Parinov I.A. Microstructure and Properties of High-Temperature Superconductors
Подождите немного. Документ загружается.

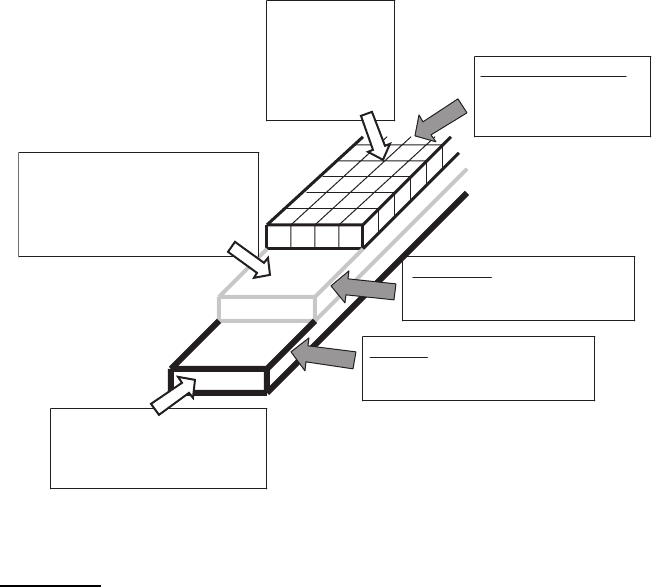
58 2 Composition Features and HTSC Preparation Techniques
obtained layer-by-layer (metal substrate–buffer layer–superconducting layer),
see Fig. 2.3. Every layer including metallic substrate plays a special role in
the long length conductors. The candidate materials and the thickness of each
layer are also presented in Fig. 2.3.
3
In general, thick YBCO-coated conductors consist of epitaxially grown
films on appropriate substrate with or without few buffer layers. Therefore, the
substrate or the buffer layer must have a suitable textured surface structure to
provide the required in-plane alignment growth of the HTSC crystalline films,
resulting in the avoidance of weak links due to the existence of only low-angle
grain boundaries. Typical problems, decided now at design and manufacture
of coated conductors, are presented in the Table 2.1.
The general problem of films, prepared by the liquid phase epitaxy and
electrophoretic deposition, demonstrating high rates of precipitation and suf-
ficiently low cost, is the crack formation due to stress relaxation during sample
cooling and different thermal properties of buffer layer and film. If we assume
that fixed cracks are extended through the whole film thickness, we may fol-
low a calculation for cracks in brittle films on elastic substrates [1057,1060].
According to the model, the total energy is the sum of the strain energy in the
Biaxial In-plane.
Alignment
Homogeneity.
High Production
Rate.
Thickening.
Alignment Control for HTSC
Layer. Prevention of Reaction.
Suppression of Crack Formation.
Homogeneity. Flatness. High
Production Rate.
High Strength. Non-magnetic
Materials. Thinning.
Alignment Control for Buffer
Layer.
Superconducting Layer
Y–123, Nd–123, Sm–123,
RE(mix)–123
Thickness: 1–10 µm
Buffer Layer YSZ, MgO, CeO
2
,
NiO, ZrO
2
, BaZrO
3
, etc
Thickness:< 3 µm
Substrate: Ni–Alloy (Hastelloy),
Ni, Ag, Ni–Cr Alloy, Zr, etc
Thickness: 25–125 µm
Fig. 2.3. The problems, solved in the design of high-qualitative-coated conductors
and including the development of basic structure, estimation of key factors and use
of candidate materials for each layer [985]
3
For simplicity and clearness, a thin passivation layer for stabilization, insulation
and encapsulation is excluded.
2.1 YBCO Films and Coated Conductors 59
Table 2.1. Nearest goals of R&D for three different types of YBCO-coated conductors [985]
Type Structure or
technique
features
Substrate Buffer
layer
HTSC layer Key
processes
Target
Textured
substrate
Non-reactive,
high strength
Ni, Ag clad
materials:
Ni–Cr, Ni–V,
Ni-based alloys,
etc.
None or
NiO, ZrO
2
,
BaZrO
3
,
MgO,
YSZ,
Y
2
O
3
,
CeO
2
etc.
Y-123,
RE-123,
etc.
Rolling/annealing;
surface
polishing;
surface oxidation
epitaxy; buffer
layer; HTSC
layer; evaluation
(J
c
,etc.)
Length: 10–100 m;
substrate thickness:
≤ 100 μm;
J
c
≥ 10
5
− 10
6
A/cm
2
(77 K)
Aligned buffer
layer
ISPLD, IBAD Polycrys-talline
Hastelloy,
Ni-based alloys,
etc.
