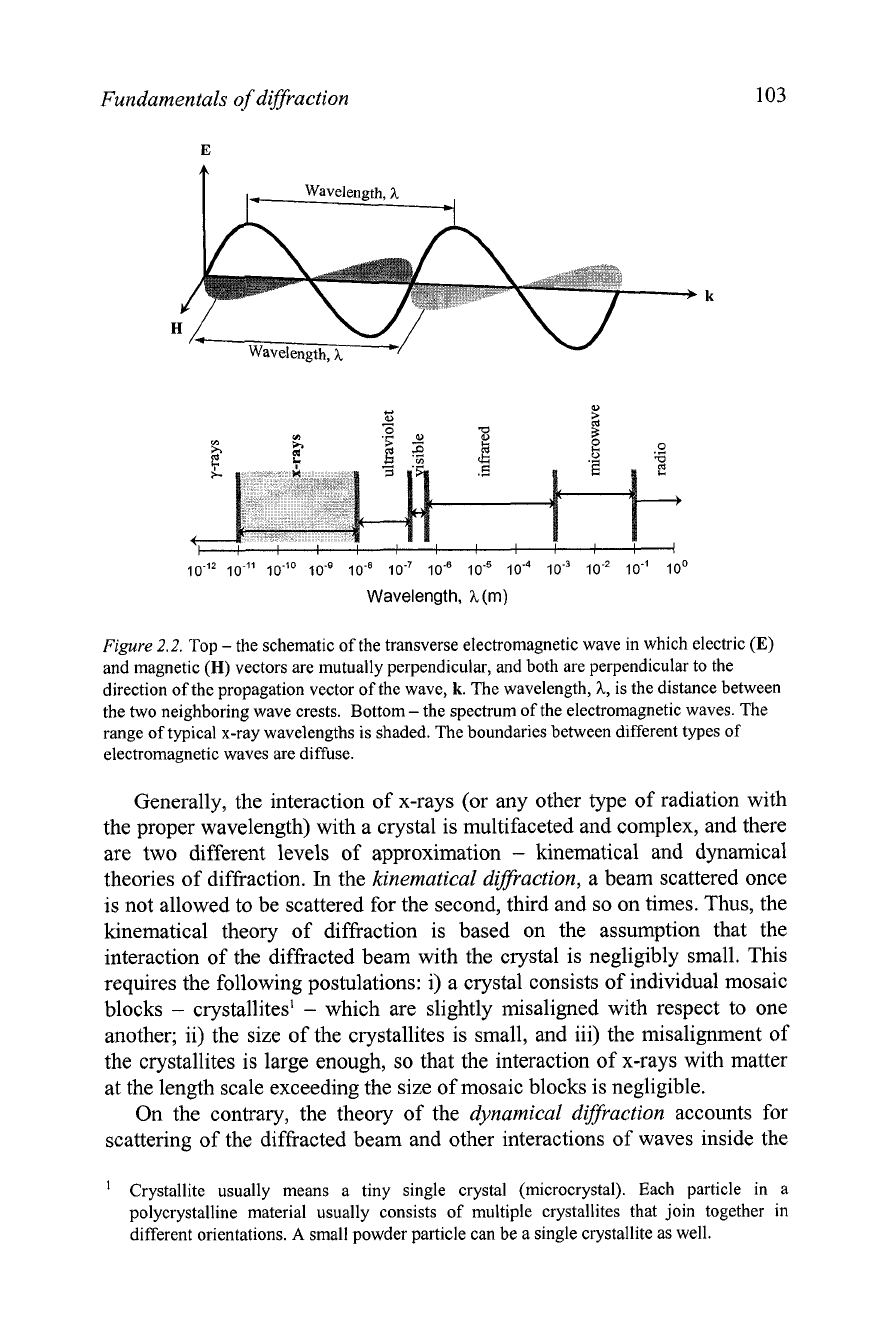
Electromagnetic radiation is generated every time when electric charge
accelerates or decelerates. It consists of transverse waves where electric
(E)
and magnetic
(H)
vectors are perpendicular to one another and to the
propagation vector of the wave
(k),
see
Figure
2.2, top. The x-rays have
wavelengths from -0.1 to -100 A, which are located between y-radiation and
ultraviolet rays as also shown in
Figure
2.2, bottom. The wavelengths, most
commonly used in crystallography, range between -0.5 and -2.5
A
since
they are of the same order of magnitude as the shortest interatomic distances
observed in both organic and inorganic materials. Furthermore, these
wavelengths can be easily produced in almost every research laboratory.
When x-rays propagate through a substance, the occurrence of the
following processes should be considered in the phenomenon of diffraction:
1. Coherent scattering (section
2.5), which produces beams with the same
wavelength as the incident (primary) beam. In other words, the energy of
the photons in a coherently scattered beam remains unchanged when
compared to that in the primary beam.
