Parinov I.A. Microstructure and Properties of High-Temperature Superconductors
Подождите немного. Документ загружается.

310 7 Modeling of YBCO Oxide Superconductors
Crystal
Diffusion Layer
Atmosphere
Δμ
Δμ
1
Δμ
2
a
b
c
μ
L
μ
S
Chemical Potential
C
I
C
L
211
C
L
123
Y-Concentration
Growing
Interface
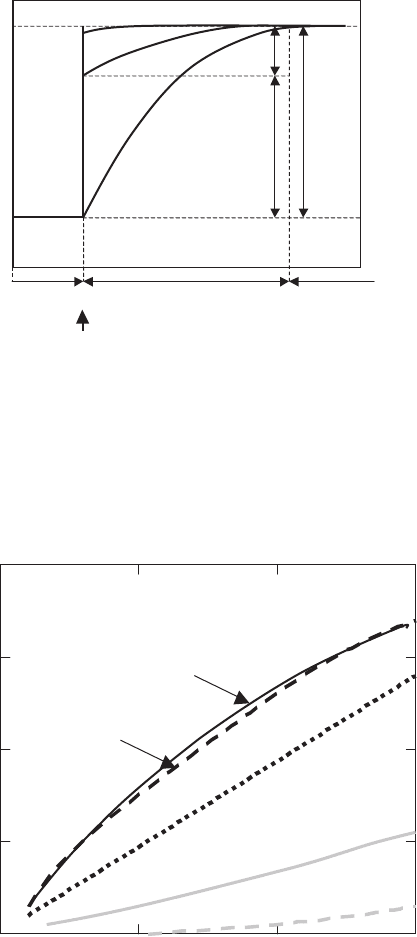
Fig. 7.6. Yttrium concentration profiles in front of the growing interface [759] and
differences in chemical potential in the diffusion layer: (a) kinetics governed by inter-
face phenomena, (b) kinetics governed by both diffusion and interface phenomena
and (c) kinetics governed by diffusion
2.0
1.5
1.0
0.5
0.0
Diffusion Control
k
=
10
–3
k
=
10
–4
k
=
10
–5
k
=
10
–6
ΔT, °C
R (10
–5
cm
/s)
0102030
Fig. 7.7. Effect of kinetic coefficient, k,ongrowthrate,R, as a function of the
undercooling in the case of a linear dependence on the supersaturation [759]
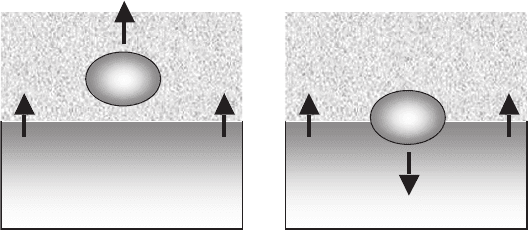
7.1 Modeling of 123 Phase Solidification from Liquid 311
a quasi-linear dependence of the growth rate vs supercooling. On the contrary,
in the case of a square power law dependence vs supersaturation, two behav-
iors are possible, depending on the k value: for k<10
−4
cm/sthegrowth
rate would show a square power law dependence vs supersaturation, and for
k>10
−3
cm/s the growth rate would demonstrate a linear dependence vs
undercooling, irrespective of the square law dependence of the growth rate vs
supersaturation.
In the case of pure 123 phase, a good agreement with experiment [759]
is observed for R
ab
and R
c
vs the undercooling. Hence, one can conclude
that growth of 123 phase is generally controlled simultaneously by yttrium
diffusion and interface kinetics processes. If a 211 properitectic phase is added
to 123 phase before the melt process, for a given undercooling the larger
amount of 211 phase can supply more yttrium diffusion to the growing front,
corresponding to the growth rate of solidification process. At the same time,
the change from a quadratic dependence of R
ab
vs supersaturation to a linear
dependence (and vice versa for R
c
), observed in [759], is indeed unexpected if
one assumes that the yttrium flux reaching the interface is proportional to the
211 phase concentration. Obviously, this means that the growth mechanisms
in the considered directions are different for both compositions, in spite of
identical growth conditions. Thus, one can modify the growth rate and its
anisotropy, changing solidification process and superconductor composition.