YSZ,
MgO,
CeO
2
,etc.
Y-123,
RE-123,
etc.
Substrate
polishing; IBAD
process; ISPLD
process; HTSC
layer; evaluation
(J
c
,etc.).
Length: 100–1000 m;
substrate thickness:
≤ 100 μm;
J
c
≥ 10
4
–10
5
A/cm
2
(77 K); production
rate: > 1m/h
Rapidly grown
HTSC layer
MOD, LPE Ni, Ag clad
materials:
Ni-based alloys,
etc.
None or
MgO,
YSZ, NiO,
BaZrO
3
,
CeO
2
,etc.
Y-123,
RE-123,
etc.
Substrate
polishing;
buffer layer;
homogeneous
seed film;
MOD process;
LPE process;
evaluation (J
c
,
etc.)
Length: 1–10 m;
substrate thickness:
≤ 100μm; HTSC
thickness: ≥ 5μm;
J
c
≥ 10
5
–10
6
A/cm
2
(77 K); production
rate: > 1m/h
60 2 Composition Features and HTSC Preparation Techniques
cracked region and the energy necessary to create the cracks, that is, the new
surface energy. The crack spacing, l, will be adjusted in order to minimize the
total energy of the system to [1060]
l ≈ 5.6
K
2
Ic
h/(Eε)
2
, (2.1)
where K
Ic
is the fracture toughness of the mode I, h the film thickness, E
the Young’s modulus and ε the strain. The total energy of the cracked film
cannot exceed the strain energy of the uncracked film. This results in minimum
crack spacing and is consistent with the requirement that the energy release
by cracking should be higher than the energy necessary to create the cracks.
This means that below a critical thickness
h
c
=0.5K
2
Ic
/(Eε)
2
, (2.2)
no cracks will appear.
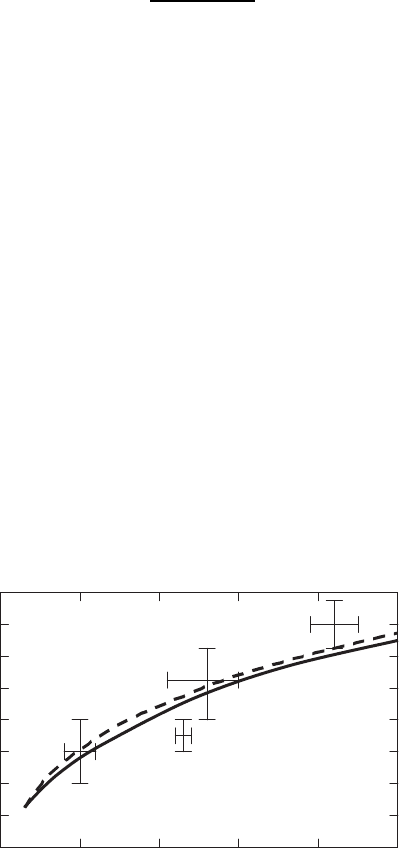
Based on this approach, the predicted dependence of the average crack
spacing l on the thickness h of a-axis-oriented YBCO films on (100)SrPrGaO
4
substrates is shown in Fig. 2.4. At the same time, the decreasing micron-
sized particles to sub-micron-sized particles in colloids may diminish the film
cracking at its electrophoretic deposition consideraldy (see Fig. 2.5).
In order to obtain YBCO samples with high properties, a preparation of
qualitative precursor powders is also very important. A number of techniques
for processing YBCO powders have been reported, including conventional
solid-state reaction, precipitation [106], plasma spray [415], freeze drying [495],
spray drying, combustion synthesis [571], sol–gel method [722], acetate method
[686] and flame synthesis [889].
