Pecharsky V.K., Zavalij P.Y. Fundamentals of Powder Diffraction and Structural Characterization of Materials
Подождите немного. Документ загружается.

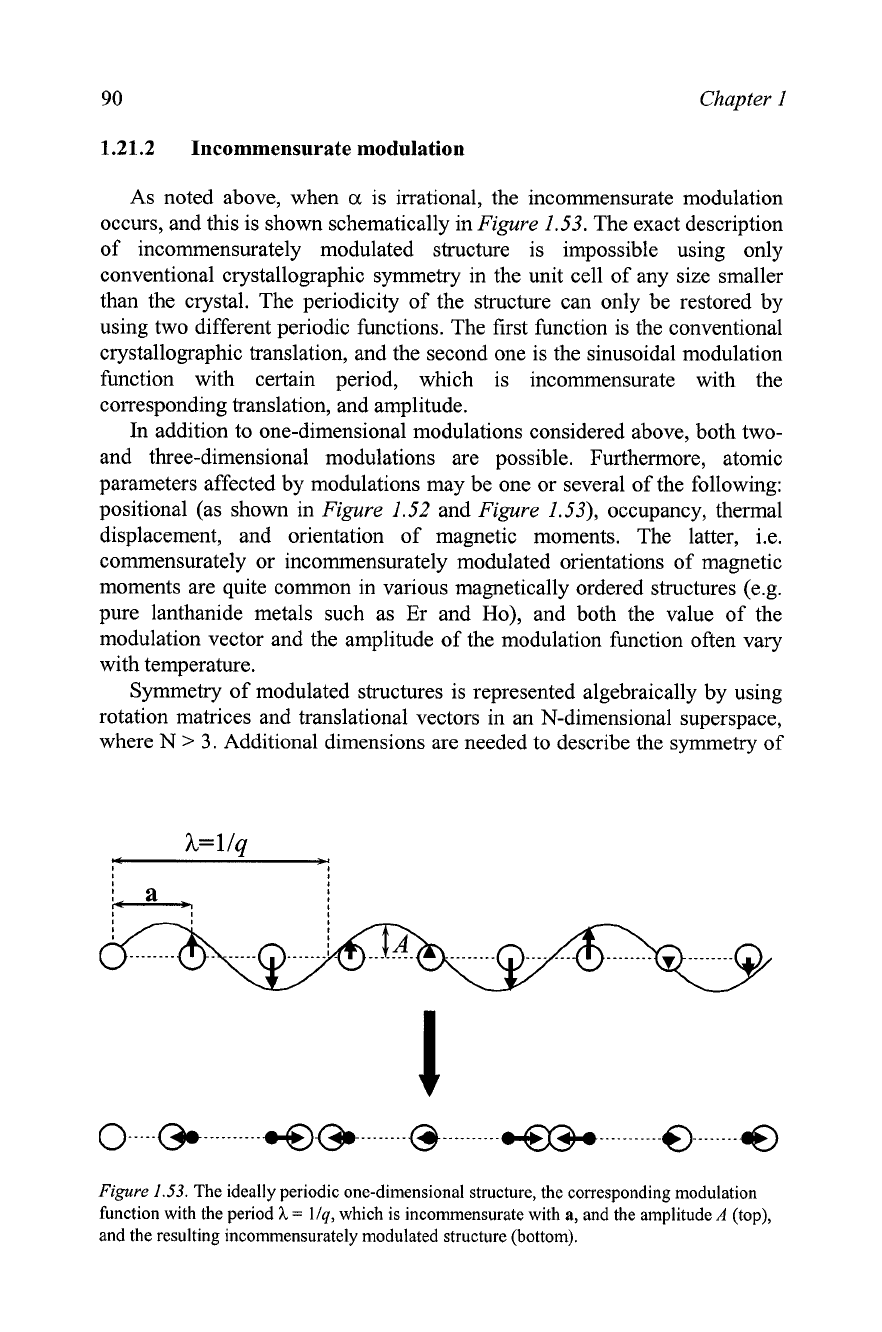
90
Chapter
I
1.21.2
Incommensurate modulation
As noted above, when
a
is irrational, the incommensurate modulation
occurs, and this is shown schematically in
Figure
1.53.