2. Incoherent (or Compton) scattering, in which the wavelength of the
scattered beam increases due to partial loss of photon energy in collisions
with core electrons (the Compton effect).
3.
Absorption of the x-rays, see section 2.3.2.1, in which some photons are
dissipated in random directions due to scattering, and some photons lose
their energy by ejecting electron(s) from an atom (i.e. ionization) andlor
due to the photoelectric effect (i.e. x-ray fluorescence).
Incoherent scattering is not essential when the interaction of x-rays with
crystal lattices is of concern, and it is generally neglected. When absorption
becomes significant, it is usually taken into account as a separate effect.
Thus, in the first approximation only coherent scattering results in the
diffraction from periodic lattices and will be considered in this chapter.
Fundamentals of diffraction
10.12 10-11 10.10 10.8 10-8
10-7
10-8 10.5 i0.4
lo-3
lo-2
10.1 100
Wavelength,
h
(m)
Figure
2.2.
Top
-
the schematic of the transverse electromagnetic wave in which electric
(E)
and magnetic
(H)
vectors are mutually perpendicular, and both are perpendicular to the
direction of the propagation vector of the wave,
k.
The wavelength,
h,
is the distance between
the two neighboring wave crests. Bottom
-
the spectrum of the electromagnetic waves. The
range of typical x-ray wavelengths is shaded. The boundaries between different types of
electromagnetic waves are diffuse.
Generally, the interaction of x-rays (or any other type of radiation with
the proper wavelength) with a crystal is multifaceted and complex, and there
are two different levels of approximation
-
kinematical and dynamical
theories of diffraction.
In the kinematical diffraction, a beam scattered once
is not allowed to be scattered for the second, third and so on times. Thus, the
kinematical theory of diffraction is based on the assumption that the
interaction of the diffracted beam with the crystal is negligibly small. This
requires the following postulations: i) a crystal consists of individual mosaic
blocks
-
crystallites'
-
which are slightly misaligned with respect to one
another; ii) the size of the crystallites is small, and iii) the misalignment of
the crystallites is large enough, so that the interaction of x-rays with matter
at the length scale exceeding the size of mosaic blocks is negligible.
On the contrary, the theory of the dynamical
diffvaction accounts for
scattering of the diffracted beam and other interactions of waves inside the
'
Crystallite usually means a tiny single crystal (microcrystal). Each particle in a
polycrystalline material usually consists of multiple crystallites that join together in
different orientations.
A
small powder particle can be a single crystallite as well.

104
Chapter
2
crystal, and thus the mathematical apparatus of the theory is quite complex.
Dynamical effects become significant and the use of the theory of dynamical
diffraction is justified only when the crystals are nearly perfect or when there
is an exceptionally strong interaction of the radiation with the material.
In
the majority of crystalline materials, however, dynamical effects are small
and they are usually noticeable only when precise single crystal experiments
are conducted. Even then, numerous dynamical effects
(e.g. primary and/or
secondary extinction, simultaneous diffraction, thermal diffuse scattering,
and others) are usually applied as corrections to the kinematical diffraction
model.
The kinematical approach is simple, and adequately and accurately
describes the diffraction of x-rays from mosaic crystals. This is especially
true for polycrystalline materials where the size of crystallites is relatively
small. Hence, the kinematical theory of diffraction is used in this chapter and
throughout this book.
2.2.2
Production of
x-rays
The x-rays are usually generated using two different methods or sources.
The first is a device, which is called an x-ray tube, where electromagnetic
waves are generated from impacts of high-energy electrons with a metal
target. These are the simplest and the most commonly used sources of x-rays
that are available in a laboratory of any size, and thus, an x-ray tube is
known as a laboratory or a conventional x-ray source. Conventional x-ray
sources usually have low efficiency and their brightness' is fundamentally
limited by the thermal properties of the target material. The latter must be
continuously cooled because nearly all kinetic energy of the accelerated
electrons is converted into heat when they decelerate rapidly (and sometimes
instantly) during the impacts with a metal target.
The second is a much more advanced source of x-ray radiation
-
the
synchrotron, where high energy electrons are confined in a storage ring.
When they move in a circular orbit, electrons accelerate towards the center
of the ring, thus emitting electromagnetic radiation. The synchrotron sources
are extremely bright (or brilliant2) since thermal losses are minimized and
'
Brightness is measured as photon flux
-
a number of photons per second per unit area
-
where the area is expressed in terms of the corresponding solid angle in the divergent
beam. Brightness is different from intensity of the beam, which is the total number of
photons leaving the target, because intensity can be easily increased by increasing the area
of the target irradiated by electrons without increasing brightness.
The quality of synchrotron beams is usually characterized by brilliance, which is defined
as brightness divided by the product of the source area (in
rnrn2)
and a fraction of a useful
photon energy, i.e. bandwidth [see, for example, J. Als-Nielsen and
D.
