Parinov I.A. Microstructure and Properties of High-Temperature Superconductors
Подождите немного. Документ загружается.

68 2 Composition Features and HTSC Preparation Techniques
In Region I, the conductor is heated above the melting point of Bi-2212,
held at this temperature for a short time and then cooled. In this stage,
Bi-2212 powder melts incongruently, forming liquid and non-superconducting
crystalline phase. At the end of this stage, Bi-2212 formation begins. At melt-
ing, the Bi-2212 powder releases oxygen, which is not a problem for films
that are open to the environment, but can be a problem for tapes and wires,
where it can cause the silver sheath to bubble. On the other hand, at el-
evated temperatures, used during melting, Bi evaporation from the melt is
possible [924]. The silver sheath of tapes and wires prevents this process, but
this is actual danger for films. Also, it should be noted that critical condi-
tion to prepare homogeneous highly aligned microstructure is the minimum
fraction of crystalline phases in the melt at the beginning of Bi-2212 phase
formation.
In Region II, Bi-2212 forms from the melt, where growth and alignment of
Bi-2212 grains occur. Since Bi-2212 melts incongruently, at cooling it should
be formed by a reaction between liquid and crystalline phases. In this case,
in order to form a homogeneous highly aligned microstructure, the melt must
contain small grain of non-superconducting phases when Bi-2212 begins to
form. The cooling rates, used for the melt process of Bi-2212 conductors
should be sufficiently fast to intensify Bi-2212 phase formation and mini-
mize fraction of normal phases, which are present in the final product always.
The problems with having non-superconducting phases in the fully processed
conductor are that the phases are too large to pin flux, they block the su-
percurrent path and diminish useful properties of the conductor. In order to
obtain high superconducting properties, a highly aligned grain structure is
necessary. Plate-like Bi-2212 grains grow from the melt because growth of
Bi-2212 is faster in the ab-plane than in the c-direction. This two-dimensional
growth is critical for the alignment that develops during cooling. The experi-
ments have shown [409] that the cooling rate and thickness of the oxide melt
affect the alignment: slower cooling and thinner oxide yield higher alignment.
The misorientation angle for a given grain size decreases with decreasing oxide
thickness. The alignment mechanism requires that the large, properly oriented
grains grow at the expense of the smaller misoriented grains. Films that are
< 20–25 μm thick align easily. In thicker films, the alignment is usually not
uniform throughout, being higher close to the free surface than near the silver
interface. It has been suggested that in the films the Bi-2212 growth and align-
ment begin at the free surface and proceed into the oxide layer. Since films
are two-dimensional, one would expect a higher alignment in thinner films be-
cause of the smaller misorientation angle a grain could have and still grow to
a given length. Moreover, as the aligned grains grow in films, they may rotate
misaligned grains into alignment near the free surface more easily compared
with the nearest neighborhood of the superconductor/metal interface. The
free surface in films is also important from the point of general alignment of
the film structure. Thick films (50–100 μm) can have a 20–25 μmthicklayerof
aligned Bi-2212 grains at the free surface with poorly aligned Bi-2212 below
2.2 BSCCO Films, Tapes and Wires 69
this layer [409]. In general, the grain alignment and J
c
are higher in films
than tapes.
In Region III, the formation and alignment of Bi-2212 is maximized. At
the same time, in Region IV, where the sample cools to room temperature, the
critical alteration of microstructure occurs. High J
c
requires fast cooling rate
for films (of order 1200
◦
C/h [984]) and analogous rates for tapes and wires
[977]. However, at very high cooling rates the conductors may crack, leading
to low J
c
[984]. At the same time, the Bi-2212 can decompose with slow cool-
ing rates (at < 300
◦
C/h) forming Bi-2201, possessing lower superconducting
properties.