0
•
•
•
•
20
15
10
5
0
246810
Film Thickness, h (µm)
Average Crack Spacing,
l (µm)
Fig. 2.4. Theoretical crack spacing (solid curve) as a function of the film thickness
compared with the experimental data and a fit (dashed curve) to the data obtained
from a-axis-oriented YBCO LPE films grown on (100) SrPrGaO
4
[05]

2.1 YBCO Films and Coated Conductors 61
Film A
Film B
500 µm
500 µm
(a)
(b)
Fig. 2.5. Scanning electron microscopy images of sintered films. The images of (a )
and (b) show films fabricated from colloids, consisting of micron-sized particles and
sub-micron-sized particles, respectively. The heat treatment conditions for both are
the same [926]
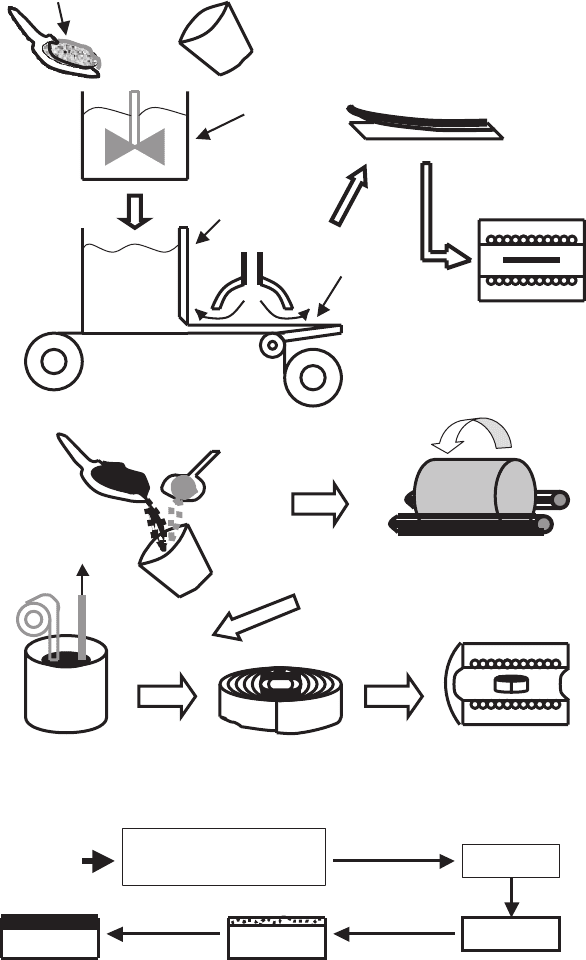
The fabrication of a ceramic superconducting powder is presented in the
flow chart of Fig. 2.6. First, the raw materials are weighed in a desired mo-
lar ratio and then mixed by conventional mixing/milling or liquid-solution
mixing. The homogeneity, obtained by conventional mixing, is limited by the
particle size of the powders, but the best mixing is generally obtained from
powders with a particle size less than 1 μm. For ultra-fine powders (particle
size much smaller than 1 μm), the particles tend to segregate, thus resulting
in poor mixing. This problem may be minimized by liquid-solution mixing,
ensuring the precise compositional control and molecular-level chemical ho-
mogeneity. Moreover, this technique eliminates contamination from grinding
and milling media that occurs during a conventional mix/milling process. For
a multi-component system like HTSC, the mixing plays a key role in obtaining
high phase purity. Better mixing also translates into faster reaction kinetics.

62 2 Composition Features and HTSC Preparation Techniques
Raw Materials
Mixing Grinding
Drying or Solvent
Removal
Calcination
Grinding Comminution
Fig. 2.6. A flow chart for the synthesis of ceramic superconducting powders
These powders can be calcined at lower temperatures and/or shorter time to
achieve the desired phase purity.
The next step is drying or solvent removal, that is necessary to preserve
the chemical homogeneity obtained by mixing. For multi-component systems,
solvent removal by slow evaporation can lead to a very inhomogeneous residue
due to the difference in solubilities of various components. In order to minimize
this effect, various techniques are used, including, in particular, the processes
of filtration, sublimation, etc. [960].
After drying, the powders are calcined in a controlled atmosphere for re-
action until reaching a final composition and phase assemblage. The reaction
Melt
Melt +
Y
2
BaCuO
5
Melt +
YBa
2
Cu
3
O
7–x
Melt +
CuO + BaCuO
2
Y
2
BaCuO
5
+
YBa
2
Cu
3
O
7–x
Y
2
BaCuO
5
+
CuO + BaCuO
2
Y
2
BaCuO
5
YBa
2
Cu
3
O
7–x
CuO + BaCuO
2
T
Fig. 2.7. A phase diagram of Y
2
O
3
–CuO–BaO system [318]
2.2 BSCCO Films, Tapes and Wires 63
path for HTSC systems depends on various processing parameters, such as
calcination temperature and time, heating rate, atmosphere (oxygen partial
pressure) and starting phases. The powders can also be synthesized directly
from solution using pyrolysis techniques [889] or an electro-deposition tech-
nique [65].
The phase diagram, presented for YBa
2
Cu
3
O
x
in Fig. 2.7, shows that even
small fluctuations of compositions can lead to the formation of normal (non-
superconducting) phases, namely Y
2
BaCuO
5
, CuO and BaCuO
2
. An applica-
tion of precursors with carbon content also complicates the formation of the
YBa
2
Cu
3
O
x
phase and may lead to diminishing superconducting properties.
