Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

Chemical Equilibrium
In this part of the chapter, the equilibrium criterion dG]
T, p
5 0 introduced in Sec. 14.1
is used to study the equilibrium of reacting mixtures. The objective is to establish the
composition present at equilibrium for a specified temperature and pressure. An
important parameter for determining the equilibrium composition is the equilibrium
constant. The equilibrium constant is introduced and its use illustrated by several
solved examples. The discussion is concerned only with equilibrium states of reacting
systems, and no information can be deduced about the rates of reaction. Whether an
equilibrium mixture would form quickly or slowly can be determined only by con-
sidering the chemical kinetics, a topic that is not treated in this text.
14.2 Equation of Reaction Equilibrium
In Chap. 13 the conservation of mass and conservation of energy principles are applied
to reacting systems by assuming that the reactions can occur as written. However, the
extent to which a chemical reaction proceeds is limited by many factors. In general, the
composition of the products actually formed from a given set of reactants, and the relative
amounts of the products, can be determined only from experiment. Knowledge of the
composition that would be present were a reaction to proceed to equilibrium is frequently
useful, however. The equation of reaction equilibrium introduced in the present section
provides the basis for determining the equilibrium composition of a reacting mixture.
14.2.1
Introductory Case
Consider a closed system consisting initially of a gaseous mixture of hydrogen and
oxygen. A number of reactions might take place, including
1H
2
1
1
2
O
2
S
d
1H
2
O (14.18)
1H
2
S
d
2H (14.19)
1O
2
S
d
2O (14.20)
Let us consider for illustration purposes only the first of the reactions given above,
in which hydrogen and oxygen combine to form water. At equilibrium, the system
will consist in general of three components: H
2
, O
2
, and H
2
O, for not all of the hydro-
gen and oxygen initially present need be reacted. Changes in the amounts of these
components during each differential step of the reaction leading to the formation of
an equilibrium mixture are governed by Eq. 14.18. That is
dn
H
2
52dn
H
2
O
,
dn
O
2
52
1
2
dn
H
2
O
(14.21a)
where dn denotes a differential change in the respective component. The minus signs
signal that the amounts of hydrogen and oxygen present decrease when the reaction
proceeds toward the right. Equations 14.21a can be expressed alternatively as
2dn
H
2
1
5
2dn
O
2
1
2
5
dn
H
2
O
1
(14.21b)
which emphasizes that increases and decreases in the components are proportional
to the stoichiometric coefficients of Eq. 14.18.
Equilibrium is a condition of balance. Accordingly, as suggested by the direction of
the arrows in Eq. 14.18, when the system is at equilibrium, the tendency of the hydrogen
and oxygen to form water is just balanced by the tendency of water to dissociate into
oxygen and hydrogen. The equilibrium criterion dG]
T, p
5 0 can be used to determine
the composition at an equilibrium state where the temperature is T and the pressure is p.
This requires evaluation of the differential dG]
T, p
in terms of system properties.
14.2 Equation of Reaction Equilibrium 853
c14ChemicalandPhaseEquilibrium.i853 Page 853 7/26/10 11:43:21 PM users-133c14ChemicalandPhaseEquilibrium.i853 Page 853 7/26/10 11:43:21 PM users-133 /Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New/Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New
854 Chapter 14
Chemical and Phase Equilibrium
For the present case, Eq. 14.10 giving the difference in the Gibbs function of the
mixture between two states having the same temperature and pressure, but composi-
tions that differ infinitesimally, takes the following form:
dG4
T, p
5 m
H
2
dn
H
2
1 m
O
2
dn
O
2
1 m
H
2
O
dn
H
2
O
(14.22)
The changes in the mole numbers are related by Eqs. 14.21. Hence
dG4
T, p
5 121m
H
2
2
1
2
m
O
2
1 1m
H
2
O
2 dn
H
2
O
At equilibrium, dG]
T, p
5 0, so the term in parentheses must be zero. That is
21m
H
2
2
1
2
m
O
2
1 1m
H
2
O
5 0
When expressed in a form that resembles Eq. 14.18, this becomes
1m
H
2
1
1
2
m
O
2
5 1m
H
2
O
(14.23)
Equation 14.23 is the equation of reaction equilibrium for the case under consideration.
The chemical potentials are functions of temperature, pressure, and composition. Thus,
the composition that would be present at equilibrium for a given temperature and
pressure can be determined, in principle, by solving this equation. The solution proce-
dure is described in Sec. 14.3.
