Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

14.3.3
Equilibrium Constant for Mixtures and Solutions
The procedures that led to the equilibrium constant for reacting ideal gas mixtures
can be followed for the general case of reacting mixtures by using the fugacity and
activity concepts introduced in Sec. 11.9. In principle, equilibrium compositions of
such mixtures can be determined with an approach paralleling the one for ideal gas
mixtures.
Equation 11.141 can be used to evaluate the chemical potentials appearing in the
equation of reaction equilibrium (Eq. 14.26). The result is
v
A
1g8
A
1 RT ln a
A
21 v
B
1g8
B
1 RT ln a
B
25 v
C
1g 8
C
1 RT ln a
C
21 v
D
1g 8
D
1 RT ln a
D
2
(14.36)
where g8
i
is the Gibbs function of pure component i at temperature T and the pres-
sure p
ref
5 1 atm, and a
i
is the activity of that component.
Collecting terms and employing Eq. 14.29a, Eq. 14.36 becomes
2
¢G8
RT
5 ln
a
a
v
C
C
a
v
D
D
a
v
A
A
a
v
B
B
b
(14.37)
This equation can be expressed in the same form as Eq. 14.31 by defining the equi-
librium constant as
K 5
a
C
v
C
a
v
D
D
a
v
A
A
a
v
B
B
(14.38)
The enthalpy of formation of O
2
is zero by definition;
¢h
for the CO
2
at the inlet vanishes because CO
2
enters
at 258C.
With enthalpy of formation values from Tables A-25 and ¢
h
values for O
2
, CO, and CO
2
, from Table A-23
Q
#
cv
n
#
CO
2
5 0.42232393,520 1 1174,695 2 9364241 0.57832110,530 1 1109,667 2 866924
1 0.289
1
114,809 2 8682
2
2
1
2393,520
2
➊
5 322,385 kJ
/
kmol
1
CO
2
2
➊
For comparison, let us determine the heat transfer if we assume no dissociation—
namely, when CO
2
alone exits the reactor. With data from Table A-23, the heat
transfer is
Q
#
cv
n
#
CO
2
5 h
CO
2
13200 K22 h
CO
2
1298 K2
5 174,695 2 9364 5 165,331 kJ
/
kmol
1
CO
2
2
The value is much less than the value obtained in the solution above because
the dissociation of CO
2
requires more energy input (an endothermic reac-
tion).
Determine the heat transfer rate, in kW, and the molar flow
rate of mixture exiting, in kmol/s, for a flow rate of 3.1 3 10
25
kmol/s of
CO
2
entering. Ans. 10 kW, 4 3 10
25
kmol/s.
Ability to…
❑
apply Eq. 14.35 together
with the energy balance for
reacting systems to determine
heat transfer for a reactor.
❑
retrieve and use data from
Tables A-23, A-25, and A-27.
✓
Skills Developed
TAKE NOTE...
Study of Sec. 14.3.3
requires content from
Sec. 11.9
14.3 Calculating Equilibrium Compositions 863
c14ChemicalandPhaseEquilibrium.i863 Page 863 7/26/10 11:46:38 PM users-133c14ChemicalandPhaseEquilibrium.i863 Page 863 7/26/10 11:46:38 PM users-133 /Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New/Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New
864 Chapter 14 Chemical and Phase Equilibrium
Since Table A-27 and similar compilations are constructed simply by evaluating
2¢
G
8
/
RT for specified reactions at several temperatures, such tables can be employed
to evaluate the more general equilibrium constant given by Eq. 14.38. However,
before Eq. 14.38 can be used to determine the equilibrium composition for a known
value of K, it is necessary to evaluate the activity of the various mixture compo-
nents. Let us illustrate this for the case of mixtures that can be modeled as ideal
solutions.