The exact description
of incommensurately modulated structure is impossible using only
conventional crystallographic symmetry in the unit cell of any size smaller
than the crystal. The periodicity of the structure can only be restored by
using two different periodic functions. The first function is the conventional
crystallographic translation, and the second one is the sinusoidal modulation
function with certain period, which is incommensurate with the
corresponding translation, and amplitude.
In
addition to one-dimensional modulations considered above, both two-
and three-dimensional modulations are possible. Furthermore, atomic
parameters affected by modulations may be one or several of the following:
positional (as shown in
Figure
1.52
and
Figure
1.53),
occupancy, thermal
displacement, and orientation of magnetic moments. The latter, i.e.
commensurately or incommensurately modulated orientations of magnetic
moments are quite common in various magnetically ordered structures
(e.g.
pure lanthanide metals such as Er and Ho), and both the value of the
modulation vector and the amplitude of the modulation function often vary
with temperature.
Symmetry of modulated structures is represented algebraically by using
rotation matrices and translational vectors in an N-dimensional superspace,
where N
>
3.
Additional dimensions are needed to describe the symmetry of
Figure
1.53.
The ideally periodic one-dimensional structure, the corresponding modulation
function with the period
h
=
llq,
which is incommensurate with
a,
and the amplitude
A
(top),
and the resulting incommensurately modulated structure (bottom).

Fundamentals
of
crystalline state
9
1
the modulation functions. Thus, one-dimensional modulation is described in
a four-dimensional superspace using
4x4
rotation matrices and
4x
1 vectors
(3
dimensions for a normal space plus one for the modulation function),
while two- and three-dimensional modulations require
5x5
and
6x6
rotation
matrices and
5x
1 and
6x
1
vectors, respectively.
1.21.3
Quasicrystals
The symmetry of quasicrystals can be represented by introducing a
different perturbation function, which is based on the Fibonacci1 numbers.
An
infinite Fibonacci sequence is derived from two numbers,
0
and 1, and is
formed according to the following rule:
This results in the series of numbers
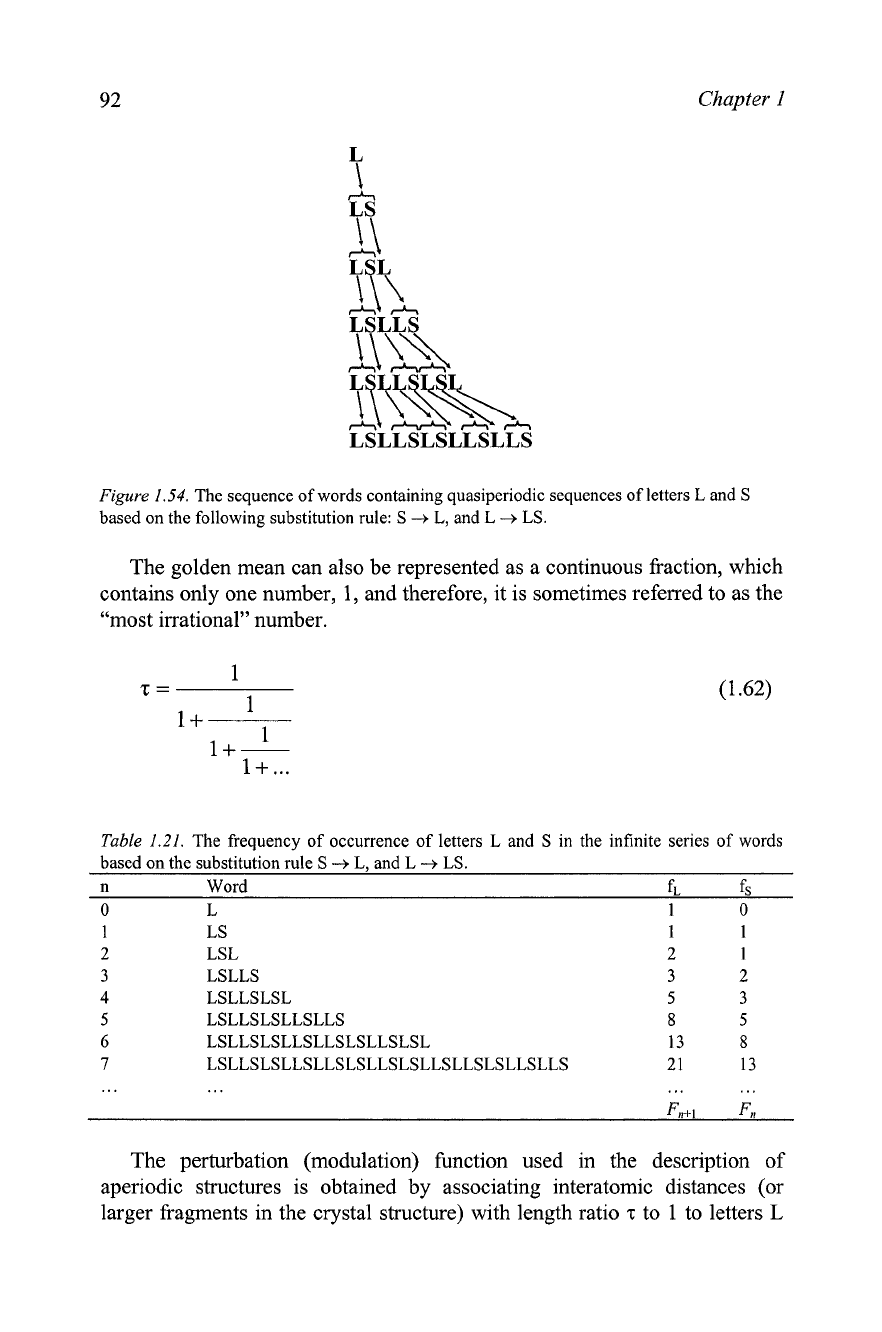
Assume that we have a sequence of words containing letters
L
(for long