McMorrow,
Elements of modern x-ray physics, John Wiley
&
Sons, New York
(2001)l.

Fundamentals
of
diffraction
105
there is no target to cool. Their brightness is only limited by the flux of
electrons in the high energy beam. Today, the so-called third generation of
synchrotrons is in operation and their brilliance exceeds that of the
conventional x-ray tube by nearly ten orders of magnitude.
Obviously, given the cost of both the construction and maintenance of a
synchrotron source, owning one would be prohibitively expensive and
inefficient for an average crystallographic laboratory. All synchrotron
sources are multiple user facilities, which are constructed and maintained
using governmental support
(e.g. they are supported by the United States
Department of Energy in the US and by similar agencies in Europe and
Japan), which is fully justified by the unique structural information that can
be obtained using brilliant, variable energy x-ray beams.
In
general, there is no principal difference in the diffraction phenomena
using the synchrotron and conventional x-ray sources, except for the
presence of several highly intense peaks with fixed wavelengths in the
conventionally obtained x-ray spectrum and their absence,
i.e. the
continuous distribution of photon energies when using synchrotron sources.
Here and throughout the book, the x-rays from conventional sources are of
concern unless noted otherwise.
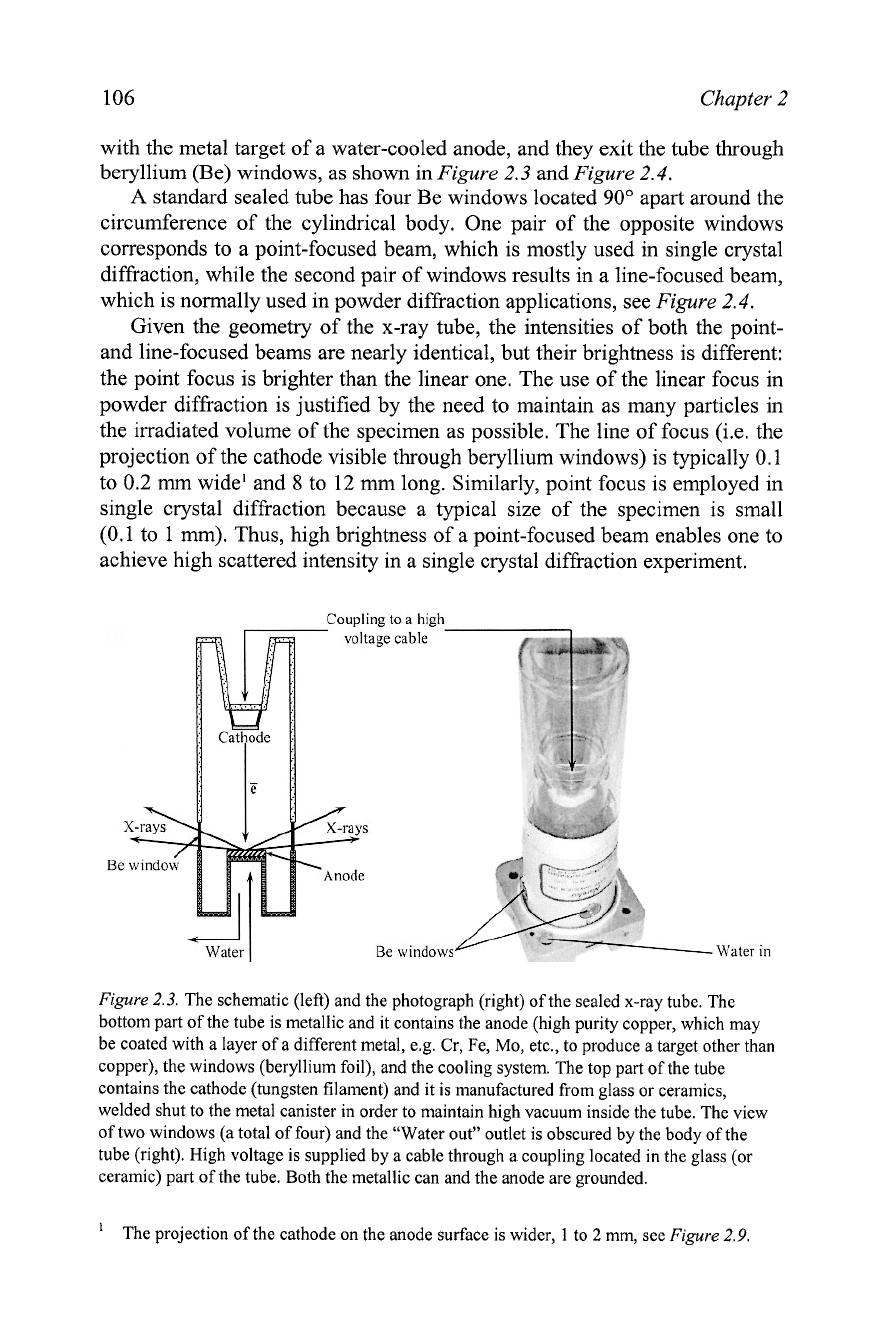
2.2.3 Conventional sealed x-ray sources
As noted above, the x-ray tube is a conventional laboratory source of x-
rays. The two types of x-ray tubes in common use today are the sealed tube
and the rotating anode tube. The sealed tube consists of a stationary anode
coupled with a cathode, and both are placed inside a metallglass or a
metallceramic container sealed under high vacuum as shown in
Figure
2.3.