14.2.2
General Case
The foregoing development can be repeated for reactions involving any number of
components. Consider a closed system containing five components, A, B, C, D, and E,
at a given temperature and pressure, subject to a single chemical reaction of the form
n
A
A 1 n
B
B
S
d
n
C
C 1 n
D
D (14.24)
where the n’s are stoichiometric coefficients. Component E is assumed to be inert
and thus does not appear in the reaction equation. As we will see, component E does
influence the equilibrium composition even though it is not involved in the chemical
reaction. The form of Eq. 14.24 suggests that at equilibrium the tendency of A and
B to form C and D is just balanced by the tendency of C and D to form A and B.
The stoichiometric coefficients n
A
, n
B
, n
C
, and n
D
do not correspond to the respec-
tive number of moles of the components present. The amounts of the components
present are designated n
A
, n
B
, n
C
, n
D
, and n
E
. However, changes in the amounts of the
components present do bear a definite relationship to the values of the stoichiomet-
ric coefficients. That is
2dn
A
n
A
5
2dn
B
n
B
5
dn
C
n
C
5
dn
D
n
D
(14.25a)
where the minus signs indicate that A and B would be consumed when C and D are
produced. Since E is inert, the amount of this component remains constant, so dn
E
5 0.
Introducing a proportionality factor d´, Eqs. 14.25a take the form
2
dn
A
n
A
5
2dn
B
n
B
5
dn
C
n
C
5
dn
D
n
D
5 de
from which the following expressions are obtained:
dn
A
52n
A
de, dn
B
52n
B
de
dn
C
5 n
C
de, dn
D
5 n
D
de
(14.25b)
The parameter ´ is sometimes referred to as the extent of reaction.
extent of reaction
c14ChemicalandPhaseEquilibrium.i854 Page 854 7/26/10 11:43:22 PM users-133c14ChemicalandPhaseEquilibrium.i854 Page 854 7/26/10 11:43:22 PM users-133 /Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New/Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New
For the system under present consideration, Eq. 14.10 takes the form
dG4
T, p
5 m
A
dn
A
1 m
B
dn
B
1 m
C
dn
C
1 m
D
dn
D
1 m
E
dn
E
Introducing Eqs. 14.25b and noting that dn
E
5 0, this becomes
dG4
T, p
5 12n
A
m
A
2 n
B
m
B
1 n
C
m
C
1 n
D
m
D
2 de
At equilibrium, dG]
T, p
5 0, so the term in parentheses must be zero. That is
2n
A
m
A
2 n
B
m
B
1 n
C
m
C
1 n
D
m
D
5 0
or when written in a form resembling Eq. 14.24
n
A
m
A
1 n
B
m
B
5 n
C
m
C
1 n
D
m
D
(14.26)
For the present case, Eq. 14.26 is the equation of reaction equilibrium. In principle,
the composition that would be present at equilibrium for a given temperature and
pressure can be determined by solving this equation. The solution procedure is simpli-
fied through the equilibrium constant concept introduced in the next section.
equation of reaction
equilibrium
14.3 Calculating Equilibrium Compositions
The objective of the present section is to show how the equilibrium composition of a
system at a specified temperature and pressure can be determined by solving the equation
of reaction equilibrium. An important part is played in this by the equilibrium constant.
14.3.1
Equilibrium Constant for Ideal Gas Mixtures
The first step in solving the equation of reaction equilibrium, Eq. 14.26, for the equi-
librium composition is to introduce expressions for the chemical potentials in terms
of temperature, pressure, and composition. For an ideal gas mixture, Eq. 14.17 can be
used for this purpose. When this expression is introduced for each of the components
A, B, C, and D, Eq. 14.26 becomes
n
A
ag 8
A
1 RT ln
y
A
p
p
ref
b1 n
B
ag 8
B
1 RT ln
y
B
p
p
ref
b
5 n
C
ag 8
C
1 RT ln
y
C
p
p
ref
b1 n
D
ag 8
D
1 RT ln
y
D
p
p
ref
b
(14.27)
where g8
i
is the Gibbs function of component i evaluated at temperature T and the
pressure p
ref
5 1 atm. Equation 14.27 is the basic working relation for chemical equi-
librium in a mixture of ideal gases. However, subsequent calculations are facilitated
if it is written in an alternative form, as follows.
Collect like terms and rearrange Eq. 14.27 as
1n
C
g8
C
1 n
D
g8
D
2 n
A
g8
A
2 n
B
g8
B
2
52
RT an
C
ln
y
C
p
p
ref
1 n
D
ln
y
D
p
p
ref
2 n
A
ln
y
A
p
p
ref
2 n
B
ln
y
B
p
p
ref
b
(14.28)
The term on the left side of Eq. 14.28 can be expressed concisely as DG8. That is
¢G85n
C
g8
C
1 n
D
g8
D
2 n
A
g8
A
2 n
B
g8
B
(14.29a)
which is the change in the Gibbs function for the reaction given by Eq. 14.24 if
each reactant and product were separate at temperature T and a pressure of 1 atm.