distance or fragment) and S (for short distance or fragment), which are
constructed by replacing each letter in the previous word using the following
substitution rule: letter S is replaced by letter L, while letter
L
is replaced by
the word LS. Starting from
L
as the first word, the infinite sequence of
words is obtained, and the first six members of this sequence are shown in
Figure 1.54.
The fi-equency of occurrence of letters L and
S
in this sequence is
represented in Table 1.21, and it is easy to recognize that they are identical
to the consecutive members
(Fn+, and F,) of the Fibonacci series. The
corresponding limit when the number of words, n, approaches infinity is the
golden mean,
T
Leonardo Pisano Fibonacci (1 170
-
1250). Medieval Italian mathematician who in 1202
wrote
Liber
abaci
-
"Book of the abacus"
-
in which he formulated the problem leading to
the sequence of numbers 1, 1, 2,
3,
5,
8,
13,
21,
34,
55,
.. .
(without the first term, i.e.
without 0): "How many pairs of rabbits can be produced in a year from one pair of rabbits
assuming that every month each pair produces one new pair of rabbits, which becomes
productive one month after birth?"
Chapter
I
Figure
1.54.
The sequence of words containing quasiperiodic sequences of letters
L
and
S
based on the following substitution rule:
S
-+
L,
and
L
-+
LS.
The golden mean can also be represented as a continuous fraction, which
contains only one number, 1, and therefore, it is sometimes referred to as the
"most irrational" number.
Table
1.21.
The frequency of occurrence of letters
L
and
S
in the infinite series of words
based on the substitution rule
S
-+
L,
and
L
-+
LS.
n Word f~ fs
0
L
1
0
1
LS
1
1
2
LSL
2
1
3 LSLLS 3
2
4
LSLLSLSL
5
3
5
LSLLSLSLLSLLS
8
5
G
LSLLSLSLLSLLSLSLLSLSL 13
8
7
LSLLSLSLLSLLSLSLLSLSLLSLLSLSLLSLLS
21 13
The perturbation (modulation) function used in the description of
aperiodic structures is obtained by associating interatomic distances (or
larger fragments in the crystal structure) with length ratio
z
to 1 to letters
L

Fundamentals
of
crystalline state
93
and S and the resulting modulation function is no longer a sinusoidal wave,
but is saw-tooth-like. It is worth noting that the periodicity in this simple
one-dimensional case
(Figure
1.54
and
Table
1.21)
is absent but the order is
perfect: as soon as the law has been established, the "structure" of the series,
i.e. the location of S and
L
can be predicted at any point starting from the
origin or any other known location.