In detail, the carbon problem will be considered in Chap. 4.
2.2 BSCCO Films, Tapes and Wires
Now for the preparation of Bi-2212 thick films on Ag and MgO substrates,
the set of techniques are applied, namely melt-processing [405,407], elec-
trophoretic deposition [49], doctor-bladed [520], dip-coated [1067] and organic
precursor [397] films. Last three procedures are presented in Fig. 2.8. It should
be noted that the fabrication of Bi-2223, possessing higher superconducting
properties, by using melt processing is impossible because of PbO loss in high-
temperature process [253]. The powder for doctor-bladed and dip-coated films
can be made using any of the processes listed in Table 2.2.
In the doctor-bladed process, one makes a “green” film (i.e., film before heat
treatment) from the organic/powder mixture by pouring a pool of the slurry
on a flat surface (e.g., a piece of glass), then leveling the slurry with a straight-
edged blade, located above the flat surface at the desired film thickness (this
allows to carry out corresponding control during its preparation). The film
is dried, cut into strips that are placed on the silver foil and finally melt
processed.
For the dip-coated films, the silver foil is passed through the organic/powder
mixture, which adheres to the foil. The thickness of the film is controlled,
changing the organic compounds, modifying their proportions in the mixture
and adjusting the solids loading in the mixture. After passing through the or-
ganic/powder mixture, the coating is dried, the organics are burned out and
the film is melt processed.
For the organic precursor films, a solution of organometallic compounds
of Bi, Sr, Ca and Cu is deposited on the Ag foil, the solvent is burned out and
the process is repeated until the desired layer thickness is built up. Finally,
the film is melt processed. A Bi-2212 film can also be made, painting Bi-2212
powder onto a silver foil. Here, the powder is mixed with an organic liquid
having a high vapor pressure (e.g., butanol). This slurry is brushed onto the
foil and finally melt processed.
Since the pioneering work of Heine et al. [405] on Bi-2212 conductors, al-
most all Bi-2212 conductors have been melt processed. This method is also
64 2 Composition Features and HTSC Preparation Techniques
(a)
(b)
(c)
Oxide
Powder
Organic
Formulation
Mixer
‘‘Green’’ Tape
‘‘Green’’ Tape
“Green” Tape
Doctor-Blade
Slurry
Ag Foil Substrate
Carrier Sheet
Furnace
Organic Binder
Calcined Powder
Organic Solvent
Mixing
Continuous Dipping
Cooling
Heat Treatment
Bi(C
7
H
15
COO)
3
Ca(C
7
H
15
COO)
2
Sr(C
7
H
15
COO)
2
Cu(C
7
H
15
COO)
2
Organo-metallic
Compounds
Dissolve into
Organic Solution
Solution
Ag
HTSC Tape
Heat
Treatment
Ag
Pyrolyze
500ºC, in Air
Ag
Fig. 2.8. Schematic diagram to make (a)doctor-bladed,(b) dip-coated and (c)
organic precursor films [397]
2.2 BSCCO Films, Tapes and Wires 65
Table 2.2. Synthesis techniques to make Bi-2212 and Bi-2223 powders
Method Description Advantages Disadvantages
12 3 4
Solid-state
reaction
[409]
Mix oxides, peroxides, carbonates or nitrates of
Bi, (Pb), Sr, Ca and Cu. React at elevated
temperature where no melting occurs. Grind
sample and refire. Repeat until reaction is
complete
Simple, inexpensive
technique
Large grain size of reactants
can cause slow reactions.
Product can have large
grain size. Impurities can
introduce during grinding
Co-
precipitation
[409]
Dissolve Bi, (Pb), Sr, Ca and Cu compounds in
acid. Add base to precipitate cations. Fire
precipitate to yield the desired phase
Intimate mixing of
cations
Not all cations may
precipitate out at the same
rate, causing segregation.
Initial composition and
precipitate composition may
be different
Aerosol spray
pyrolysis [409]
Make solution, containing cations. Produce fine
mist of the solution and pass it through a hot
furnace to form a powder of mixed oxides. Fire
mixed powder to yield the desired phase
Intimate mixing of
cations. Product has
very fine grain size
(1–2 μm). Product can
have low carbon
content (using nitrates)
Species can lose, partially
Pb, during pyrolysis.
Powder, formed in pyrolysis
is not fully reacted to the
desired phase
Burn
technique [07]
Form nitrate solution of cations. Add organic
species, such as sugar, to solution. Heat solution
to remove water then heat powder at elevated
temperature. The sugar (fuel) and nitrate
ion oxidant react (i.e., burn) at elevated
temperature, yielding a high temperature that
forms mixed oxides. Fire this powder to yield
the desired phase
Intimate mixing of
cations. Powder can
have fine grain size
Species can lose, partially
Pb, during burn process.