14.3 Calculating Equilibrium Compositions 855
c14ChemicalandPhaseEquilibrium.i855 Page 855 7/26/10 11:43:23 PM users-133c14ChemicalandPhaseEquilibrium.i855 Page 855 7/26/10 11:43:23 PM users-133 /Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New/Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New
856 Chapter 14 Chemical and Phase Equilibrium
This expression can be written alternatively in terms of specific enthalpies and
entropies as
¢G8 5 n
C
1h
C
2 T s8
C
21 n
D
1h
D
2 Ts8
D
22 n
A
1h
A
2 Ts8
A
22 n
B
1h
B
2 T s 8
B
2
5 1n
C
h
C
1 n
D
h
D
2 n
A
h
A
2 n
B
h
B
22 T1n
C
s8
C
1 n
D
s 8
D
2 n
A
s 8
A
2 n
B
s 8
B
2 (14.29b)
Since the enthalpy of an ideal gas depends on temperature only, the h’s of Eq. 14.29b
are evaluated at temperature T. As indicated by the superscript 8, each of the entro-
pies is evaluated at temperature T and a pressure of 1 atm.
Introducing Eq. 14.29a into Eq. 14.28 and combining the terms involving loga-
rithms into a single expression gives
2
¢G8
RT
5 ln c
y
n
C
C
y
n
D
D
y
n
A
A
y
n
B
B
a
p
p
ref
b
n
C
1n
D
2n
A
2n
B
d
(14.30)
Equation 14.30 is simply the form taken by the equation of reaction equilibrium,
Eq. 14.26, for an ideal gas mixture subject to the reaction Eq. 14.24. As illustrated
by subsequent examples, similar expressions can be written for other reactions.
Equation 14.30 can be expressed concisely as
2
¢G8
RT
5 ln K1T2
(14.31)
where K is the equilibrium constant defined by
K1T25
y
n
C
C
y
n
D
D
y
n
A
A
y
n
B
B
a
p
p
ref
b
n
C
1n
D
2n
A
2n
B
(14.32)
Given the values of the stoichiometric coefficients, n
A
, n
B
, n
C
, and n
D
and the tem-
perature T, the left side of Eq. 14.31 can be evaluated using either of Eqs. 14.29
together with the appropriate property data. The equation can then be solved for the
value of the equilibrium constant K. Accordingly, for selected reactions K can be
evaluated and tabulated against temperature. It is common to tabulate log
10
K or ln
K versus temperature, however. A tabulation of log
10
K values over a range of tem-
peratures for several reactions is provided in Table A-27, which is extracted from a
more extensive compilation.
The terms in the numerator and denominator of Eq. 14.32 correspond, respectively,
to the products and reactants of the reaction given by Eq. 14.24 as it proceeds from
left to right as written. For the inverse reaction n
C
C 1 n
D
D
S
d
n
A
A 1 n
B
B, the equi-
librium constant takes the form
K
*
5
y
n
A
A
y
n
B
B
y
n
C
C
y
n
D
D
a
p
p
ref
b
n
A
1n
B
2n
C
2n
D
(14.33)
Comparing Eqs. 14.32 and 14.33, it follows that the value of K
*
is just the reciprocal
of K: K
*
5 1/K. Accordingly,
log
10
K
*
52log
10
K (14.34)
Hence, Table A-27 can be used both to evaluate K for the reactions listed proceeding
in the direction left to right and to evaluate K
*
for the inverse reactions proceeding
in the direction right to left.
Example 14.1 illustrates how the log
10
K values of Table A-27 are determined. Sub-
sequent examples show how the log
10
K values can be used to evaluate equilibrium
compositions.
equilibrium constant
c14ChemicalandPhaseEquilibrium.i856 Page 856 7/26/10 11:43:25 PM users-133c14ChemicalandPhaseEquilibrium.i856 Page 856 7/26/10 11:43:25 PM users-133 /Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New/Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New
Evaluating the Equilibrium Constant at a Specified Temperature
c c c c EXAMPLE 14.1 c
Evaluate the equilibrium constant, expressed as log
10
K, for the reaction CO 1
1
2
O
2
d
S
CO
2
at (a) 298 K and
(b) 2000 K. Compare with the value obtained from Table A-27.
SOLUTION
Known:
The reaction is CO 1
1
2
O
2
S
d
CO
2
.
Find: Determine the equilibrium constant for T 5 298 K 1258C2 and T 5 2000 K.