IDEAL SOLUTIONS. For an ideal solution, the activity of component i is given by
a
i
5
y
i
f
i
f
8
i
(11.142)
where f
i
is the fugacity of pure i at the temperature T and pressure p of the mixture,
and f8
i
is the fugacity of pure i at temperature T and the pressure p
ref
. Using this
expression to evaluate a
A
, a
B
, a
C
, and a
D
, Eq. 14.38 becomes
K 5
1
y
C
f
C
/
f 8
C
2
v
C
1
y
D
f
D
/
f 8
D
2
v
D
1
y
A
f
A
/
f 8
A
2
v
A
1
y
B
f
B
/
f 8
B
2
v
B
(14.39a)
which can be expressed alternatively as
K 5 c
1f
C
/
p2
v
C
1f
D
/
p2
v
D
1
f
A
/
p
2
v
A
1
f
B
/
p
2
v
B
dc
1f 8
A
/
p
ref
2
v
A
1f 8
B
/
p
ref
2
v
B
1
f 8
C
/
p
ref
2
v
C
1
f 8
D
/
p
ref
2
v
D
dc
y
v
C
C
y
v
D
D
y
v
A
A
y
v
B
B
a
p
p
ref
b
v
C
1v
D
2v
A
2v
B
d
(14.39b)
The ratios of fugacity to pressure in this equation can be evaluated, in principle,
from Eq. 11.124 or the generalized fugacity chart, Fig. A-6, developed from it. In
the special case when each component behaves as an ideal gas at both T, p and T,
p
ref
, these ratios equal unity and Eq. 14.39b reduces to the underlined term, which
is just Eq. 14.32.
While carbon dioxide is often mentioned by the media
because of its effect on global climate change, and
rightly so, other gases released to the atmosphere also
contribute to climate change but get less publicity. In particular,
methane, CH
4
, which receives little notice as a greenhouse gas,
has a Global Warming Potential of 25, compared to carbon diox-
ide’s GWP of 1 (see Table 10.1).
Sources of methane related to human activity include fossil-fuel
(coal, natural gas, and petroleum) production, distribution, com-
bustion, and other uses. Wastewater treatment, landfills, and agri-
culture, including ruminant animals raised for food, are also human-
related sources of methane. Natural sources of methane include
wetlands and methane hydrate deposits in seafloor sediments.
For decades, the concentration of methane in the atmosphere
has increased significantly. But some observers report that the
increase has slowed recently and may be ceasing. While this
could be only a temporary pause, reasons have been advanced
to explain the development. Some say governmental actions
aimed at reducing release of methane have begun to show
results. Changes in agricultural practices, such as the way rice
is produced, also may be a factor in the reported reduction of
methane in the atmosphere.
Another view is that the plateau in atmospheric methane may
at least in part be due to chemical equilibrium: Methane released
to the atmosphere is balanced by its consumption in the atmo-
sphere. Methane is consumed in the atmosphere principally by its
reaction with the hydroxyl radical (OH), which is produced through
decomposition of atmospheric ozone by action of solar radiation.
For instance, OH reacts with methane to yield water and CH
3
, a
methyl radical, according to
C
H
4
1
O
H S H
2
O
1
C
H
3
. Other reac-
tions follow this, leading eventually to water-soluble products that
are washed out of the atmosphere by rain and snow.
Understanding the reasons for the apparent slowing rate of
growth of methane in the atmosphere will take effort, including
quantifying changes in the various sources of methane and pin-
pointing natural mechanisms by which it is removed from the
atmosphere. Better understanding will enable us to craft mea-
sures aimed at curbing release of methane, allowing the atmo-
sphere’s natural ability to cleanse itself to assist in maintaining
a healthier balance.
Methane, Another Greenhouse Gas
c14ChemicalandPhaseEquilibrium.i864 Page 864 8/12/10 8:24:15 AM user-s146c14ChemicalandPhaseEquilibrium.i864 Page 864 8/12/10 8:24:15 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
14.4 Further Examples of the Use
of the Equilibrium Constant
Some additional aspects of the use of the equilibrium constant are introduced in this
section: the equilibrium flame temperature, the van’t Hoff equation, and chemical
equilibrium for ionization reactions and simultaneous reactions. To keep the presen-
tation at an introductory level, only the case of ideal gas mixtures is considered.
14.4.1
Determining Equilibrium Flame Temperature
In this section, the effect of incomplete combustion on the adiabatic flame tempera-
ture, introduced in Sec. 13.3, is considered using concepts developed in the present
chapter. We begin with a review of some ideas related to the adiabatic flame tem-
perature by considering a reactor operating at steady state for which no significant
heat transfer with the surroundings takes place.
Let carbon monoxide gas entering at one location react completely with the theo-
retical amount of air entering at another location as follows:
CO 1
1
2
O
2
1 1.88N
2
S
CO
2
1 1.88N
2
As discussed in Sec. 13.3, the products would exit the reactor at a temperature we
have designated the maximum adiabatic flame temperature. This temperature can be
determined by solving a single equation, the energy equation. At such an elevated
temperature, however, there would be a tendency for CO
2
to dissociate
CO
2
S
CO 1
1
2
O
2
Since dissociation requires energy (an endothermic reaction), the temperature of the
products would be less than the maximum adiabatic temperature found under the
assumption of complete combustion.