Powder formed in burn
process is not fully reacted
to the desired phase.
Product may contain carbon
(continued)
66 2 Composition Features and HTSC Preparation Techniques
Table 2.2. (continued)
Method Description Advantages Disadvantages
12 3 4
Freeze drying
[409]
Spray aqueous nitrate solution of Bi, (Pb),
Sr, Ca and Cu into liquid nitrogen. Collect
frozen droplets and freeze dry them to
remove water. Fire dried powder to yield
the desired phase
Intimate mixing of
cations. Product can
have low carbon
content
Cations may demix during
freeze drying, if the
temperature is not carefully
controlled. Nitrates,
presented after freeze drying,
may melt during firing,
leading to large grains of
non-superconducting phases
Liquid mix
method [838]
Form nitrate solution of cations then add
glycol or citric acid. Heat to remove water
and form polymerized gel, then heat to
elevated temperature to yield the desired
phase
Intimate mixing of
cations. Powder can
have fine grain size
Product may contain carbon
Micro-
emulsion
[587]
Form suspension of micro-droplets of
aqueous nitrate solution of Bi, (Pb), Sr,
Ca and Cu in oil. Add base to form
precipitates. Separate precipitate from oil
by washing in solvent. Fire precipitate to
yield the desired phase
Intimate mixing of
cations. Powder can
have fine grain size
Product may contain carbon
Sol–gel [91] Form alkoxide solution of cations. Add
water or alcohol to cross-link molecules,
forming gel through polymerization and
condensation reactions. Heat to elevated
temperature to burn the organics and yield
the desired phase
Intimate mixing of
cations. Powder can
have fine grain size
Method is better suited to
making films, than bulk
powders
2.2 BSCCO Films, Tapes and Wires 67
named by partial melt processing, that reflects the fact that Bi-2212 melts
incongruently forming liquid and crystalline phases (i.e., partial melt).
4
Nu-
merous heating schedules that are currently used for the melt process of Bi-
2212 can be generalized for tapes and wires (Fig. 2.9a), and also for films
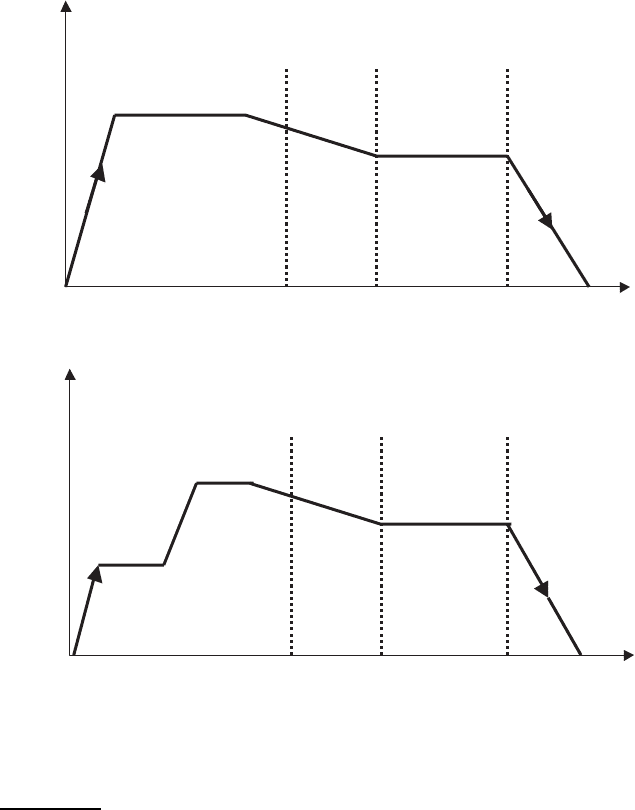
(Fig. 2.9a). Both schedules could be divided into four stages [409].
T
max
30 min
5 min
10°/h
~840°C
72 hours
5°/min
I
II
III
IV
Room Temperature
Temperature
Time
T
max
10°/h
100°/h
840°C
2 hours
I
II
III
IV
Room Temperature
Temperature
Time
600°C
1 h
(a)
(b)
Fig. 2.9. Generic Bi-2212 melt-processing schedules used for (a) tapes and wires
and (b) films. The schedules have been divided into four regions that are described
in the text [409]
4
It should be noted that the term melt processing clearly distinguishes from a
lower-temperature processing, where only a portion of the Bi-2212 is melted,
which is called liquid-assisted processing [410].