In order to minimize the number of intergranular weak links, that are
proper for oxide superconductors, a high degree of crystallographic texture
must be obtained. One possible route by which a strong crystallographic
texture can be produced is to melt process the material under the effect
of an elevated magnetic field [275]. In this case, the driving force for grain
alignment is provided by the anisotropic paramagnetic susceptibility, exhib-
ited by the superconductor grains. When a superconductor grain is placed in
a magnetic field, the axis of maximum susceptibility aligns with the magnetic
field direction. As a result, in the case of superconductors, such as BSCCO and
YBCO the grains should align with the c-axis parallel to the external magnetic
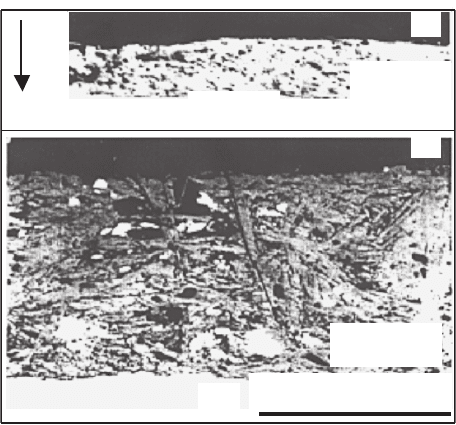
field [53,1100]. Scanning electron microscopy (SEM) backscattered images of
polished cross-sections of Bi-2212 thick films with two different thicknesses,
processed in zero field and at 10 T magnetic field, are shown in Figs. 2.10
H
0T
Ag
100 µm
Ag
Bi-2212
Bi-2212
(a)
(b)
Fig. 2.10. SEM images of cross-sections of Bi-2212 films melt processed in the
absence of magnetic field [275]
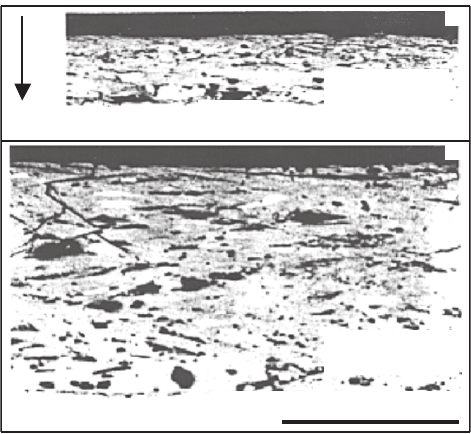
70 2 Composition Features and HTSC Preparation Techniques
Ag
H
10T
Ag
100
µm
Bi-2212
Bi-2212
(a)
(b)
Fig. 2.11. SEM images of cross-sections of Bi-2212 films melt processed under 10 T
magnetic field [275]
and 2.11, respectively. In the first case, the degree of texture decreases with
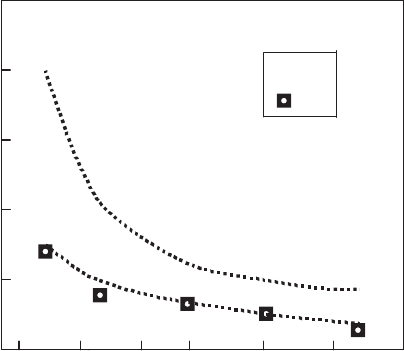
the increasing film thickness. In Fig. 2.12, the transport critical current den-
sities of the films measured at 4.2 K are plotted as function of their thickness
for films processed under zero and 10 T magnetic field. Analogous results of
a higher degree of alignment at the higher magnetic field are also observed in
Bi-2212 tapes (see Fig. 2.13).
The superconductors Bi-2212/Ag are also prepared by using melt-solidifica-
tion method [396]. In its modification, pre-annealing and intermediate rolling
are applied to improved the alignment of superconducting grains [699]. At
the same time, monocore and multifilament superconducting tapes and wires
in silver sheath (Bi-2212/Ag and Bi-2223/Ag) are prepared most successively
by using the oxide-powder-in-tube (OPIT) method [216,1091,1159]. Short Bi-
2223/Ag multifilament tapes have demonstrated J
c
> 80 kA/cm
2
at 77 K us-
ing this technique [287]. However, this multistage method is characterized