The particle motion near an advancing solidification front can be described
in terms of an interfacial energy relationship between a particle, solid and
liquid in the framework of the pushing/trapping mechanism of the particle by
the solidification front (see Fig. 7.8) [1092]:
σ
PS
= σ
PL
+ σ
SL
, (7.15)
where σ
PS
, σ
PL
and σ
SL
are particle/solid, particle/liquid and solid/liquid
interfacial free energies, respectively. According to this criterion, a particle
present at a growing solid phase is trapped when σ
PS
<σ
PL
+σ
SL
. In contrast,
211
123 Crystal
Liquid
123 Crystal
Liquid
211
(a) Pushing (b) Trapping
σ
PS
> σ
PL
+ σ
LS
σ
PS
< σ
PL
+ σ
LS
Fig. 7.8. Schematic of interfacial energy criteria on particle pushing and trapping
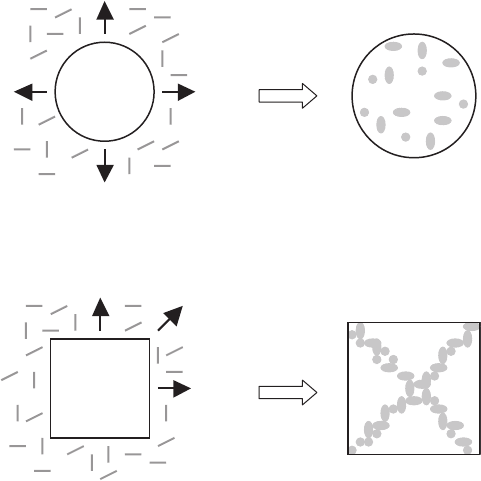
312 7 Modeling of YBCO Oxide Superconductors
when σ
PS
>σ
PL
+ σ
SL
, a particle is pushed out from a growing solid by the
fast diffusion of the liquid.
This interfacial energy criterion has been applied to melt-processed YBCO
samples in order to explain trapping of 211 particles within 123 crystals [475,
532, 534, 540]. However, this is not enough because it assumes the interaction
of the front with only one particle pushed out or trapped by the interface.
Moreover, the above criterion should be modified for anisotropically growing
crystals of melt-processed YBCO samples. The modified interfacial energy
relationship is given as [534]
σ
PS(hkl)
= σ
PL
+ σ
S(hkl)L
, (7.16)
where σ
PS(hkl)
, σ
PL
and σ
S(hkl)L
are particle/(hkl) interface, particle/liquid
and (hkl) interface/liquid interfacial free energies, respectively. Then, in the
isotropically growing system or directionally growing interface, 211 particles
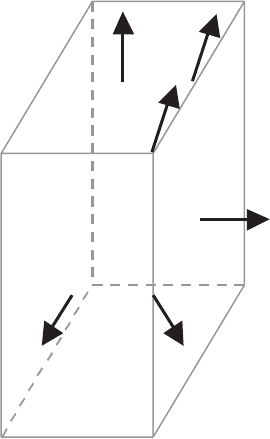
will be pushed out or trapped within a 123 domain with a random mode (see
Fig. 7.9). In this case, for an anisotropically growing 123 crystal, trapping
criteria for each growing plane are different.
123
σ
σ
σ
σ
123
Random 211
Trapping
(a) Isotropic Growth
211 + Liquid
(b) Anisotropic Growth
σ
(010)
211 + Liquid
123
123
211 Segregation
σ
(110)
σ
(100)
Fig. 7.9. Schematic of particle trapping modes in isotropically (a) and anisotropi-
cally (b) growing systems
7.1 Modeling of 123 Phase Solidification from Liquid 313
The crystal structure of 123 domains at the peritectic temperature has
a tetragonal symmetry. Therefore, there are three main growth interfaces of
(100), (010) and (001) planes (see Fig. 7.10). In the tetragonal crystal struc-
ture, the atomic arrangement of a (100) plane coincides with that of the (010)
plane. But the atomic arrangement of a (001) plane is different from that of
the (100)/(010) plane. The boundary condition of particle trapping is inferred
from the interfacial energy difference among growing (hkl) planes. The criteria
for 211 particle trapping in a melt-processed YBCO system are given as [530]
Δσ
(hkl)
= σ
PS(hkl)
− (σ
PL
+ σ
S(hkl)L
); (7.17)
Δσ
(100)
=Δσ
(010)
=Δσ
(001)
; (7.18)
Δσ
(101)
=Δσ
(011)
=Δσ
(110)
=Δσ
(111)
. (7.19)
From the interfacial energy model and experimental observations of 211
patterns [481, 532, 534, 1105], it is followed that the crystallographic planes of
X-like 211 tracks are the six diagonal planes of 123 domain, which divide the
cubic space into three pairs of pyramids (see Fig. 7.11). These diagonal planes
may vary with the aspect ratio of grown 123 domains. For example, there
may be [110] planes for a cubic domain or [103] planes for an orthorhombic
domain [534] (see Fig. 7.12). In the case of planar segregation of 211 phase,
a pair of pyramids having (001) growth fronts corresponds to 211-free spaces,
while the other two pairs of pyramids having (100) and (010) growth fronts
are filled with 211 particles.