The x-ray tube assembly is
a
simple and maintenance-free device.
However, the overall efficiency of an x-ray tube is very low
-
approximately
1% or less. Most of the energy supplied to the tube is converted into heat,
and therefore, the anode must be continuously cooled with chilled water to
avoid target meltdown. The input power to the sealed x-ray tube (-0.5 to
3
kW) is therefore, limited by the tube's ability to dissipate heat, but the
resultant energy of the usable x-ray beam is much lower than 1% of the input
power because only a small fraction of the generated photons exits through
each window. Additional losses occur during the monochromatization and
collimation of the beam (see section
2.3).
In
the x-ray tube, electrons are emitted by the cathode, usually
electrically heated tungsten filament, and they are accelerated towards the
anode by a high electrostatic potential (30 to 60 kV) maintained between the
cathode and the anode. The typical current in a sealed tube is between 10 and
50
mA.
The x-rays are generated by the impacts of high-energy electrons
106
Chapter
2
with the metal target of a water-cooled anode, and they exit the tube through
beryllium (Be) windows, as shown in
Figure
2.3
and
Figure
2.4.
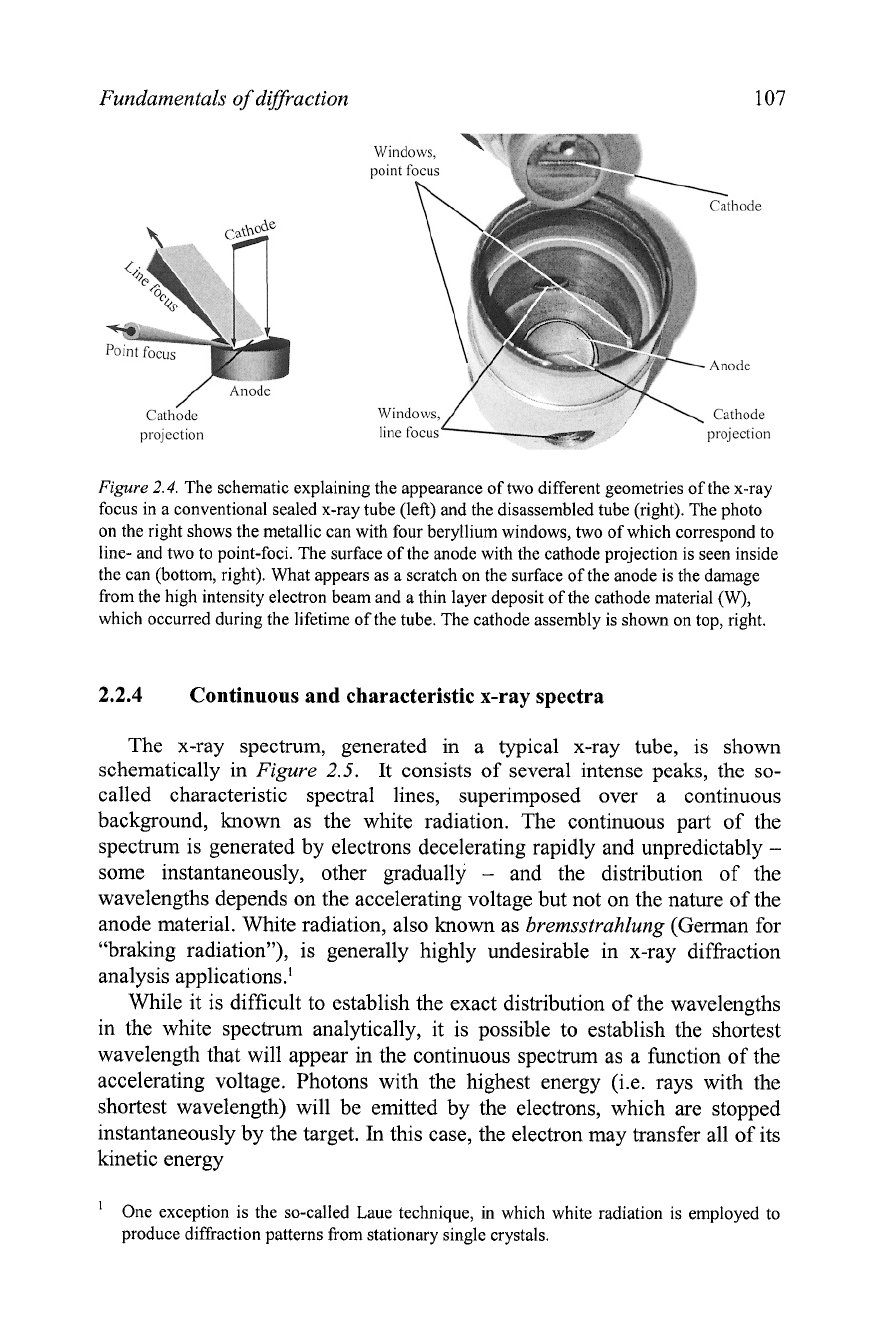
A
standard sealed tube has four Be windows located 90" apart around the
circumference of the cylindrical body. One pair of the opposite windows
corresponds to a point-focused beam, which is mostly used in single crystal
diffraction, while the second pair of windows results in a line-focused beam,
which is normally used in powder diffraction applications, see
Figure
2.4.