Engineering Model: The ideal gas model applies.
Analysis: The equilibrium constant requires the evaluation of ¢G8 for the reaction. Invoking Eq. 14.29b for this
purpose, we have
¢G851h
CO
2
2 h
CO
2
1
2
h
O
2
22 T1s8
CO
2
2 s8
CO
2
1
2
s 8
O
2
2
where the enthalpies are evaluated at temperature T and the absolute entropies are evaluated at temperature T
and a pressure of 1 atm. Using Eq. 13.9, the enthalpies are evaluated in terms of the respective enthalpies of
formation, giving
¢G8531h8
f
2
CO
2
2 1h8
f
2
CO
2
1
2
1h8
f
0
2
O
2
41 31¢h2
CO
2
2 1¢h2
CO
2
1
2
1¢h2
O
2
42 T1s8
CO
2
2 s8
CO
2
1
2
s 8
O
2
2
where the ¢h terms account for the change in specific enthalpy from T
ref
5 298 K to the specified temperature
T. The enthalpy of formation of oxygen is zero by definition.
(a) When T 5 298 K, the ¢
h
terms of the above expression for DG8 vanish. The required enthalpy of formation
and absolute entropy values can be read from Table A-25, giving
¢G85312393,52022 12110,53022
1
2
10242 2983213.69 2 197.54 2
1
2
1205.0324
52257,253 kJ
/
kmol
With this value for DG8, Eq. 14.31 gives
ln K 52
1
2257,253 kJ
/
kmol
2
1
8.314 kJ
/
kmol ? K
21
298 K
2
5 103.83
which corresponds to log
10
K 5 45.093.
Table A-27 gives the logarithm to the base 10 of the equilibrium constant for the inverse reaction:
CO
2
S
d
CO 1
1
2
O
2
. That is, log
10
K
*
5 245.066. Thus, with Eq. 14.34, log
10
K 5 45.066, which agrees closely with
the calculated value.
(b) When T 5 2000 K, the ¢h and s8 terms for O
2
, CO, and CO
2
required by the above expression for DG8 are
evaluated from Tables A-23. The enthalpy of formation values are the same as in part (a). Thus
¢G85312393,52022 12110,53022
1
2
10241 31100,804 2 936422 165408 2 86692
2
1
2
167,881 2 8682242 20003309.210 2 258.600 2
1
2
1268.65524
52282,990 1 5102 1 167,435 52110,453 kJ
/
kmol
With this value, Eq. 14.31 gives
ln K 52
12110,453
2
18.3142120002
5 6.643
which corresponds to log
10
K 5 2.885.
14.3 Calculating Equilibrium Compositions 857
c14ChemicalandPhaseEquilibrium.i857 Page 857 8/12/10 9:43:08 AM user-s146c14ChemicalandPhaseEquilibrium.i857 Page 857 8/12/10 9:43:08 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
858 Chapter 14 Chemical and Phase Equilibrium
If ln K 5 23.535 for the given reaction, use Table A-27 to deter-
mine T, in K. Ans. 1000 K.
At 2000 K, Table A-27 gives log
10
K
*
5 22.884. With Eq. 14.34, log
10
K 5 2.884,
which is in agreement with the calculated value.
Using the procedures described above, it is straightforward to determine log
10
K
versus temperature for each of several specified reactions and tabulate the results
as in Table A-27.
Ability to…
❑
evaluate log
10
K based on
Eq. 14.31 and data from
Tables A-23 and A-25.
❑
use the relation of Eq.
14.34 for inverse reactions.
✓Skills Developed
14.3.2
Illustrations of the Calculation of Equilibrium
Compositions for Reacting Ideal Gas Mixtures
It is often convenient to express Eq. 14.32 explicitly in terms of the number of moles
that would be present at equilibrium. Each mole fraction appearing in the equation has
the form y
i
5 n
i
/n, where n
i
is the amount of component i in the equilibrium mixture
and n is the total number of moles of mixture. Hence, Eq. 14.32 can be rewritten as
K 5
n
n
C
C
n
n
D
D
n
n
A
A
n
n
B
B
a
p
/
p
ref
n
b
n
C
1n
D
2n
A
2n
B
(14.35)
The value of n must include not only the reacting components A, B, C, and D but
also all inert components present. Since inert component E has been assumed pres-
ent, we would write n 5 n
A
1 n
B
1 n
C
1 n
D
1 n
E
.