Similar to modulated structures, symmetry of quasicrystals also can be
described in space with more than three dimensions. For example, two-
dimensional and three-dimensional quasiperiodicity can be described in
five-
and six-dimensional space, respectively, using
5x5
and
6x6
rotation
matrices. Yet, the properties of these matrices (Eqs. 1.44 to
1.47)
remain the
same for both modulated structures and quasicrystals.
The more detailed description of the non-conventional symmetry goes
beyond the scope of this book' as it has little use in powder diffraction,
because even the three-dimensional diffraction from aperiodic crystals is
quite complex. When the diffraction picture is projected along one
dimension, its treatment becomes too complicated and the crystal structure
of aperiodic crystals is rarely, if ever, completely studied by means of
powder diffraction techniques beyond simple phase identification.
Nevertheless, this section has been included here for completeness, and to
give the reader a flavor of recent developments in
~rystallography.~
'
A more complete description of de Wolffs approach to treatment of various types of
aperiodic crystals can be found in the International Tables for Crystallography, vol. B,
Second edition,
U.
Shmueli, Ed., Published for the International Union of Crystallography
by Kluwer Academic Publishers, Boston/Dordrecht/London (2001).
The discovery of five-fold symmetry prompted the ad-interim Commission on Aperiodic
Crystals of the International Union of Crystallography to change the definition of a crystal
as
a
periodic three-dimensional arrangement of identical unit cells to the following: "...by
'crystal' we mean any solid having an essentially discrete diffraction diagram, and by
'aperiodic crystal' we mean any crystal in which three-dimensional lattice periodicity can
be considered to be absent". International Union of Crystallography. Report of the
Executive Committee for 1991,
Acta Cryst. A48,922
-
946 (1992).

94
Chapter
I
1.22 Additional reading
1. C. Giacovazzo, H.L. Monaco, D. Viterbo, F. Scordari, G. Gilli, G.
Zanotti, and M. Catti, Fundamentals of crystallography. IUCr texts on
crystallography 7, Second Edition, Oxford University Press, Oxford,
New York (2002).
2.
D.
Schwarzenbach, Crystallography, John Wiley
&
Sons, New York
(1996).
3.
M.B. Boisen and G.V. Gibbs. Mathematical crystallography
-
an
introduction to the mathematical foundations of crystallography. Reviews
in mineralogy, Vol. 15 (revised). Washington, DC: The Mineralogical
Society of America (1992).
4.
D.
Farmer, Groups and symmetry, Amer. Math. Soc., Providence,
RI
(1995).
5. C. Hammond, The basics of crystallography and diffraction. IUCr texts
on crystallography
3.
Oxford University Press, Oxford, New York
(1997).
6.
E. Prince, Mathematical techniques in crystallography and materials
science. Second edition, Springer-Verlag, Berlin, Heidelberg, Germany
(1 992).
7.
C. Janot, Quasicrystals.
A
primer, Clarendon Press, Oxford (1992).
8.
IUCr Teaching Pamphlets:
http://www.iucr.org/iucr-top/comm/cteachlpamphlets.html
9. International Tables for Crystallography, vol. A, Fifth revised edition,
Theo Hahn, Ed., Published for the International Union of Crystallography
by Kluwer Academic Publishers,
Boston/Dordrecht/London,
(2002).
10. International Tables for Crystallography. Brief teaching edition of
volume A, Fifth revised edition. Theo Han, Ed., Kluwer Academic
Publishers, Boston/Dordrecht/London (2002).
Fundamentals of crystalline state
95
1.23 Problems
Answers to all problems are located in the file Chapter-l-Problems-
Solutions.pdf on the CD accompanying this book.