σ
(010)
σ
(111)
σ
(001)
σ
(011)
σ
(110)
σ
(100)
Fig. 7.10. Boundary planes for 211 particle trapping, based on an interfacial energy
model
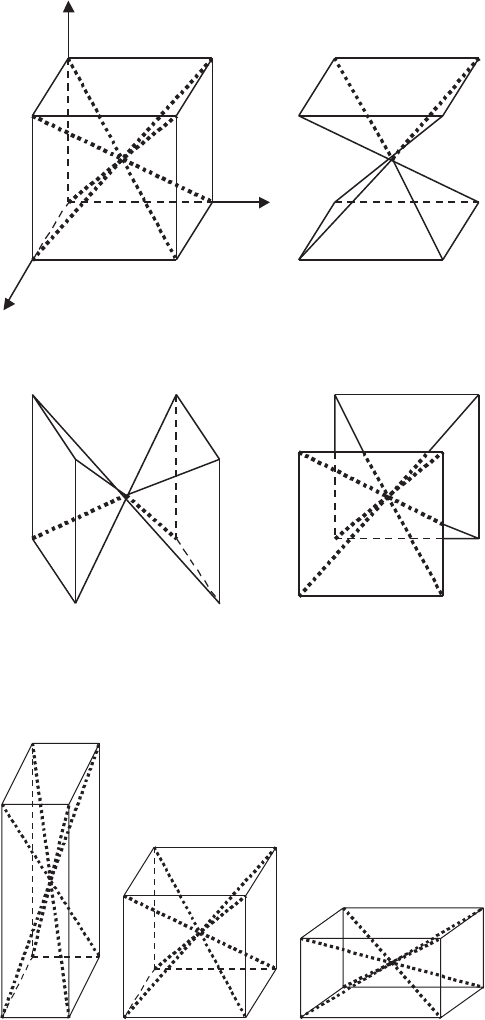
314 7 Modeling of YBCO Oxide Superconductors
[100]
[001]
[010]
(001)
(010)
(100)
(a) (b)
(c)
(d)
Fig. 7.11. (a) Three-dimensional demonstration of the planes, where 211 particles
are trapped in a stoichiometric YBCO system, (b) the 211-free spaces and (c, d)
the 211-filled spaces in a 211 phase excess system
(c)
(a)
(b)
Fig. 7.12. Variation of diagonal planes as a function of grain anisotropy
7.1 Modeling of 123 Phase Solidification from Liquid 315
The crystallographic alignment of 211 particles may also be explained in
terms of an interfacial energy relationship between the (uvw) plane of a 211
particle, (hkl) plane of interface and liquid [530]. The corresponding criterion
is given as
σ
P(uvw)S(hkl)
= σ
P(uvw)L
+ σ
S(hkl)L
, (7.20)
where σ
P(uvw)
is an interfacial energy of the (uvw) plane of a 211 particle. In
this case, these particles have a polygonal shape with faceted surfaces with
interfacial anisotropy.