Equation 14.35 provides a relationship among the temperature, pressure, and compo-
sition of an ideal gas mixture at equilibrium. Accordingly, if any two of temperature,
pressure, and composition are known, the third can be found by solving this equation.
suppose that the temperature T and pressure p are known and
the object is the equilibrium composition. With temperature known, the value of K
can be obtained from Table A-27. The n’s of the reacting components A, B, C, and
D can be expressed in terms of a single unknown variable through application of
the conservation of mass principle to the various chemical species present. Then,
since the pressure is known, Eq. 14.35 constitutes a single equation in a single
unknown, which can be solved using an equation solver or iteratively with a hand
calculator. b b b b b
In Example 14.2, we apply Eq. 14.35 to study the effect of pressure on the equi-
librium composition of a mixture of CO
2
, CO, and O
2
.
Determining Equilibrium Composition Given Temperature and Pressure
c c c c EXAMPLE 14.2 c
One kilomole of carbon monoxide, CO, reacts with
1
2
kmol of oxygen, O
2
, to form an equilibrium mixture of CO
2
, CO,
and O
2
at 2500 K and (a) 1 atm, (b) 10 atm. Determine the equilibrium composition in terms of mole fractions.
SOLUTION
Known:
A system initially consisting of 1 kmol of CO and
1
2
kmol of O
2
reacts to form an equilibrium mixture
of CO
2
, CO, and O
2
. The temperature of the mixture is 2500 K and the pressure is (a) 1 atm, (b) 10 atm.
Find: Determine the equilibrium composition in terms of mole fractions.
c14ChemicalandPhaseEquilibrium.i858 Page 858 8/12/10 8:24:05 AM user-s146c14ChemicalandPhaseEquilibrium.i858 Page 858 8/12/10 8:24:05 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
Engineering Model: The equilibrium mixture is modeled as an ideal gas mixture.
Analysis: Equation 14.35 relates temperature, pressure, and composition for an ideal gas mixture at equilibrium.
If any two are known, the third can be determined using this equation. In the present case, T and p are known,
and the composition is unknown.
Applying conservation of mass, the overall balanced chemical reaction equation is
1CO 1
1
2
O
2
S zCO 1
z
2
O
2
1 11 2 z2CO
2
where z is the amount of CO, in kmol, present in the equilibrium mixture. Note that 0 # z # 1.
The total number of moles n in the equilibrium mixture is
n 5 z 1
z
2
1 11 2 z25
2 1 z
2
Accordingly, the molar analysis of the equilibrium mixture is
y
CO
5
2z
2 1 z
,
y
O
2
5
z
2 1 z
,
y
CO
2
5
211 2 z
2
2 1 z
At equilibrium, the tendency of CO and O
2
to form CO
2
is just balanced by the tendency of CO
2
to form
CO and O
2
, so we have CO
2
d
S
CO 1
1
2
O
2
. Accordingly, Eq. 14.35 takes the form
K 5
z1z
/
22
1
y
2
1
1 2 z
2
c
p
y
p
ref
1
2 1 z
2
y
2
d
111
y
221
5
z
1 2 z
a
z
2 1 z
b
1
y
2
a
p
p
ref
b
1
/
2
At 2500 K, Table A-27 gives log
10
K 5 21.44. Thus, K 5 0.0363. Inserting this value into the last expression
0.0363 5
z
1 2 z
a
z
2 1 z
b
1
y
2
a
p
p
ref
b
1
y
2
(a)
(a) When p 5 1 atm, Eq. (a) becomes
0.0363 5
z
1 2 z
a
z
2 1 z
b
1
y
2
Using an equation solver or iteration with a hand calculator, z 5 0.129. The equilibrium composition in terms
of mole fractions is then
y
CO
5
210.129
2
2.129
5 0.121,
y
O
2
5
0.129
2.129
5 0.061,
y
CO
2
5
211 2 0.129
2
2.129
5 0.818
(b) When p 5 10 atm, Eq. (a) becomes
0.0363 5
z
1 2 z
a
z
2 1 z
b
1
y
2
1102
1
/
2
➊
Solving, z 5 0.062. The corresponding equilibrium composition in terms of
mole fractions is y
CO
5 0.06, y
O
2
5 0.03, y
CO
2
5 0.91.
➊
Comparing the results of parts (a) and (b) we conclude that the extent to
which the reaction proceeds toward completion (the extent to which CO
2
is formed) is increased by increasing the pressure.
If z 5 0.0478 (corresponding to p 5 22.4 atm, T 5 2500 K),
what would be the mole fraction of each constituent of the equilibrium
mixture? Ans. y
CO
5 0.0467, y
O
2
5 0.0233, y
CO
2
5 0.9300.
Ability to…
❑
apply Eq. 14.35 to deter-
mine equilibrium composition
given temperature and
pressure.
❑
retrieve and use data from
Table A-27.