Given the geometry of the x-ray tube, the intensities of both the point-
and line-focused beams are nearly identical, but their brightness is different:
the point focus is brighter than the linear one. The use of the linear focus in
powder diffraction is justified by the need to maintain as many particles in
the irradiated volume of the specimen as possible. The line of focus (is. the
projection of the cathode visible through beryllium windows) is typically 0.1
to 0.2 mm wide' and
8
to 12 mm long. Similarly, point focus is employed in
single crystal diffraction because a typical size of the specimen is small
(0.1 to 1 mm). Thus, high brightness of a point-focused beam enables one to
achieve high scattered intensity in a single crystal diffraction experiment.
\
X-rays
7
Be
window
Cathode
-
e
Figure
2.3.
The schematic (left) and the photograph (right) of the sealed x-ray tube. The
bottom part of the tube is metallic and it contains the anode (high purity copper, which may
be coated with a layer of a different metal, e.g. Cr, Fe, Mo, etc., to produce a target other than
copper), the windows (beryllium foil), and the cooling system. The top part of the tube
contains the cathode (tungsten filament) and it is manufactured from glass or ceramics,
welded shut to the metal canister in order to maintain high vacuum inside the tube. The view
of two windows (a total of four) and the "Water out" outlet is obscured by the body of the
tube (right). High voltage is supplied by a cable through a coupling located in the glass (or
ceramic) part of the tube. Both the metallic can and the anode are grounded.
'
The projection of the cathode on the anode surface is wider,
1
to
2
mm,
see
Figure
2.9.
Fundamentals of diffraction
107
Cathode Wmdows,
i
Cathode
projection
lme focus
projection
Figure
2.4. The schematic explaining the appearance of two different geometries of the x-ray
focus in a conventional sealed x-ray tube (left) and the disassembled tube (right). The photo
on the right shows the metallic can with four beryllium windows, two of which correspond to
line- and two to point-foci. The surface of the anode with the cathode projection is seen inside
the can (bottom, right). What appears as a scratch on the surface of the anode is the damage
from the high intensity electron beam and a thin layer deposit of the cathode material
(W),
which occurred during the lifetime of the tube. The cathode assembly is shown on top, right.
2.2.4
Continuous and characteristic x-ray spectra