by numerous technical parameters and procedures which define (together
with initial composition) useful properties of the final sample. In this case,
it is very important to prepare superconducting Bi-2212 and Bi-2223 pow-
ders, possessing better phase composition, grain size and shape, and also
chemical purity. Corresponding technical operations have been presented in
Table 2.2. Today, the standard approaches to obtain Bi-2223 precursor pow-
ders include the so-called methods of one-powder synthesis and two-powder
2.2 BSCCO Films, Tapes and Wires 71
•
•
•
•
•
20 40 60 80 100 120
•
0 T
10 T
140
Thickness (µm)
J
c
(10
4
A/cm
2
)
5
4
3
2
1
0
Fig. 2.12. Transport current densities at 4.2 K, zero field, as a function of thickness
for films processed under 0 and 10 T magnetic fields [275]
synthesis [213]. In the first case, the precursor is prepared as the result
of calcination of the oxides and carbonates mixture. In the second case,
sintering of the mixture of two cuprate compositions is carried out. The
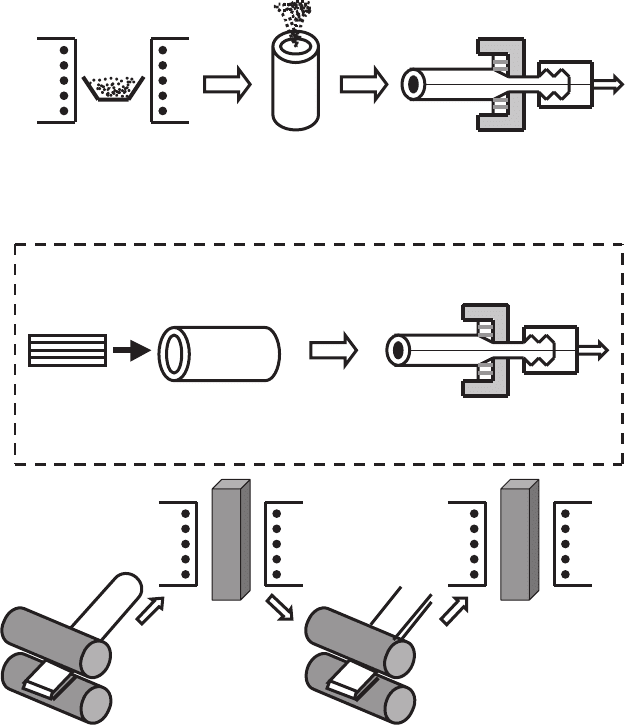
OPIT method for making conductors is shown schematically in Fig. 2.14
and includes the following procedures. After the preparation of precursor
powder, it is packed in a tube from Ag or its alloy. Here, silver is used,
in particular, because of its property to diffuse oxygen at high tempera-
tures. This permits to control the oxygen pressure during superconductor
fabrication [409]. The tube is sealed and mechanically worked into desired
conductor form. Usually the Ag tube is drawn to a small diameter (∼ 2 mm),
using set of cone holes. Then, this wire is rolled into a flat tape with
thickness ∼ 0.1 mm. Multifilament superconductors are prepared, placing
separate wires in a tube with a large diameter and carrying out analo-
gous technical operations. At final stage, the samples are subjected to set
of thermal treatments. During chemical reaction, necessary superconduct-
ing phases form at this stage. Mechanical deforming helps to align and con-
struct the texture of crystalline structure in this technological link. Thus, the
brittle superconducting oxide is encircled by the sheath from silver or its
alloy which protects the superconducting core from chemical and thermody-
namic influences. Figure 2.15 shows different OPIT conductor forms that have
been made.
The use of silver as sheath material of BSCCO tapes and wires is caused by
the compatibility of Ag with superconducting powders at high temperatures