✓
Skills Developed
14.3 Calculating Equilibrium Compositions 859
c14ChemicalandPhaseEquilibrium.i859 Page 859 8/12/10 9:43:28 AM user-s146c14ChemicalandPhaseEquilibrium.i859 Page 859 8/12/10 9:43:28 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
860 Chapter 14 Chemical and Phase Equilibrium
In Example 14.3, we determine the temperature of an equilibrium mixture when
the pressure and composition are known.
In Example 14.4, we consider the effect of an inert component on the equilibrium
composition.
Considering the Effect on Equilibrium of an Inert Component
c c c c EXAMPLE 14.4 c
One kilomole of carbon monoxide reacts with the theoretical amount of air to form an equilibrium mixture of
CO
2
, CO, O
2
, and N
2
at 2500 K and 1 atm. Determine the equilibrium composition in terms of mole fractions,
and compare with the result of Example 14.2.
Measurements show that at a temperature T and a pressure of 1 atm, the equilibrium mixture for the system of
Example 14.2 has the composition y
CO
5 0.298, y
O
2
5 0.149, y
CO
2
5 0.553. Determine the temperature T of the
mixture, in K.
SOLUTION
Known:
The pressure and composition of an equilibrium mixture of CO, O
2
, and CO
2
are specified.
Find: Determine the temperature of the mixture, in K.
Engineering Model: The mixture can be modeled as an ideal gas mixture.
Analysis: Equation 14.35 relates temperature, pressure, and composition for an ideal gas mixture at equilibrium.
If any two are known, the third can be found using this equation. In the present case, composition and pressure
are known, and the temperature is the unknown.
Equation 14.35 takes the same form here as in Example 14.2. Thus, when p 5 1 atm, we have
K1T25
z
1 2 z
a
z
2 1 z
b
1
y
2
where z is the amount of CO, in kmol, present in the equilibrium mixture and T is the temperature of the
mixture.
The solution to Example 14.2 gives the following expression for the mole
fraction of the CO in the mixture: y
CO
5 2z/(2 1 z). Since y
CO
5 0.298,
z 5 0.35.
Inserting this value for z into the expression for the equilibrium constant
gives K 5 0.2078. Thus, log
10
K 5 20.6824. Interpolation in Table A-27 then gives
T 5 2881 K.
➊
Comparing this example with part (a) of Example 14.2, we conclude that the
extent to which the reaction proceeds to completion (the extent to which
CO
2
is formed) is decreased by increasing the temperature.
Determining Equilibrium Temperature Given Pressure and Composition
c c c c EXAMPLE 14.3 c
Determine the temperature, in K, for a pressure of 2 atm if the
equilibrium composition were unchanged. Ans. <2970 K.
Ability to…
❑
apply Eq. 14.35 to deter-
mine temperature given
pressure and equilibrium
composition.
❑
retrieve and use data from
Table A-27.
✓
Skills Developed
➊
c14ChemicalandPhaseEquilibrium.i860 Page 860 7/26/10 11:46:32 PM users-133c14ChemicalandPhaseEquilibrium.i860 Page 860 7/26/10 11:46:32 PM users-133 /Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New/Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New
Determine the amounts, in kmol, of each component of the equi-
librium mixture. Ans. n
CO
5 0.175, n
O
2
5 0.0875, n
CO
2
5 0.8250, n
N
2
5 1.88.
SOLUTION
Known:
A system initially consisting of 1 kmol of CO and the theoretical amount of air reacts to form an equi-
librium mixture of CO
2
, CO, O
2
, and N
2
. The temperature and pressure of the mixture are 2500 K and 1 atm.
Find: Determine the equilibrium composition in terms of mole fractions, and compare with the result of Example
14.2.
Engineering Model: The equilibrium mixture can be modeled as an ideal gas mixture wherein N
2
is inert.
Analysis: For a complete reaction of CO with the theoretical amount of air
CO 1
1
2
O
2
1 1.88N
2
S
CO
2
1 1.88N
2
Accordingly, the reaction of CO with the theoretical amount of air to form CO
2
, CO, O
2
, and N
2
is
CO 1
1
2
O
2
1 1.88N
2
S
zCO 1
z
2
O
2
1 11 2 z2CO
2
1 1.88N
2
where z is the amount of CO, in kmol, present in the equilibrium mixture.