When dissociation takes place, the gaseous products exiting the reactor would not
be CO
2
and N
2
, but a mixture of CO
2
, CO, O
2
, and N
2
. The balanced chemical reac-
tion equation would read
CO 1
1
2
O
2
1 1.88N
2
S z CO 1 11 2 z2CO
2
1
z
2
O
2
1 1.88N
2
(14.40)
where z is the amount of CO, in kmol, present in the exiting mixture for each kmol
of CO entering the reactor.
Accordingly, there are two unknowns: z and the temperature of the exiting stream.
To solve a problem with two unknowns requires two equations. One is provided by
an energy equation. If the exiting gas mixture is in equilibrium, the other equation
is provided by the equilibrium constant, Eq. 14.35. The temperature of the products
may then be called the equilibrium flame temperature. The equilibrium constant used
to evaluate the equilibrium flame temperature would be determined with respect to
CO
2
S
d
CO 1
1
2
O
2
.
Although only the dissociation of CO
2
has been discussed, other products of com-
bustion may dissociate, for example
H
2
O
S
d
H
2
1
1
2
O
2
H
2
O
S
d
OH 1
1
2
H
2
O
2
S
d
2O
H
2
S
d
2H
N
2
S
d
2N
When there are many dissociation reactions, the study of chemical equilibrium is
facilitated by the use of computers to solve the simultaneous equations that result.
equilibrium flame
temperature
14.4 Further Examples of the Use of the Equilibrium Constant 865
c14ChemicalandPhaseEquilibrium.865 Page 865 7/26/10 11:37:36 PM users-133 c14ChemicalandPhaseEquilibrium.865 Page 865 7/26/10 11:37:36 PM users-133 /Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New/Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New
866 Chapter 14 Chemical and Phase Equilibrium
Simultaneous reactions are considered in Sec. 14.4.4. The following example illustrates
how the equilibrium flame temperature is determined when one dissociation reaction
occurs.
Determining the Equilibrium Flame Temperature
c c c c EXAMPLE 14.6 c
Carbon monoxide at 258C, 1 atm enters a well-insulated reactor and reacts with the theoretical amount of air
entering at the same temperature and pressure. An equilibrium mixture of CO
2
, CO, O
2
, and N
2
exits the reactor
at a pressure of 1 atm. For steady-state operation and negligible effects of kinetic and potential energy, determine
the composition and temperature of the exiting mixture in K.
SOLUTION
Known:
Carbon monoxide at 258C, 1 atm reacts with the theoretical amount of air at 258C, 1 atm to form an
equilibrium mixture of CO
2
, CO, O
2
, and N
2
at temperature T and a pressure of 1 atm.
Find: Determine the composition and temperature of the exiting mixture.
Schematic and Given Data:
Analysis:
The overall reaction is the same as in the solution to Example 14.4
CO 1
1
2
O
2
1 1.88N
2
S z CO 1
z
2
O
2
1 11 2 z2CO
2
1 1.88N
2
By assumption 3, the exiting mixture is an equilibrium mixture. The equilibrium constant expression developed
in the solution to Example 14.4 is
K1T25
z1z
/
22
1
/
2
11 2 z2
a
p
/
p
ref
15.76 1 z2
/
2
b
1
/
2
(a)
Since p 5 1 atm, Eq. (a) reduces to
K1T25
z
11 2 z2
a
z
5.76 1 z
b
1
/
2
(b)
This equation involves two unknowns: z and the temperature T of the exiting equilibrium mixture.