of sintering. However, for tapes with silver sheath, there is the problem of
72 2 Composition Features and HTSC Preparation Techniques
0 T
10 T
H
Fig. 2.13. SEM images of cross-sections of Bi-2212 tapes melt processed under 0
and 10 T magnetic fields [275]
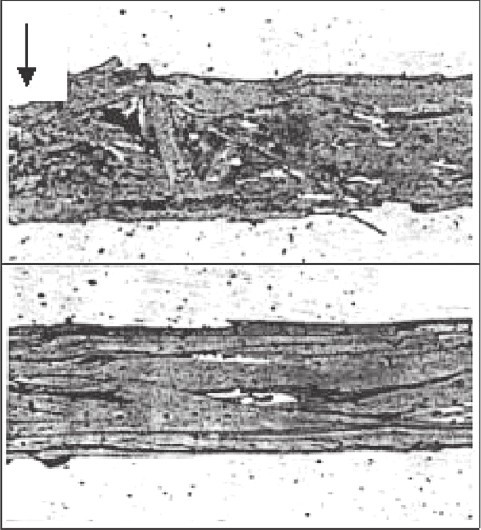
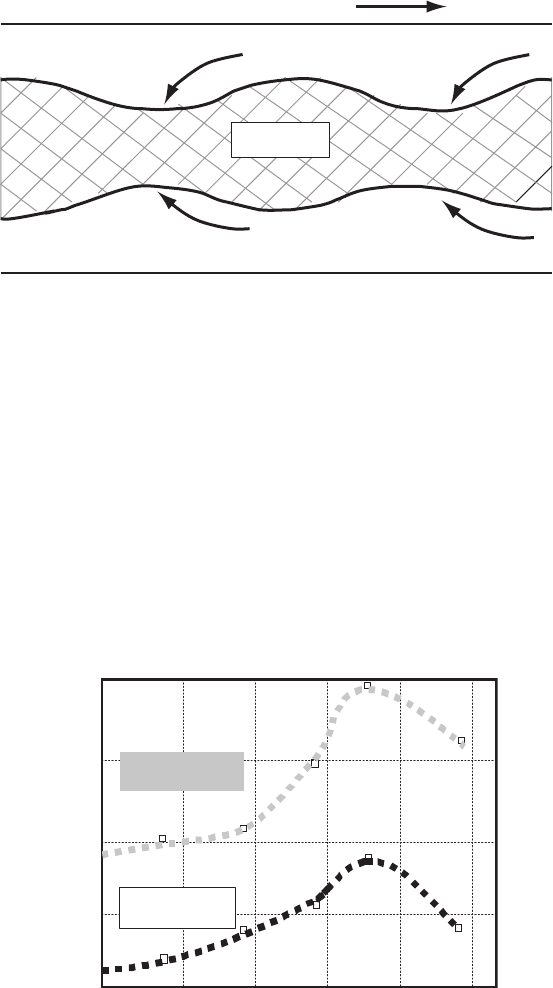
formation of an undulating oxide/Ag interface, which is known as sausaging.
This occurs along the tape after its drawing, rolling and pressing (Fig. 2.16)
[385,665], and also silver creeps at high temperatures [320]. Moreover, HTSC
tape sheath, used in concrete devices or samples, must satisfy definite de-
mands, namely (i) they must possess sufficient mechanical strength in order
to bear strains, stated by high electric and magnetic fields, that may crack
brittle superconducting core; (ii) they must possess low heat conduction in
current conductor and high electric resistance to decrease current losses in
cables [1177]. In order to solve these problems, the silver alloys with some
metals, namely Au, Cu, Mg, Mn, Pd, Zr, (Ni, Y), (Zr, Al) and (Mg, Ni) are
used [06,16,954,991,1063]. Thus, in total, there is a problem to obtain optimum
microstructures of superconducting composite, taking into account different
behaviours of the silver (alloy) sheath and oxide core at thermal treatment
[385,1130].
Now, OPIT method is generally applied to prepare BSCCO superconduc-
tors. The advantage of this HTSC system is that the texturing and alignment
of crystallites, influencing significantly superconducting properties of tapes,
may be simply achieved at the final stage of the sample fabrication. In this
2.2 BSCCO Films, Tapes and Wires 73
Powder
Sintering
Packaging
Tube
Drawing
Multi-filamentary Samples
Restack
Second Drawing
Annealing
Rollin
g
Re-rolling
Re-annealing
Fig. 2.14. Schematic diagram of the oxide-powder-in-tube method to make HTSC
wires and tapes [409]
case, the tape deformation leads to shear in BSCCO structure along the double
layer Bi–O, acting as glass phase, due to the weak joints of the adjacent layers
to each other. As a result, the grains form an aligned structure at the next
heating. The YBCO family has a worse structure of grains and demonstrates
higher isotropy compared with BSCCO and also possesses small intergranular
transport currents that are caused by weak links. Therefore, all attempts to
use OPIT method for this family were not successful [649,1021].