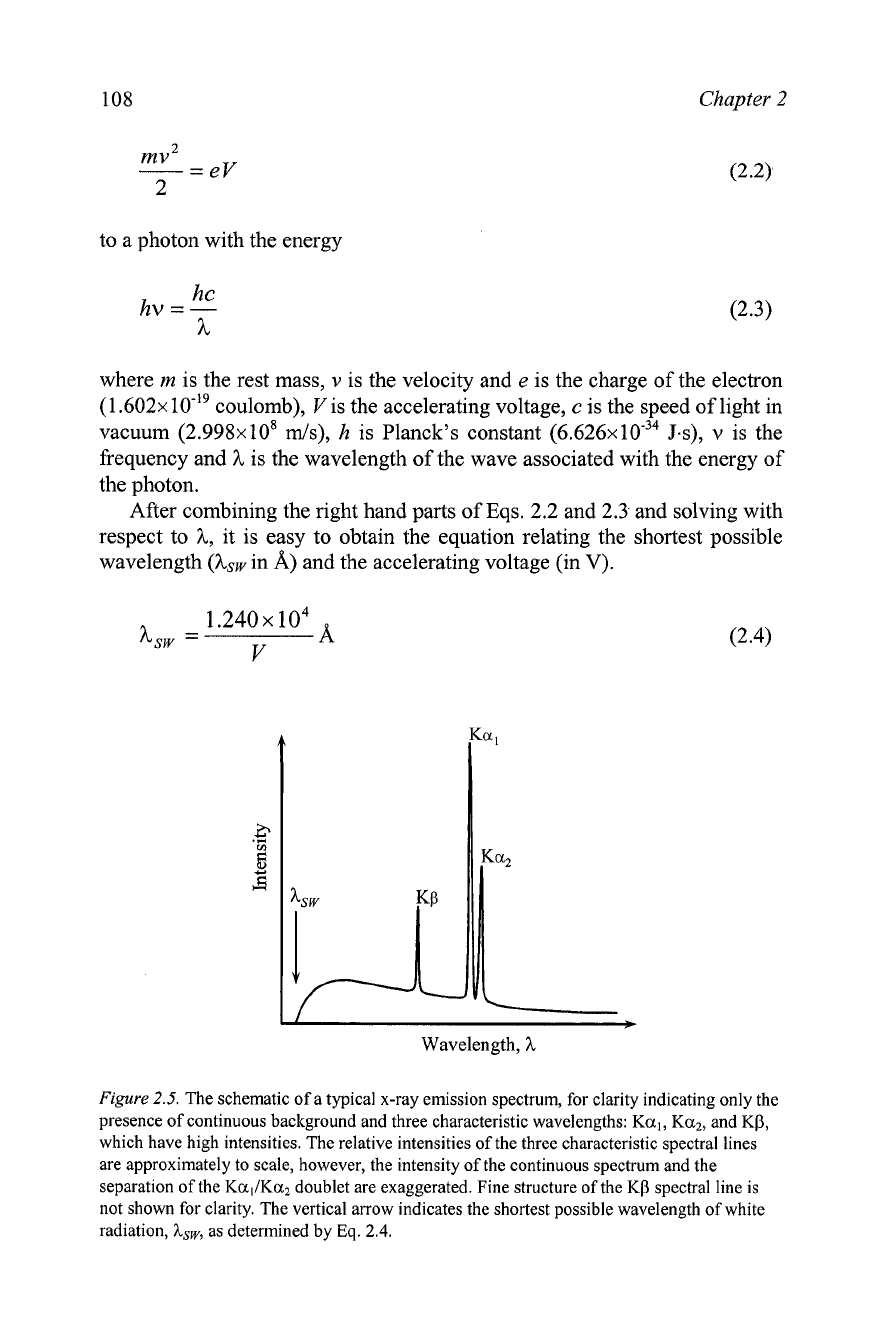
The x-ray spectrum, generated in a typical x-ray tube, is shown
schematically in
Figure
2.5.
It consists of several intense peaks, the so-
called characteristic spectral lines, superimposed over a continuous
background, known as the white radiation. The continuous part of the
spectrum is generated by electrons decelerating rapidly and unpredictably
-
some instantaneously, other gradually
-
and the distribution of the
wavelengths depends on the accelerating voltage but not on the nature of the
anode material. White radiation, also known as
bremsstrahlung
(German for
"braking radiation"), is generally highly undesirable in x-ray diffraction
analysis applications.'
While it is difficult to establish the exact distribution of the wavelengths
in the white spectrum analytically, it is possible to establish the shortest
wavelength that will appear in the continuous spectrum as a function of the
accelerating voltage. Photons with the highest energy (i.e. rays with the
shortest wavelength) will be emitted by the electrons, which are stopped
instantaneously by the target.
In this case, the electron may transfer all of its
kinetic energy
'
One exception is the so-called Laue technique, in which white radiation is employed to
produce diffraction patterns from stationary single crystals.
to a photon with the energy
Chapter
2
(2.2)
where
m
is the rest mass,
v
is the velocity and
e
is the charge of the electron
(1.602~ lo-'' coulomb),
V
is the accelerating voltage,
c
is the speed of light in
vacuum (2.998~10~ mls),
h
is Planck's constant (6.626~10"~ J.s),
v
is the
frequency and
h
is the wavelength of the wave associated with the energy of
the photon.
After combining the right hand parts of Eqs. 2.2 and 2.3- and solving with
respect to A, it is easy to obtain the equation relating the shortest possible
wavelength (Asw in A) and the accelerating voltage (in
V).
Wavelength,
h
Figure
2.5.
The schematic of a typical x-ray emission spectrum, for clarity indicating only the
presence of continuous background and three characteristic wavelengths:
Ka,, Ka2,
and
KP,
which have high intensities. The relative intensities of the three characteristic spectral lines
are approximately to scale, however, the intensity of the continuous spectrum and the
separation of the
KallKa2
doublet are exaggerated. Fine structure of the
K(3
spectral line is
not shown for clarity. The vertical arrow indicates the shortest possible wavelength of white
radiation,
hsw,
as determined by
Eq.
2.4.