The total number of moles n in the equilibrium mixture is
n 5 z 1
z
2
1 11 2 z21 1.88 5
5.76 1 z
2
The composition of the equilibrium mixture in terms of mole fractions is
y
CO
5
2z
5.76 1
z
,
y
O
2
5
z
5.76 1
z
,
y
CO
2
5
2
1
1 2 z
2
5.76 1
z
,
y
N
2
5
3.76
5.76 1
z
At equilibrium we have CO
2
d
S
CO 1
1
2
O
2
. So Eq. 14.35 takes the form
K 5
z1z
/
22
1
y
2
1
1 2 z
2
c
p
/
p
ref
1
5.76 1 z
2
y
2
d
1
y
2
The value of K is the same as in the solution to Example 14.2, K 5 0.0363.
Thus, since p 5 1 atm, we have
0.0363 5
z
1 2 z
a
z
5.76 1 z
b
1
y
2
Solving, z 5 0.175. The corresponding equilibrium composition is y
CO
5 0.059,
y
CO
2
5 0.278, y
O
2
5 0.029, y
N
2
5 0.634.
Comparing this example with Example 14.2, we conclude that the presence
of the inert component nitrogen reduces the extent to which the reaction pro-
ceeds toward completion at the specified temperature and pressure (reduces
the extent to which CO
2
is formed).
Ability to…
❑
apply Eq. 14.35 to deter-
mine equilibrium composition
for given temperature and
pressure in the presence of
an inert component.
❑
retrieve and use data from
Table A-27.
✓
Skills Developed
In the next example, the equilibrium concepts of this chapter are applied together
with the energy balance for reacting systems developed in Chap. 13.
c c c c EXAMPLE 14.5 c
Using Equilibrium Concepts with the Energy Balance
Carbon dioxide at 258C, 1 atm enters a reactor operating at steady state and dissociates, giving an equilibrium
mixture of CO
2
, CO, and O
2
that exits at 3200 K, 1 atm. Determine the heat transfer to the reactor, in kJ per
kmol of CO
2
entering. The effects of kinetic and potential energy can be ignored and W
#
cv
5 0.
14.3 Calculating Equilibrium Compositions 861
c14ChemicalandPhaseEquilibrium.i861 Page 861 8/12/10 9:43:41 AM user-s146c14ChemicalandPhaseEquilibrium.i861 Page 861 8/12/10 9:43:41 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

862 Chapter 14
Chemical and Phase Equilibrium
SOLUTION
Known:
Carbon dioxide at 258C, 1 atm enters a reactor at steady state. An equilibrium mixture of CO
2
, CO, and
O
2
exits at 3200 K, 1 atm.
Find: Determine the heat transfer to the reactor, in kJ per kmol of CO
2
entering.