74 2 Composition Features and HTSC Preparation Techniques
(a)
(b)
(c)
(f)
(d)
(e)
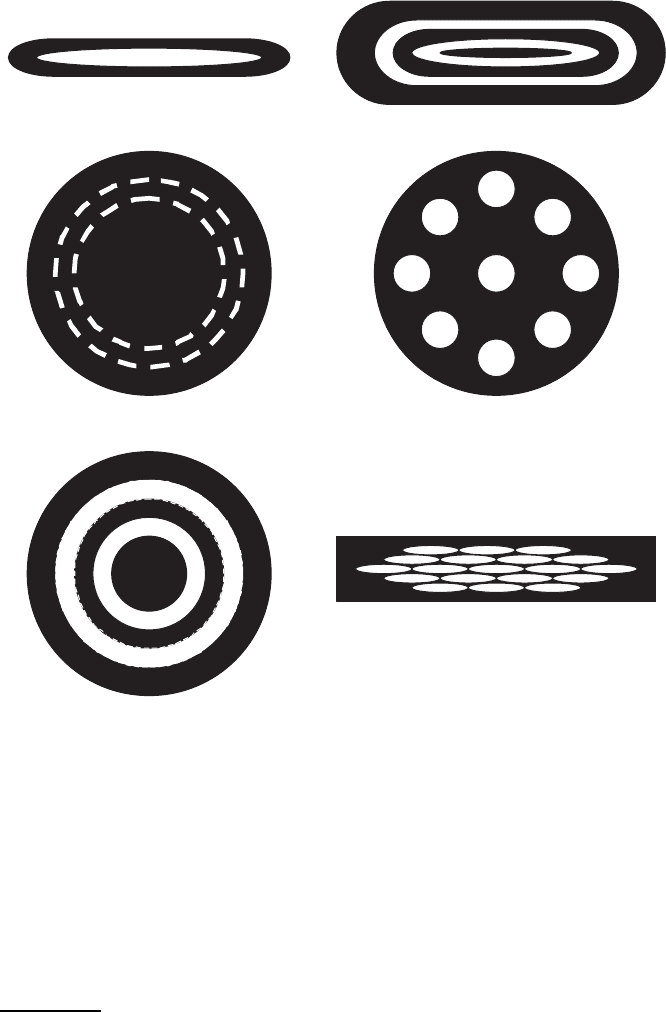
Fig. 2.15. Representative configurations for Ag-sheathed wires and tapes. Black
regions are Ag and white regions are BSCCO: (a) monocore tape, (b) coaxial mul-
tifilament wire, (c) wire with two BSCCO cores, (d) tape rolled from the wire in
(c), (e)filamentwireand(f)filamenttape
At long sintering of Bi-2223/Ag monocore tapes, a diminishing of magnetic
flux pinning with corresponding decrease in critical current, I
c
(Fig. 2.17
5
),
which could be caused by decreasing Pb content in the superconducting
core at increasing calcination time [670,671], is observed. However, we pro-
pose the more probable causes decreasing I
c
to be inevitable processes of
pore transformation at intergranular boundaries with pore displacement into
5
Obviously, the initial increase in critical current is connected with improvement
in quality of intercrystalline boundaries. However, this factor has secondary value
at longer sintering.
2.2 BSCCO Films, Tapes and Wires 75
Ag
BSCCO
Rolling Direction
Fig. 2.16. Longitudinal cross-section of a BSCCO tape, showing undulations in the
Ag/oxide interface. This is known as sausaging
growing grains, forming close porosity at prolonged sintering of the sample
[801,1185].
In total, an increasing of low pinning, which is intrinsic to BSCCO family
(compared to YBCO family), is one of the main goals of thermal treatment,
applied to prepare BSCCO/Ag samples by OPIT technique [87,282,493].