Another equation involving the two unknowns is obtained from an energy rate balance of the form Eq. 13.12b,
which reduces with assumption 1 to
h
R
5 h
P
(c)
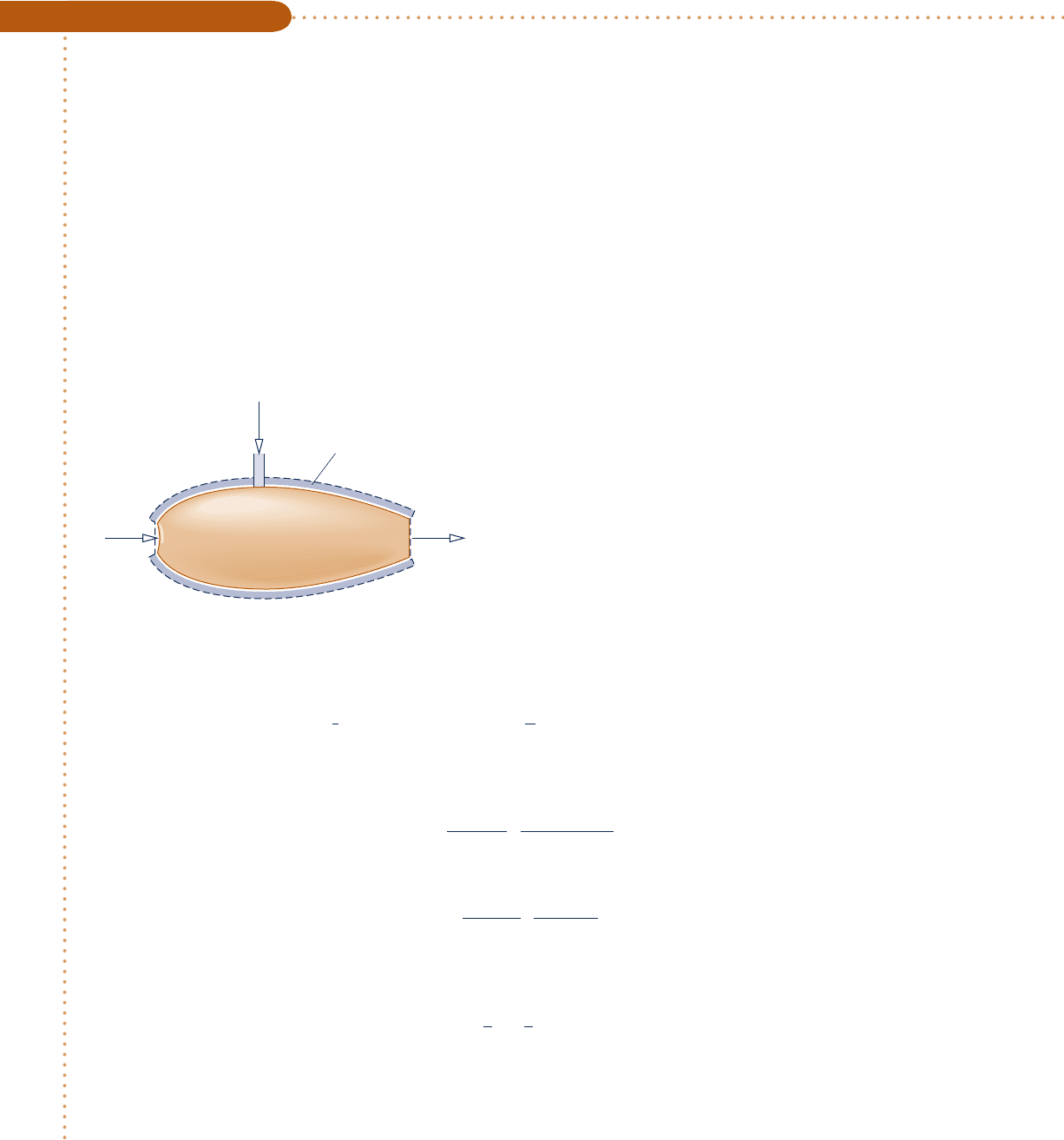
Engineering Model:
1.
The control volume shown on the accompany-
ing sketch by a dashed line operates at steady
state with Q
#
cv
5 0, W
#
cv
5 0, and negligible
effects of kinetic and potential energy.
2. The entering gases are modeled as ideal gases.
3. The exiting mixture is an ideal gas mixture at
equilibrium wherein N
2
is inert.
Fig. E14.6
Air
25°C, 1 atm
Insulation
(CO
2
, CO, O
2
, N
2
)
T, 1 atm
CO
25°
C, 1 atm
c14ChemicalandPhaseEquilibrium.866 Page 866 8/12/10 8:54:56 AM user-s146 c14ChemicalandPhaseEquilibrium.866 Page 866 8/12/10 8:54:56 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
As illustrated by Example 14.7, the equation solver and property retrieval features
of Interactive Thermodynamics: IT allow the equilibrium flame temperature and com-
position to be determined without the iteration required when using table data.
c c c c EXAMPLE 14.7 c
Solve Example 14.6 using Interactive Thermodynamics: IT and plot equilibrium flame temperature and z, the
amount of CO present in the exiting mixture, each versus pressure ranging from 1 to 10 atm.