Fundamentals
of
diffraction 109
The three characteristic lines are quite intense and they result from the
transitions of upper level electrons in the atom core to vacant lower energy
levels, from which an electron was ejected by the impact with an electron
accelerated in the x-ray tube. The energy differences between various energy
levels in an atom are element-specific and therefore, each chemical element
emits x-rays with a constant,
i.e, characteristic, distribution of wavelengths
that appear due to excitations of core electrons by high energy electrons
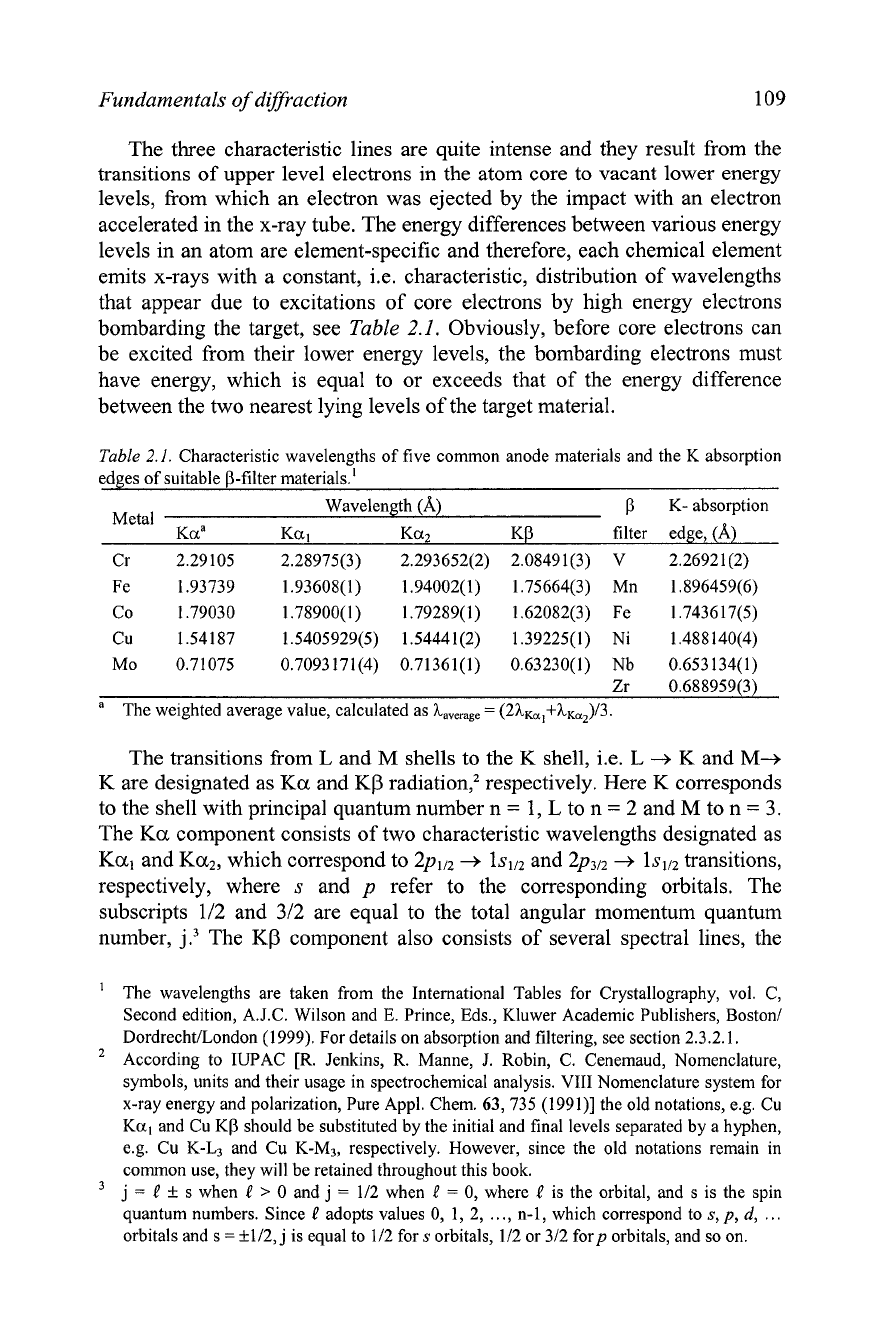
bombarding the target, see Table
2.1.
Obviously, before core electrons can
be excited from their lower energy levels, the bombarding electrons must
have energy, which is equal to or exceeds that of the energy difference
between the two nearest lying levels of the target material.
Table
2.1.
Characteristic wavelengths of five common anode materials and the K absorption
edges of suitable P-filter materials.'
Wavelength (A)
p
K-
absorption
Metal
Kaa K~I Ka2 KP filter edge, (A)
Cr 2.29105 2.28975(3) 2.293652(2)
2.08491(3) V 2.26921 (2)
Fe 1.93739 l.93608(1) 1.94002(1)
1.75664(3) Mn l.896459(6)
Co 1.79030 1.789OO(l) l.79289(1) l.62082(3) Fe 1.7436 U(5)
Cu
1
.%I187 1.5405929(5) 1.54441(2) l.39225(1) Ni l.488140(4)
Mo 0.71075 0.7093171(4) 0.71361(1) 0.63230(1) Nb 0.653134(1)
Zr 0.688959(3)
a
The weighted average value, calculated as ha,,,,
=
(2hK,,+hK,,)/3.