There are other problems demanding their solution during tape processing by
using OPIT technique, namely (i) an initiation of the local heterogeneities into
powders during mechanical deformation (e.g., pressing) [673]; (ii) the bubble
formation into silver sheath due to isolation of gases [384,568]; (iii) a possi-
bility of the microstructure perturbations because of violation in crystallite
alignment, initiated near secondary phases due to the formation of crack-like
500 100 150 200 250
120
70
20
10
0
Time (h)
Ic(A)
T = 4.2 K
T = 77 K
B = 0 T
Fig. 2.17. The critical current vs sintering time (B =0T,T =4.2and77K)
76 2 Composition Features and HTSC Preparation Techniques
defects during inelastic strain of pressed powder [831], and also due to various
deviations from optimum-phase composition [783]; (iv) difficulties in the tex-
ture formation of HTSC connected with the existence of secondary phases, het-
erogeneity and redistribution of material during thermal treatment [385,624];
(v) impossibility to totally heal the microcracks formed during mechanical de-
formation using high-temperature treatment [854]. Finally, an increased length
of tape initiates an action of scaling factor, which influences considerable de-
crease in the critical current density. Due to this, the doping additions of Ag
(Ag
2
O, AgNO
3
) are added to HTSC for improvement of structure-sensitive
properties [915,1001]. The complexity and multistage character of the OPIT
technique, and also smaller more than order of magnitude the critical cur-
rent density of tapes of compare with films, define modifications of this stan-
dard technique of the BSCCO/Ag tape processing. As examples, we point the
following:
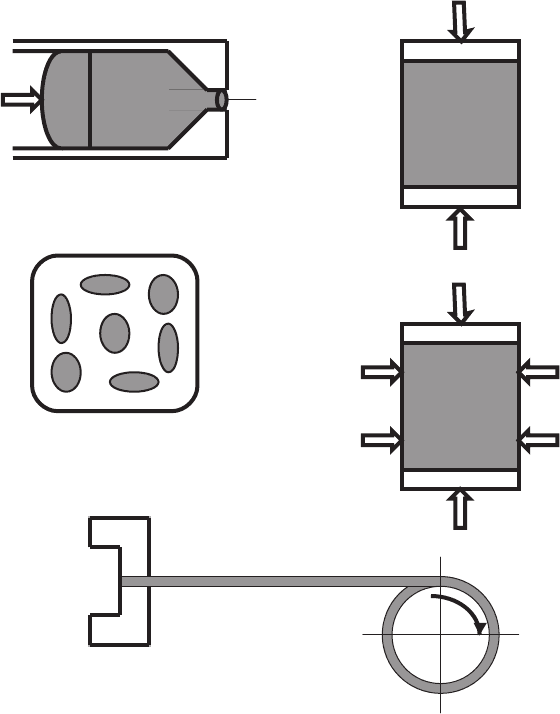
– using the hot extrusion (Fig. 2.18a) to obtain long-scale (l>150 m) Bi-
2223/Ag tapes [673];
– intermediate mechanical deforming (one-axis pressing [341] (Fig. 2.18b) or
cold rolling [831]) of tapes after first sintering, that form Bi-2223 phase in
order to increase the density of superconducting core and corresponding J
c
,
– application of deformation methods which are alternative to rolling, namely
semi-continuous pressing [592], consecutive pressing [32] and periodic
pressing [672];
– modifications of the standard rolling: (i) out-center rolling with eccentric-
ity, using two concentric rollers [565], (ii) sandwich rolling, in which a
tape locates between two thick steel plates fastened with springs [1133],
(iii) transversal (differing from standard or longitudinal) rolling [359], (iv)
two-axis rolling, that replaces the wire-drawing [450], (v) groove rolling
(Fig. 2.18c) [457];
– pressing Bi-2223/Ag tapes at cryogenic temperatures, based on relative
increase in silver hardness compared with superconducting oxide at liquid
nitrogen temperatures [164];
– application of hot isostatic pressing (Fig. 2.18d) to solve the problem of
porosity and microcracks in Bi-2223/Ag tapes [77];
– using an expensive pressure to pack a superconducting core at the tem-
peratures of Bi-2223/Ag phase formation [888];
–usingthewind-and-react (W&R), react-and-wind (R&W) and react-wind-
sinter (RWS) techniques of continuous winding at the final stage of Bi-
2212/Ag tape processing, which decrease the damage of long-scale samples
during their winding [78];
–usingthewind-react-and-tighten (WRAT) technique, that combines the
advantages of the W&R and R&W techniques and consists of free wind-
ing and reaction in the winding with final introduction of isolation after
completion of thermal processes and tightness of the winding to its final
size [765].
2.2 BSCCO Films, Tapes and Wires 77
(a)
(c)
(d)
(e)
(b)
Fig. 2.18. Some additional technical operations used to improve quality of HTSC
prepared by OPIT technique: (a)extrusion,(b) one-axis pressing (c) groove rolling
(a cross-section of seven-filament wire is shown), (d) isostatic pressing and (e)con-
tinuous winding
Finally, new techniques to prepare the composite tapes and wires are
designed, that use optimum geometry and placement of components or
advantages of superconducting and ceramic materials, namely:
– Tape-in-rectangular-tube (TIRT) technique to prepare superconducting fil-
aments with c-axis oriented in the direction that is not perpendicular to
the tape surface (Fig. 2.19) [850].
– Processing round Bi-2212/Ag wires by using stranded-and-formed method
(SAFM), in which first, filaments are bundled to consist of a segment, and