SOLUTION
Known:
See Example 14.6.
Find: Using IT, plot the equilibrium flame temperature and the amount of CO present in the exiting mixture of
Example 14.6, each versus pressure ranging from 1 to 10 atm.
Engineering Model: See Example 14.6.
Analysis: Equation (a) of Example 14.6 provides the point of departure for the IT solution
K1T 25
z1z
/
22
1
/
2
11 2 z2
c
p
/
p
ref
15.76 1 z2
/
2
d
1
/
2
(a)
For a given pressure, this expression involves two unknowns: z and T.
Also, from Example 14.6, we use the energy balance, Eq. (c)
h
R
5 h
P
(c)
where
h
R
5 1h
CO
2
R
1
1
2
1h
O
2
2
R
1 1.881h
N
2
2
R
Determining the Equilibrium Flame Temperature Using Software
If the CO and air each entered at 5008C, would the equilibrium
flame temperature increase, decrease, or stay constant? Ans. Increase.
where
h
R
5 1h8
f
1 ¢h
0
2
CO
1
1
2
1h8
f
0
1 ¢h
0
2
O
2
1 1.881h8
f
0
1 ¢h
0
2
N
2
and
h
P
5 z1h8
f
1 ¢h2
CO
1
z
2
1h8
f
0
1 ¢h2
O
2
1 11 2 z21h8
f
1 ¢h2
CO
2
1 1.881h8
f
0
1 ¢h2
N
2
The enthalpy of formation terms set to zero are those for oxygen and nitrogen.
Since the reactants enter at 258C, the corresponding
¢h
terms also vanish. Col-
lecting and rearranging, we get
z1¢h2
CO
1
z
2
1¢h2
O
2
1 11 2 z21¢h2
CO
2
1 1.881¢h2
N
2
1 11 2 z231h8
f
2
CO
2
2 1h8
f
2
CO
45 0 (d)
Equations (b) and (d) are simultaneous equations involving the unknowns
z and T. When solved iteratively using tabular data, the results are z 5 0.125
and T 5 2399 K, as can be verified. The composition of the equilibrium mixture,
in kmol per kmol of CO entering the reactor, is then 0.125CO, 0.0625O
2
, 0.875CO
2
,
1.88N
2
.
Ability to…
❑
apply Eq. 14.35 together
with the energy balance
for reacting systems to
determine equilibrium flame
temperature.
❑
retrieve and use data from
Tables A-23, A-25, and
A-27.
✓
Skills Developed
14.4 Further Examples of the Use of the Equilibrium Constant 867