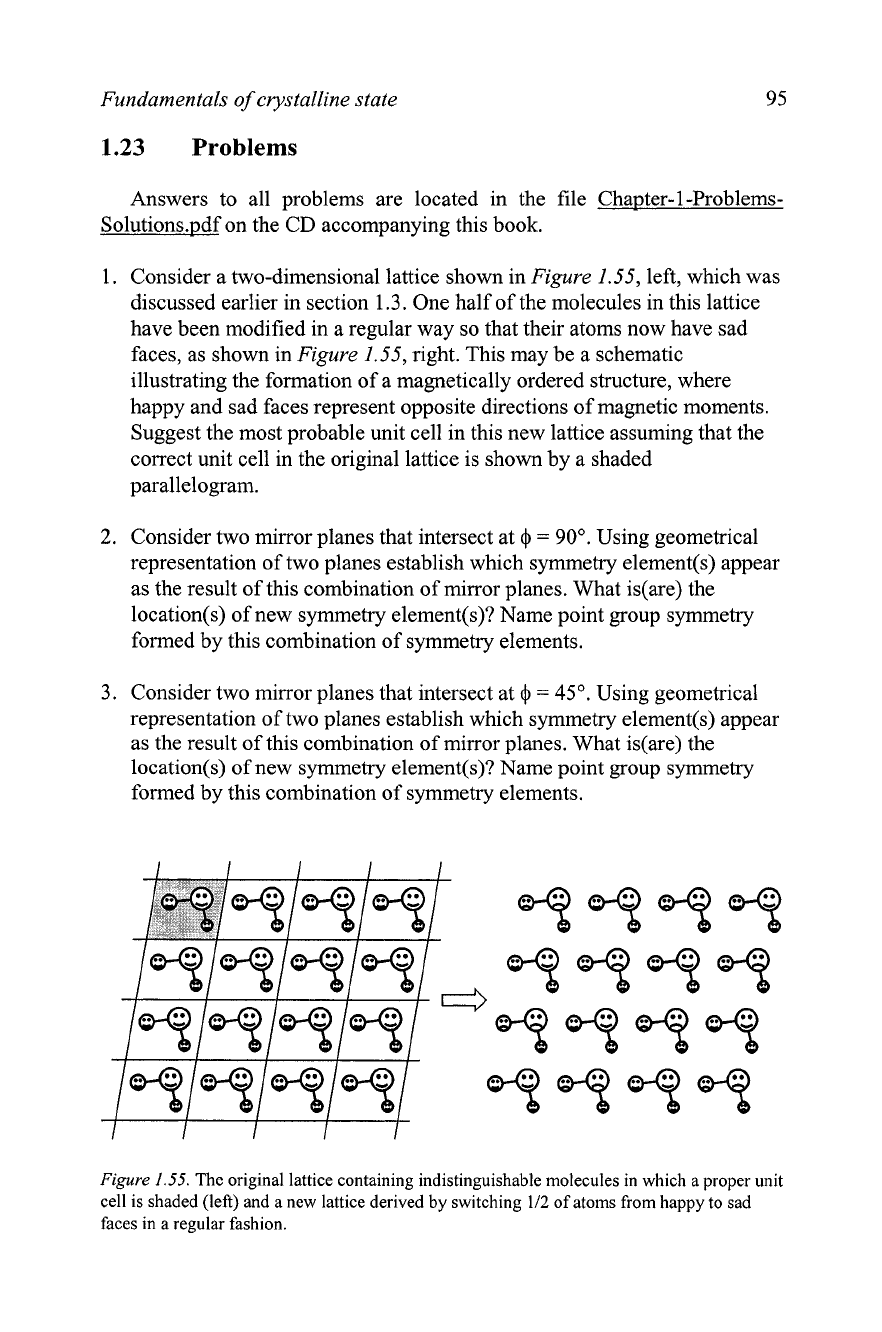
Consider a two-dimensional lattice shown in
Figure
1.55, left, which was
discussed earlier in section 1.3. One half of the molecules in this lattice
have been modified in a regular way so that their atoms now have sad
faces, as shown in
Figure
1.55, right. This may be a schematic
illustrating the formation of a magnetically ordered structure, where
happy and sad faces represent opposite directions of magnetic moments.