The transitions from
L
and
M
shells to the
K
shell, i.e.
L
-+
K
and
M+
K
are designated as Ka and
KP
radiatioq2 respectively. Here
K
corresponds
to the shell with principal quantum number n
=
1,
L
to n
=
2 and
M
to n
=
3.
The Ka component consists of two characteristic wavelengths designated as
Kal and Ka2, which correspond to 2pl12
-+
Isll2 and 2p3l2
-+
Isln transitions,
respectively, where
s
and
p
refer to the corresponding orbitals. The
subscripts 112 and 312 are equal to the total angular momentum quantum
number,
j.3
The KP component also consists of several spectral lines, the
The wavelengths are taken from the International Tables for Crystallography, vol. C,
Second edition, A.J.C. Wilson and
E.
Prince, Eds., Kluwer Academic Publishers, Boston1
DordrechtILondon (1999). For details on absorption and filtering, see section 2.3.2.1.
According to IUPAC
[R.
Jenkins,
R.
Manne, J. Robin, C. Cenemaud, Nomenclature,
symbols, units and their usage in spectrochemical analysis. VIII Nomenclature system for
x-ray energy and polarization, Pure Appl. Chem.
63,
735 (1991)l the old notations, e.g. Cu
Kal and Cu KP should be substituted by the initial and final levels separated by a hyphen,
e.g. Cu K-L3 and Cu K-M3, respectively. However, since the old notations remain in
common use, they will be retained throughout this book.
j
=
k'
f
s when
e
>
0 and
j
=
112 when
k'
=
0, where
k'
is the orbital, and s is the spin
quantum numbers. Since
k'
adopts values 0, 1, 2,
.
..,
n-1, which correspond to
s,
p,
d,
.
..
orbitals and s
=
f
112,
j
is equal to 112 for
s
orbitals, 112 or 312 forp orbitals, and so on.

110 Chapter
2
strongest being KPI and KP3, which are so close to one another that they are
practically indistinguishable in the x-ray spectra of many anode materials.
There are more characteristic lines in the emission spectrum
(e.g. La-y and
Ma-c), however, their intensities are substantially lower and their
wavelengths are greater that those of Ka and KP. Therefore, they are not
used in x-ray diffraction analysis and will not be considered here.'
In
addition to their wavelengths, the strongest characteristic spectral lines
have different intensities: the intensity of Ka, exceeds that of Ka2 by a
factor of two, and the intensity of
Ka, is approximately five times that of the
intensity of the strongest KP line, although the latter ratio varies
considerably with the atomic number. Spectral purity, i.e. the availability of
a single intense wavelength, is critical in most diffraction applications and
therefore, various monochromatization methods are used to eliminate
multiple wavelengths (and hence multiple Bragg peaks from the identical
sets of crystallographic planes), which is discussed in section 2.3. Although
the continuous x-ray emission spectrum does not result in distinct diffraction
peaks from polycrystals, its presence increases the adverse background noise
and therefore, white radiation should be minimized.
Typical anode materials that are used in x-ray tubes (Table
2.1)
produce
characteristic wavelengths between -0.5 and -2.3 A. However, only two of
them are in the most common use. These are Cu in powder and Mo in
single-
crystal diffractometry. Other anode materials can be used in special
applications, for example Ag anode
(h
Ka,
=
0.5594218 A) can be used to
increase the resolution of the atomic structure since using shorter wavelength
broadens the range of
sineI3L in which diffraction peaks can be measured.
Bragg peaks, however, are observed closer to each other and the resolution
of the diffraction pattern may deteriorate. On the other hand, Cr, Fe or Co
anodes may be used instead of a Cu anode in powder diffraction (or Cu
anode instead of Mo anode in single crystal diffractometry) to increase the
resolution of the diffraction pattern (Bragg peaks are observed further apart)
but the resolution of the atomic structure decreases.
2.2.5
Rotating anode
x-ray
sources
The low thermal efficiency of the sealed x-ray tube can be substantially
improved by using a rotating anode x-ray s~urce,~ which is shown in Figure
Except for one experimental artifact shown later in
Figure
2.18,
where two components
present in the
La
characteristic spectrum of
W
(filament material contaminating Cu anode
of a relatively old x-ray tube) are clearly recognizable in the diffraction pattern collected
from the oriented single crystalline silicon wafer.
For more details on rotating anode x-ray sources see
W.C.
Phillips, X-ray sources,
Methods Enzymol.
114,300
(1985)
and references therein.