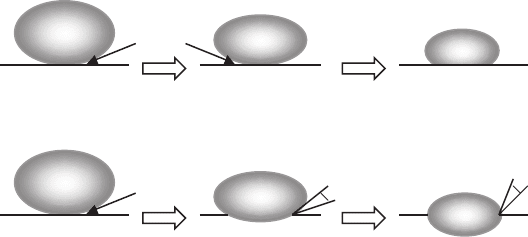
An alternative way to express the interfacial energy criteria is the liquid
wetting angle (φ) at advancing 123 interfaces. The trapping criterion of the
211 particle can be expressed with the liquid wetting angle and has been
proposed for a melt-infiltrated YBCO system [481]. As schematically shown
in Fig. 7.13, two different situations can exist for particle trapping. In the
case of φ =0
◦
, a 211 particle should be pushed toward the liquid, because
the liquid film is present between the 211 particle and the growth front. In
contrast, when φ>0
◦
and the 211 particle is not dissolved completely in the
liquid, the particle is expected to be easily trapped within the 123 domain.
7.1.4 Models of Platelets-Like Growth of 123 Phase
The model of platelets-like growth of 123 phase, which is more faster in the
ab-plane compared with the c-axis direction in the limit of single-crystalline
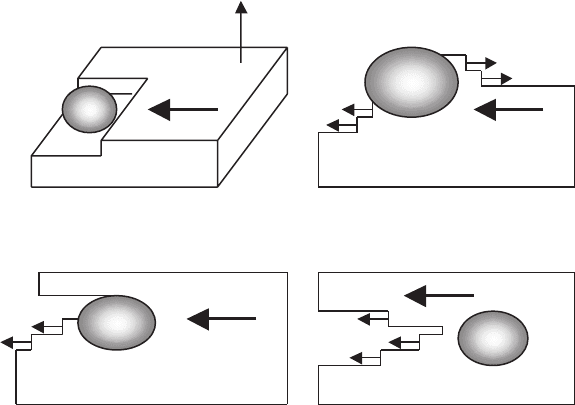
material and taking into account interaction with 211 particle, is presented in
[10]. The 211 particle may contribute to the gap-formation process, when the
growth front bipasses this particle, and the 123 material does not envelop the
211 particle completely by growing at nearly identical rates on either side of
the particle (see Fig. 7.14d). In this case, the resultant microstructure consists
(a)
(b)
211
Liquid
Film
211
211
211
123 123
Liquid
Film
211
211
φ
φ
123 123123
123
Fig. 7.13. Schematic of two different cases for 211 particle trapping within growing
123 interface [481]. Dihedral angle, φ, between 211 and 123 phases is (a) φ =0
◦
and
(b) φ>0
◦
316 7 Modeling of YBCO Oxide Superconductors
c
Growth
Direction
211
Macroscopic Growth
Direction
211
Macroscopic
Growth Direction
211
(a)
(b)
(c)
(d)
211
Fig. 7.14. Schematic of possible effect of 211 particles on the growth of melt-
processed YBCO crystal: (a) 123 platelet abuts a growing platelet; (b) heterogeneous
nucleation occurs at the “platelet–211 particle–liquid” junction; (c) once sufficient
growth along the c direction occurs such that the 211 particle can be bypassed,
rapid lateral growth will occur; (d) this process results in a gap, which may not heal
completely [10]
of platelets of identical orientation separated by gaps, which are filled in with
the rejected liquid.
The generalization of this model [334] describes the formation of linear and
plane gaps between 123 platelets and rejection of liquid at domain boundaries
in the case of 211 particle trapping by 123 matrix. As is well known, there is a
potential possibility of formation of not only the YBa
2
Cu
3
O
7−x
(123) phase,
but the BaCuO
2
(011) phase from liquid, which does not demonstrate super-
conducting properties and has solidification temperature about 1015
◦
Cthat
is near to start formation of the 123 phase [34]. Thus, solidification of either
123 phase or 011 phase is possible [317]. The situation, connected with faster
solidification of barium cuprate, may be explained on the basis of the phase
diagram and taking into account the above-described mechanism of the 123
phase formation [10, 334]. The relatively small (compared with the process of
solidification of the 011 phase) rate of establishment of equilibrium between
liquid (L) and solid 123 phase can lead to thermodynamic equilibrium in each
of these components. The liquid will demonstrate independence because of
low concentration of yttrium that is caused by low rate of change between
7.1 Modeling of 123 Phase Solidification from Liquid 317
the solid and liquid phases. In this case, a homogeneous formation of 123
nuclei is impossible, and the figurative point, describing melt, displaces into
L +BaCuO
2
+ CuO region, resulting in the solidification of barium cuprate.