Suggest the most probable unit cell in this new lattice assuming that the
correct unit cell in the original lattice is shown by a shaded
parallelogram.
Consider two mirror planes that intersect at
4
=
90'. Using geometrical
representation of two planes establish which symmetry element(s) appear
as the result of this combination of mirror planes. What
is(are) the
location(s) of new symmetry element(s)? Name point group symmetry
formed by this combination of symmetry elements.
Consider two mirror planes that intersect at
4
=
45'.
Using geometrical
representation of two planes establish which symmetry element(s) appear
as the result of this combination of mirror planes. What
is(are) the
location(s) of new symmetry element(s)? Name point group symmetry
formed by this combination of symmetry elements.
Figure
1.55.
The original lattice containing indistinguishable molecules in which a proper unit
cell is shaded (left) and a new lattice derived
by
switching
112
of atoms from happy to sad
faces in a regular fashion.

96
Chapter
1
4. Consider the following sequence of numbers: 1, 112, 113, 114,
.
.
.
,
1/N,
.
..
Is this a group assuming that the combination law is multiplication,
division, addition or subtraction? If yes, identify the combination law in
this group and establish whether this group is finite or infinite.
5.
Consider the group created by three non-coplanar translations (vectors)
using the combination law defined by Eq. 1.1. Which geometrical form
can be chosen to illustrate this group? Is the group finite?
6.
Determine both the crystal system and point group symmetry of a brick,
which is shown schematically in
Figure
1.56
and in which
a
#
b
#
c and
a=~=~=900?
7.
Determine both the crystal system and point group symmetry of benzene
molecule, C6&?
8.
Determine the point group symmetry of the octahedron. How many and
which symmetry elements are present in this point group symmetry?
9. The following relationships between lattice parameters:
a
z
b
#
c,
a
z
P
#
90 or 120•‹, and y
=
90" potentially define a "diclinic" crystal system
(two angles
#
90"). Is this an eighth crystal system? Explain your answer.
10. The relationships
a
=
b
#
c,
a
=
P
=
90•‹, and
y
#
90"
point to a
monoclinic crystal system, except that
a
=
b.
What is the reduced
(standard) Bravais lattice in this case? Provide equations that reduce this
lattice to one of the 14 standard Bravais types.
11. Consider space group symmetry Fdd2 and without using the International
Tables for Crystallography establish the following: (a) the crystal system;
(b) the corresponding point group symmetry; (c) the corresponding Laue
class; (d) the relationships between the unit cell dimensions; and (e)
explain the space group symbol.
Figure
1.56.
Illustration of a brick (parallelepiped) in which three independent edges have
different lengths.

Fundamentals
of
crystalline state
97
12. Consider independent atoms with the following coordinates in the space
group symmetry C2lm: Atoml:
x
=
0.15,
y
=
0.0,
z
=
0.33; Atom2:
x
=
.5,
y=0.11,~=0.5;andAtom3:x=0.25,y=0.25,z=0.25.Usingthe
International Tables for Crystallography carry out the following tasks:
a) Apply the coordinates and centering vectors listed for the general
equivalent position to generate all symmetrically equivalent atoms from
the three listed independent atoms (the total in each case should be the
same as the multiplicity of the general position).
b) Find atoms with equal coordinate triplets (remember that the difference
by a full translation in one, two or three directions refers to the same
atom) and cross them out. The total number of atoms left is the
multiplicity of the corresponding special position.
c) Establish both the multiplicity and the Wyckoff notation of special
position for each of the three listed independent atoms.
d) To which symmetry element(s), if any, do the independent atoms belong?
e) Which of the three original independent atoms occupies the general
equivalent position?
13. The crystal structure of a material is described in space group symmetry
P63/mmc with the following atomic coordinates
Atom
x
Y
z
Bal
0 0 0.25
Ba2
0.3333 0.6667
0.91 10
Ni
0 0 0
Sb
0.3333 0.6667 0.1510
01
0.4816 -0.0368 0.25
02
0.1685 0.3370 0.4169
Using the International Tables for Crystallography describe every atom
in terms of the multiplicities and Wyckoff letters of their site positions
and establish the content of the unit cell, the simplest chemical formula
and the number of formula units'
(Z)
per unit cell.