c14ChemicalandPhaseEquilibrium.867 Page 867 7/26/10 11:37:41 PM users-133 c14ChemicalandPhaseEquilibrium.867 Page 867 7/26/10 11:37:41 PM users-133 /Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New/Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New
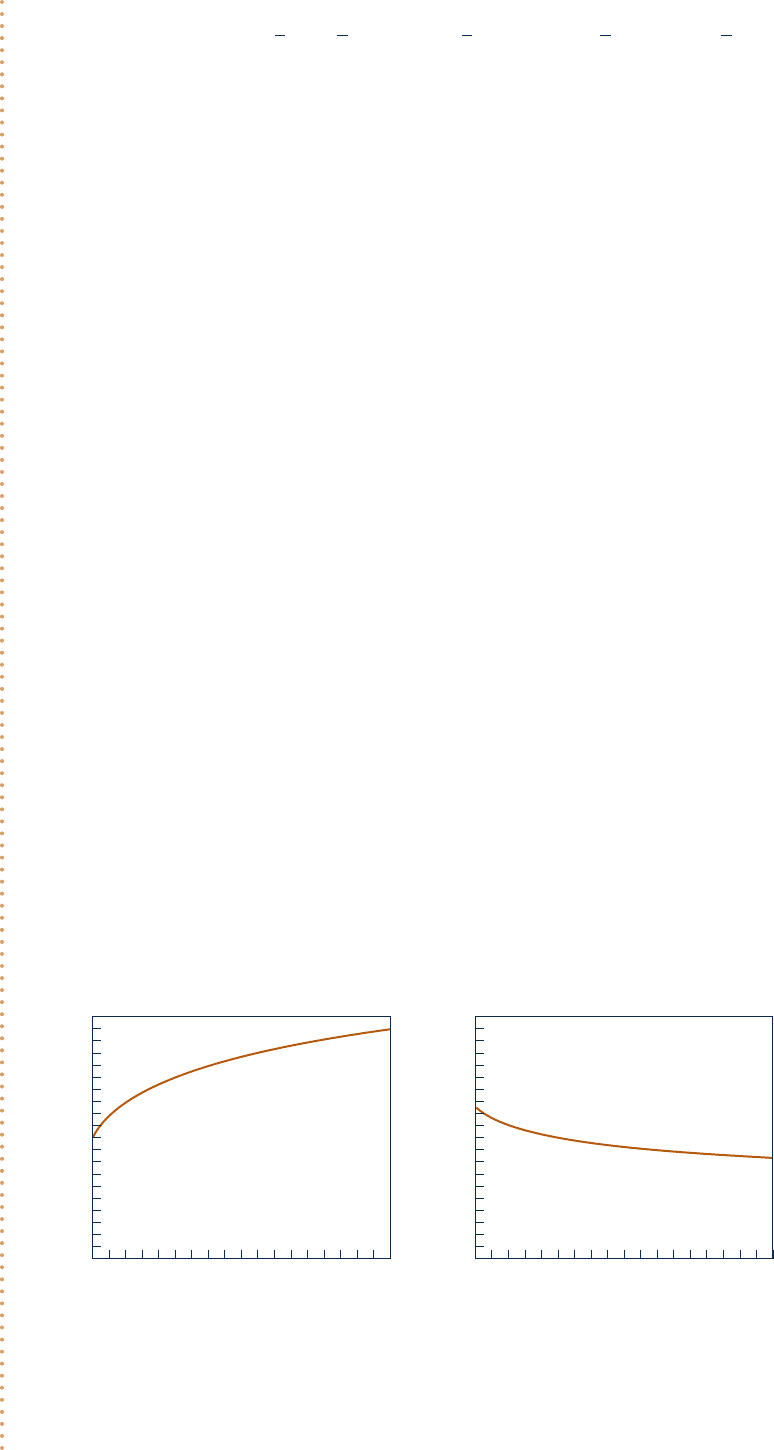
868 Chapter 14 Chemical and Phase Equilibrium
and
h
P
5 z1h
CO
2
P
1 1z
/
221h
O
2
2
P
1 11 2 z21h
CO
2
2
P
1 1.881h
N
2
2
P
where the subscripts R and P denote reactants and products, respectively, and z denotes the amount of CO in
the products, in kmol per kmol of CO entering.
With pressure known, Eqs. (a) and (c) can be solved for T and z using the following IT code. Choosing SI
from the Units menu and amount of substance in moles, and letting hCO_R denote the specific enthalpy of CO
in the reactants, and so on, we have
// Given data
TR 5 25 1 273.15 // K
p 5 1 // atm
pref 5 1 // atm
// Evaluating the equilibrium constant using Eq. (a)
K 5 ((z * (z/2)^0.5) / (1 – z)) * ((p / pref) / ((5.76 1 z) / 2))^0.5
// Energy balance: Eq. (c)
hR 5 hP
hR 5 hCO_R 1 (1/2) * hO2_R 1 1.88 * hN2_R
hP 5 z * hCO_P 1 (z /2) * hO2_P 1 (1 2 z) * hCO2_P 1 1.88 * hN2_P
hCO_R 5 h_T(“CO”,TR)
hO2_R 5 h_T(“O2”,TR)
hN2_R 5 h_T(“N2”,TR)
hCO_P 5 h_T(“CO”,T)
hO2_P 5 h_T(“O2”,T)
hCO2_P 5 h_T (“CO2”,T)
hN2_P 5 h_T (“N2”,T)
/* To obtain data for the equilibrium constant use the Look-up Table
option under the Edit menu. Load the file “eqco2.lut”. Data for
➊ CO2
S
d
CO 1 1/2 O2 from Table A-27 are stored in the look-up table
as T in column 1 and log10(K) in column 2. To retrieve the data use */
log(K) 5 lookupvall(eqco2, 1, T,2)
Obtain a solution for p 5 1 using the Solve button. To ensure rapid convergence, restrict T and K to positive
values, and set a lower limit of 0.001 and an upper limit of 0.999 for z. The results are T 5 2399 K and z 5
0.1249, which agree with the values obtained in Example 14.6.