There are two causes for breaking of usual equilibrium between liquid and
solid 123 phase: (i) a growth of the 211 particles (due to their different sizes
and well-known Ostwald ripening), reducing interphase interface with melt,
decreasing yttrium flux into liquid, leading to envelopment of these particles
by solidification front with rejection of the yttrium-depleted melt at the inter-
granular boundaries and finally initiating a de-lamination of the system; (ii)
a decreasing of the thermal stability of 123 nuclei in the liquid, leading to ab-
sence of dominating 123 solidification even at sufficient yttrium concentration
[318]. These causes change the stoichiometry of liquid in the case of trapping
of large 211 particles [334], and even solidification of pure 011 phase [318].
The development of the model [475] for the case of low G/R ratio is carried
out in [936]. The microstructure close to the quenched solid–liquid interface
exhibits bridges of 123 material between the solidifying 123 interface and
211 particles. In order to describe their morphologies, a combination of both
phenomena, namely a peritectic reaction being mediated by the liquid and a
peritectic transformation of the 211 particles, being linked to the solidification
front via bridges of 123 phase, is necessary. Note that all the above models
neglect peritectic transformation [494]. The principal difference of the model
[936] from the models [156, 475, 741] is the account of influence on the process
of the 123 phase formation of Lifschitz–Zlyozov boundary effects [291, 629,
997, 1090], and action of the capillary attraction forces between moving front
of the 123 phase solidification and 211 particles into liquid.
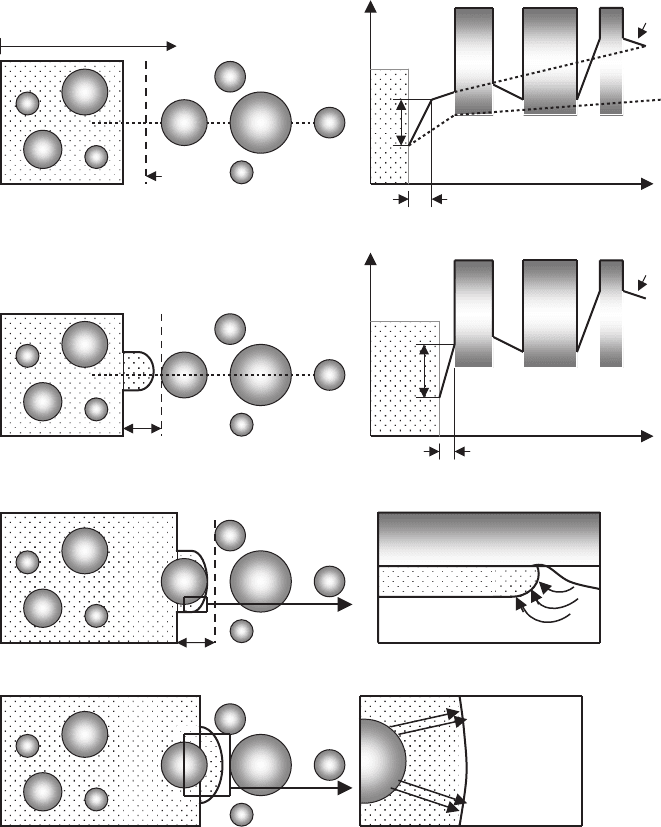
The entire local process of the engulfment of 211 particles into the so-
lidifying interface can be explained, considering four steps: (i) liquid-phase
diffusion-controlled growth, following the temperature gradient, if the 211
particle is far away from the phase boundary (see Fig. 7.15a); (ii) bridge for-
mation, when the 123 interface faces an increased Y
3+
concentration gradient,
when being approached by a 211 particle (see Fig. 7.15b); (iii) peritectic sur-
face reaction during the engulfment process (see Fig. 7.15c); and (iv) peritectic
transformation (negligible effect compared to previous steps) (see Fig. 7.15d).
Due to the peritectic character of the 123 phase creation, this phase needs
a Y-concentration that is not provided by the melt, being in equilibrium with
the 211 phase. Therefore, as in classical nucleation theory, a depletion zone
arises and the growth of the 123 phase is driven by a concentration gradient
in the depletion zone, δ, close to the 123 interface. At the same time, the
dissolving 211 particles maintain a medium yttrium concentration in liquid,
c
m
, corresponding to the Ostwald ripening theory.