14. Imagine that there is an "edge-centered" lattice (for example unit cell
edges along
Z
contain lattice points at 112~). If this were true, the
following lattice translation is present:
(0,
0, 112). Convert this lattice to
one of the standard lattices.
'
Usually, a formula unit corresponds to the simplest chemical formula or to the
stoichiometry of the molecule of a material.
Chapter 2
FUNDAMENTALS OF DIFFRACTION
2.1
Introduction
In
the previous chapter, we introduced basic concepts of symmetry and
discussed the structure of crystals in terms of three-dimensional periodic
arrays of atoms and/or molecules, sometimes perturbed by various
modulation functions.
In
doing so, we implicitly assumed that this is indeed
reality. Therefore, it is time to think about the problem from a different point
of view: how atoms or molecules can be observed
-
either directly or
indirectly
-
and thus, how is it possible to determine the crystal structure of a
material and verify the concepts of crystallographic symmetry?
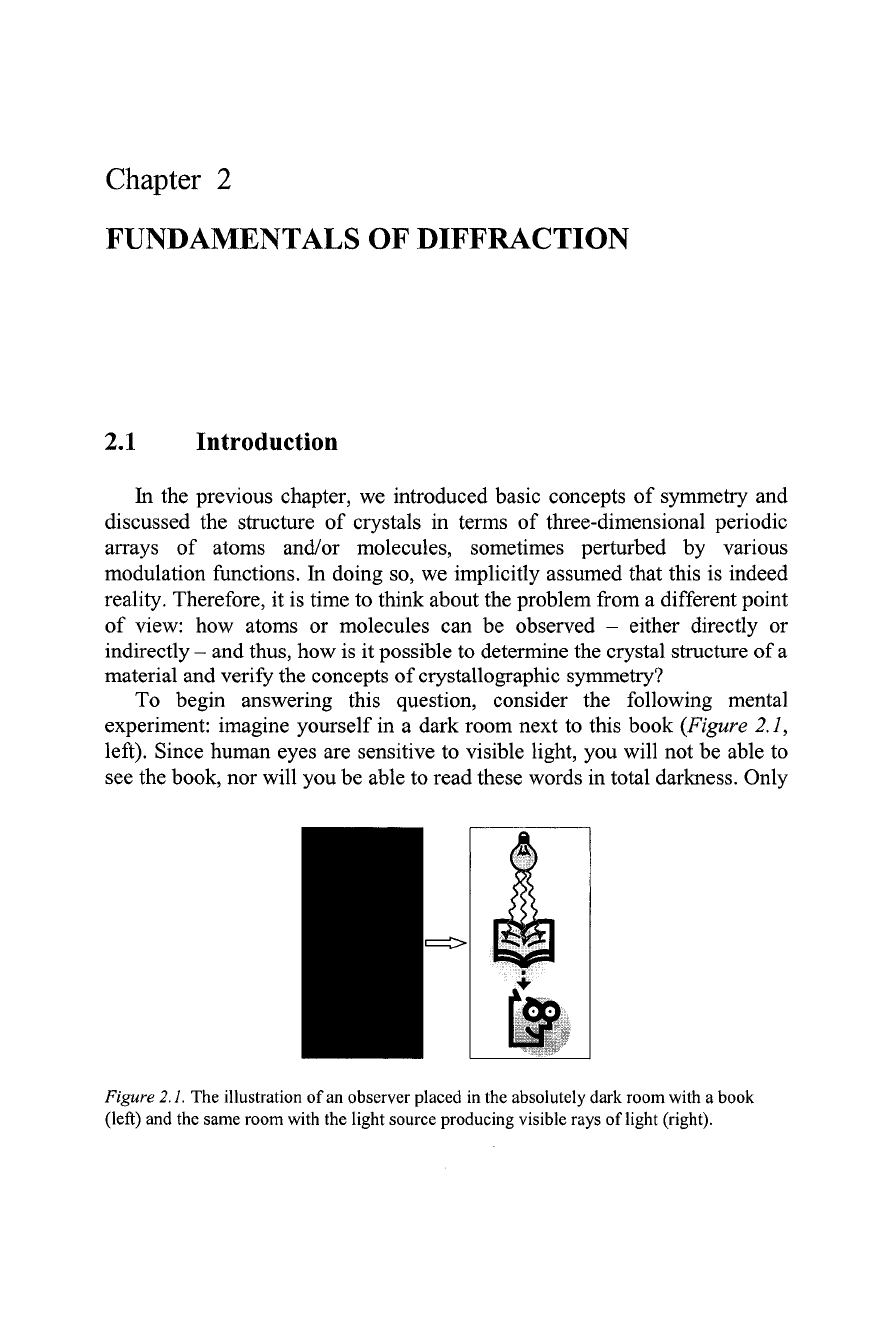
To begin answering this question, consider the following mental
experiment: imagine yourself in a dark room next to this book
(Figure
2.1,
left). Since human eyes are sensitive to visible light, you will not be able to
see the book, nor will you be able to read these words in total darkness. Only
Figure
2.1.