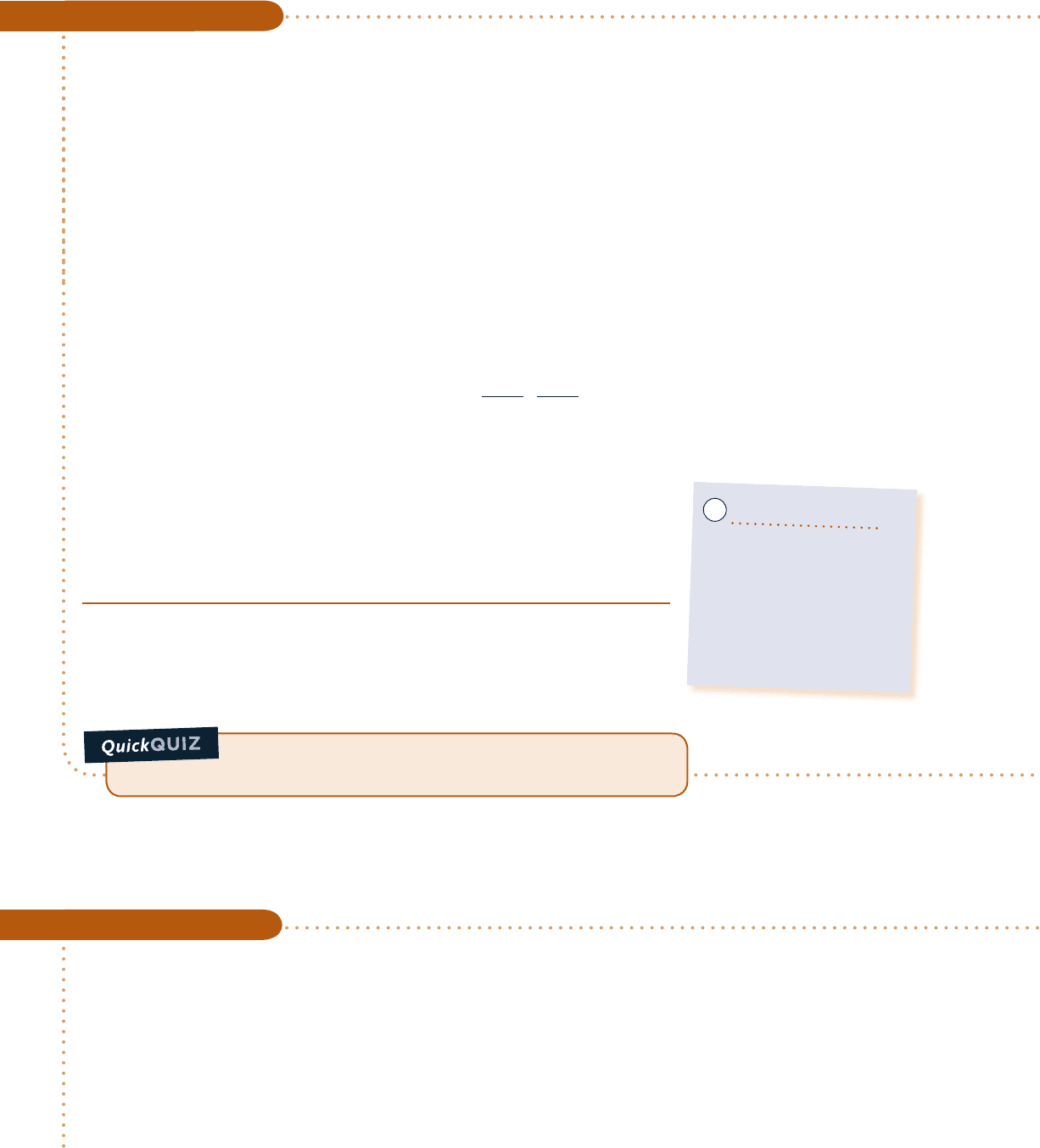
Schematic and Given Data:
Analysis:
The required heat transfer can be determined from an energy rate balance for the control volume, but
first the composition of the exiting equilibrium mixture must be determined.
Applying the conservation of mass principle, the overall dissociation reaction is described by
CO
2
S
zCO
2
1 11 2 z2CO 1
a
1 2 z
2
b
O
2
where z is the amount of CO
2
, in kmol, present in the mixture exiting the control volume, per kmol of CO
2
entering. The total number of moles n in the mixture is then
n 5 z 1 11 2 z21
a
1 2 z
2
b
5
3 2 z
2
The exiting mixture is assumed to be an equilibrium mixture (assumption 3). Thus, for the mixture we have
CO
2
d
S
CO 1
1
2
O
2
. Equation 14.35 takes the form
K 5
11 2 z2311 2 z2
/
24
1
/
2
z
c
p
/
p
ref
1
3 2 z
2
/
2
d
111
/
221
Rearranging and noting that p 5 1 atm
K 5
a
1 2 z
z
b
a
1 2 z
3 2 z
b
1
/
2
At 3200 K, Table A-27 gives log
10
K 5 20.189. Thus, K 5 0.647, and the equilibrium constant expression becomes
0.647 5
a
1 2 z
z
b
a
1 2 z
3 2 z
b
1
/
2
Solving, z 5 0.422. The composition of the exiting equilibrium mixture, in kmol per kmol of CO
2
entering, is
then 0.422CO
2
, 0.578CO, 0.289O
2
.
When expressed per kmol of CO
2
entering the control volume, the energy rate balance reduces by assumption 1 to
0 5
Q
#
cv
n
#
CO
2
2
W
#
0
c
v
n
#
CO
2
1 h
CO
2
2 10.422h
CO
2
1 0.578h
CO
1 0.289h
O
2
2
Solving for the heat transfer per kmol of CO
2
entering, and evaluating each enthalpy in terms of the respective
enthalpy of formation
Q
#
cv
n
#
CO
2
5 0.4221h
f
81¢h2
CO
2
1 0.5781h
f
81¢h2
CO
1 0.2891h
f
8
0
1 ¢h2
O
2
2 1h
f
81¢h2
0
CO
2
Engineering Model:
1.
The control volume shown on the accompanying sketch by
a dashed line operates at steady state with W
#
cv
5 0. Kinetic
energy and potential energy effects can be ignored.
2. The entering CO
2
is modeled as an ideal gas.
3. The exiting mixture of CO
2
, CO, and O
2
is an equilibrium
ideal gas mixture.
Fig. E14.5
CO
2
25°C, 1 atm
(CO
2
, CO, O
2
)
3200 K, 1 atm
Q
cv
·
c14ChemicalandPhaseEquilibrium.i862 Page 862 8/12/10 10:04:37 AM user-s146c14ChemicalandPhaseEquilibrium.i862 Page 862 8/12/10 10:04:37 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