Bridge formation starts when the depletion zone ahead of the 123 phase
boundary and dissolution region of the 211 particle begin to overlap (see
Fig. 7.15b). The increased concentration gradient leads to an accelerated
growth of the 123 phase toward the 211 particles, resulting in a bridge.
318 7 Modeling of YBCO Oxide Superconductors
123
211
211
123
Liquid
Y
123
Liquid
δ
211
211
123
Y
Y
211
123
123
Δc
Distance, ΔT
B
A
c
e
(T
)
c(x, T)
dc
––
dx
Δc
––
δ
=
211
c
m
(T)
δ
B
A
X
δ
(a)
123
123
B
A
dc
––
dx
Δc*
––
δ*
Δc*
δ*
=
c(x, T)
211
211
B
X
δ
A
(b)
(c)
(d)
Fig. 7.15. Model of local influence of 211 particles on the growth morphology of
123 phase. The parameter c(x, T ) corresponds to the local yttrium concentration, c
e
represents the equilibrium solubility, and c
m
the mean concentration, corresponding
to the Ostwald ripening theory; dc/dx denotes the concentration gradient at the 123
interface. (a) Liquid diffusion-controlled growth, (b) bridge formation, (c) peritectic
reaction and (d) peritectic transformation [936]
7.1 Modeling of 123 Phase Solidification from Liquid 319
The growing bridge, reaching the 211 interface, defines the start of the peritec-
tic reaction (in its original sense) [422], which subsequently covers the surface
of the 211 particle with solid 123 material (see Fig. 7.15c). Once the 211 sur-
face is covered, any further formation of 123 phase is governed by peritectic
transformation, that is, strongly limited by diffusion in the solid and negligible
(Fig. 7.15d).
As example of the above model, we explain the 1:1 correlation between 211
particle size and thickness of the 123 platelets, observed in [488]. A possible
explanation is based on two assumptions: (i) the plate-like growth of the 123
phase is caused by the strong anisotropic growth rates [10], ν
ab
>> ν
c
,and
(ii) the engulfment of the 211 particles is provided by the growing 123 matrix.
The anisotropy of the growth rates leads to a preferred growth of the ab-
planes parallel to the temperature gradient. At the same time, the low growth
rate along the c-axis direction results in a morphological instability, leading to
residual melt enclosed in planar defects between the ab-platelets. The platelet
A closest to the 211 particle (see Fig. 7.16a) is the first to be influenced by the
spherical diffusion region of the 211 particle and therefore will form a bridge
as explained above. Next growth in all (a, b and c) directions of this platelet A
is governed by the peritectic reaction, occurring along the 211 particle surface.
In particular, the c-axis growth of platelet A is in competition with the fast
ab-plane growth of the adjacent platelet B (see Fig. 7.16b). While the growth
of platelet B is additionally accelerated, when approaching the 211 particle,
the c-axis growth of platelet A is more and more decelerated because supply
of yttrium is more and more hindered by the growing peritectic reaction layer.
Considering the outer platelets C, note there is only competitive growth with
one platelet (e.g., platelet B), giving rise to enhanced growth as well as be-
ing parallel to the ab-planes and the c-axis. This leads to an enlarged platelet
thickness of the outer platelets after passing the particle (see Fig. 7.16c and d).
This process automatically results after some iterations in a platelet thickness,
corresponding to the mean particle diameter, which is supported by exper-
imental observation presented in Fig. 7.17b. Once the thickness is reached,
further growth of the 123 phase will be determined either by two platelets,
each passing one side of the 211 particle (see Fig. 7.18a) or one platelet passing
the whole particle, respectively (see Fig. 7.18b). Note that even a hypothetic
planar interface would change to a cellular morphology, when interacting with
211 particles, also yielding platelet dimensions, according to the 211 particle
diameter (see Fig. 7.19). The zipper-like mechanism, acting in this process,
results in an oriented growth of multiple connected plateletstoaquasi-single
crystal. Thus, the considered mechanism of solidification explains both a very
high rate of the 123 phase creation and an existence of sufficiently sharp
boundary between 211 inclusions and 123 matrix [741]. The models [10, 936]
also explain a coincidence of 211 particle sizes with thickness of 123 platelets,
observed in [488].