The illustration of an observer placed in the absolutely dark room with a book
(left) and the same room with the light source producing visible rays of light (right).

100
Chapter
2
when you turn on the light, the book becomes visible and the information
stored here becomes accessible
(Figure
2.1,
right). The fundamental
outcome of our experiment is that the book and its content can be observed
by means of a visible light after it has been scattered by the object (the book)
and detected by eyes.
In
general, a source of rays and a suitable detector (such as the light bulb
and the eye, respectively) are required to observe common objects. Atoms,
however, are too small to be discerned using any visible light source because
atomic radii' range from a few tenths of an angstrom to a few angstroms, and
they are smaller than 111000 of the wavelengths present in visible light (from
-4000 to -7000
A).
A suitable wavelength to observe individual atoms is
that of x-rays. The latter are short-wave electromagnetic radiation discovered
by W.C. Roentgen,* and they have the wavelengths that are commensurate
with both the atomic sizes and shortest interatomic distances.
Unfortunately, the index of refraction of x-rays is
-1
for all materials and
they cannot be focused by a lens in order to observe such small objects as
atoms are, as it is done by glass lenses in a visible light microscope or by
magnetic lenses in an electron microscope. Thus, in general, x-rays cannot
be used to image individual atoms
dire~tly.~ However, as was first shown by
Max von Laue in 1912 using a single crystal of hydrated copper sulphate
(CuS04.5H20), the periodicity of the crystal lattice allows atoms in a crystal
to be observed with exceptionally high resolution and precision by means of
x-ray diffraction. As we will see later, the diffraction pattern of a crystal is a
transformation of an ordered atomic structure into reciprocal space rather
than a direct image of the former, and the three-dimensional distribution of
atoms in a lattice can be restored only after the diffraction pattern has been
transformed back into direct space.
Particles in motion, such as neutrons and electrons, may be used as an
alternative to x-rays. They produce images of crystal structures in reciprocal
space because of their dual nature: as follows from quantum mechanics,
Atomic radius may be calculated self-consistently or it may be determined from
experimental structural data. Effective size of an atom varies
as
a function of its
environment and nature of chemical bonding. Several different scales
-
covalent, ionic,
metallic, and Van der Waals radii
-
are commonly used in crystallography.
Wilhelm Conrad Roentgen (1845-1923). German physicist who on November 8, 1895
discovered x-rays and was awarded the first ever Nobel Prize in Physics in 1901 "in
recognition of the extraordinary services he has rendered by the discovery of the
remarkable rays subsequently named after him". For more information about
W.C.
Roentgen see
http://www.nobel.se/physics/laureates/l90l/index.html
on the Web.
Direct imaging of atoms is feasible using x-ray holography, in which the wave after
passing through a sample is mixed with a reference wave to recover phase information and
produce three-dimensional interference patterns. For more information see R. Fitzgerald,
X-ray and y-ray holography improve views of atoms in solids, Phys. Today
54,21 (2001).