Now, use the Explore button and sweep p from 1 to 10 atm in steps of 0.01. Using the Graph button, construct
the following plots:
Fig. E14.7
T (K)
2500
2450
2400
2350
2300
24681013579
p (atm)
z
0.2
0.15
0.1
0.05
0
24681013579
p (atm)
c14ChemicalandPhaseEquilibrium.868 Page 868 7/29/10 6:00:41 PM user-s146 c14ChemicalandPhaseEquilibrium.868 Page 868 7/29/10 6:00:41 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
14.4.2
Van’t Hoff Equation
The dependence of the equilibrium constant on temperature exhibited by the values
of Table A-27 follows from Eq. 14.31. An alternative way to express this dependence
is given by the van’t Hoff equation, Eq. 14.43b.
The development of this equation begins by introducing Eq. 14.29b into Eq. 14.31
to obtain on rearrangement
RT ln K 5231n
C
h
C
1 n
D
h
D
2 n
A
h
A
2 n
B
h
B
22 T 1n
C
s 8
C
1 n
D
s 8
D
2 n
A
s 8
A
2 n
B
s8
B
24
(14.41)
Each of the specific enthalpies and entropies in this equation depends on temperature
alone. Differentiating with respect to temperature
RT
d ln K
dT
1 R ln K 52cn
C
a
d
h
C
dT
2 T
d
s 8
C
dT
b1 n
D
a
d h
D
dT
2 T
d s 8
D
dT
b
2 n
A
a
d h
A
dT
2 T
d s 8
A
dT
b2 n
B
a
d h
B
dT
2 T
d s 8
B
dT
bd
1 1n
C
s 8
C
1 n
D
s 8
D
2 n
A
s 8
A
2 n
B
s 8
B
2
From the definition of s 81T2 (Eq. 6.19), we have d s 8
/
dT 5 c
p
/
T. Moreover, dh
/
dT 5 c
p
.
Accordingly, each of the underlined terms in the above equation vanishes identically,
leaving
RT
d ln
K
dT
1
R ln K 5 1n
C
s 8
C
1 n
D
s 8
D
2 n
A
s 8
A
2 n
B
s 8
B
2
(14.42)
Using Eq. 14.41 to evaluate the second term on the left and simplifying the resulting
expression, Eq. 14.42 becomes
d ln K
dT
5
1n
C
h
C
1 n
D
h
D
2 n
A
h
A
2 n
B
h
B
2
RT
2
(14.43a)
or, expressed more concisely
d ln K
dT
5
¢H
RT
2
(14.43b)
which is the van’t Hoff equation.
In Eq. 14.43b, ¢H is the enthalpy of reaction at temperature T. The van’t Hoff equa-
tion shows that when ¢H is negative (exothermic reaction), K decreases with tempera-
ture, whereas for ¢H positive (endothermic reaction), K increases with temperature.
From Fig. E14.7, we see that as pressure increases more CO is oxidized to CO
2
(z decreases) and temperature increases.
➊ Similar files are included in IT for each of the reactions in Table A-27.
If the CO and air each entered at 5008C, determine the equi-
librium flame temperature in K using Interactive Thermodynamics: IT.
Ans. 2575.
Ability to…
❑
apply Eq. 14.35 together
with the energy balance
for reacting systems to
determine equilibrium flame
temperature.
❑
perform equilibrium calcula-
tions using Interactive
Thermodynamics: IT.
✓Skills Developed
van’t Hoff equation
14.4 Further Examples of the Use of the Equilibrium Constant 869
c14ChemicalandPhaseEquilibrium.869 Page 869 7/26/10 11:37:45 PM users-133 c14ChemicalandPhaseEquilibrium.869 Page 869 7/26/10 11:37:45 PM users-133 /Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New/Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New
870 Chapter 14 Chemical and Phase Equilibrium
The enthalpy of reaction ¢H is often very nearly constant over a rather wide
interval of temperature. In such cases, Eq. 14.43b can be integrated to yield
ln
K
2
K
1
52
¢H
R
a
1
T
2
2
1
T
1
b
(14.44)
where K
1
and K
2
denote the equilibrium constants at temperatures T
1
and T
2
, respec-
tively. This equation shows that ln K is linear in 1/T. Accordingly, plots of ln K versus
1/T can be used to determine ¢H from experimental equilibrium composition data.
Alternatively, the equilibrium constant can be determined using enthalpy data.
14.4.3
Ionization
The methods developed for determining the equilibrium composition of a reactive
ideal gas mixture can be applied to systems involving ionized gases, also known as
plasmas. In previous sections we considered the chemical equilibrium of systems where
dissociation is a factor. For example, the dissociation reaction of diatomic nitrogen
N
2
S
d
2N
can occur at elevated temperatures. At still higher temperatures, ionization may take
place according to
N
S
d
N
1
1 e
2
(14.45)
That is, a nitrogen atom loses an electron, yielding a singly ionized nitrogen atom N
1
and a free electron e
2
. Further heating can result in the loss of additional electrons
until all electrons have been removed from the atom.
For some cases of practical interest, it is reasonable to think of the neutral atoms,
positive ions, and electrons as forming an ideal gas mixture. With this idealization,
ionization equilibrium can be treated in the same manner as the chemical equilibrium
of reacting ideal gas mixtures. The change in the Gibbs function for the equilibrium
ionization reaction required to evaluate the ionization-equilibrium constant can be
calculated as a function of temperature by using the procedures of statistical thermo-
dynamics. In general, the extent of ionization increases as the temperature is raised
and the pressure is lowered.
Example 14.8 illustrates the analysis of ionization equilibrium.
Considering Ionization Equilibrium
c c c c EXAMPLE 14.8 c
Consider an equilibrium mixture at 36008R consisting of Cs, Cs
1
, and e
2
, where Cs denotes neutral cesium atoms,
Cs
1
singly ionized cesium ions, and e
2
free electrons. The ionization-equilibrium constant at this temperature for
Cs
S
d
Cs
1
1 e
2
is K 5 15.63. Determine the pressure, in atmospheres, if the ionization of Cs is 95% complete, and plot percent
completion of ionization versus pressure ranging from 0 to 10 atm.
SOLUTION
Known:
An equilibrium mixture of Cs, Cs
1
, e
2
is at 36008R. The value of the equilibrium constant at this tem-
perature is known.
Find: Determine the pressure of the mixture if the ionization of Cs is 95% complete. Plot percent completion
versus pressure.
Engineering Model: Equilibrium can be treated in this case using ideal gas mixture equilibrium considerations.
c14ChemicalandPhaseEquilibrium.870 Page 870 7/26/10 11:37:48 PM users-133 c14ChemicalandPhaseEquilibrium.870 Page 870 7/26/10 11:37:48 PM users-133 /Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New/Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New
14.4.4
Simultaneous Reactions
Let us return to the discussion of Sec. 14.2 and consider the possibility of more than
one reaction among the substances present within a system. For the present applica-
tion, the closed system is assumed to contain a mixture of eight components A, B, C,
D, E, L, M, and N, subject to two independent reactions
(1) n
A
A 1 n
B
B
S
d
n
C
C 1 n
D
D (14.24)
(2) n
A9
A 1 n
L
L
S
d
n
M
M 1 n
N
N (14.46)
As in Sec. 14.2, component E is inert. Also, note that component A has been taken
as common to both reactions but with a possibly different stoichiometric coefficient
(n
A9
is not necessarily equal to n
A
).
14.4 Further Examples of the Use of the Equilibrium Constant 871
Solving Eq. (a) for z, determine the percent of ionization of
Cs at T 5 28808R (K 5 0.78) and p 5 1 atm. Ans. 66.2%.
Analysis: The ionization of cesium to form a mixture of Cs, Cs
1
, and e
2
is described by
Cs S 11 2 z2Cs 1 z Cs
1
1 ze
2
where z denotes the extent of ionization, ranging from 0 to 1. The total number of moles of mixture n is
n 5 11 2 z21 z 1 z 5 1 1 z
At equilibrium, we have Cs
S
d
Cs
1
1 e
2
, so Eq. 14.35 takes the form
K 5
1z21z
2
11 2 z2
c
p
/
p
ref
11 1 z2
d
11121
5 a
z
2
1 2 z
2
b a
p
p
ref
b
(a)
Solving for the ratio p
/
p
ref
and introducing the known value of K
p
p
ref
5 115.632 a
1 2 z
2
z
2
b
For p
ref
5 1 atm and z 5 0.95 (95%), p 5 1.69 atm. Using an equation-solver and plotting package, the following
plot can be constructed:
Ability to…
❑
apply Eq. 14.35 to deter-
mine the extent of ioniza-
tion of cesium given tem-
perature and pressure.
✓
Skills Developed
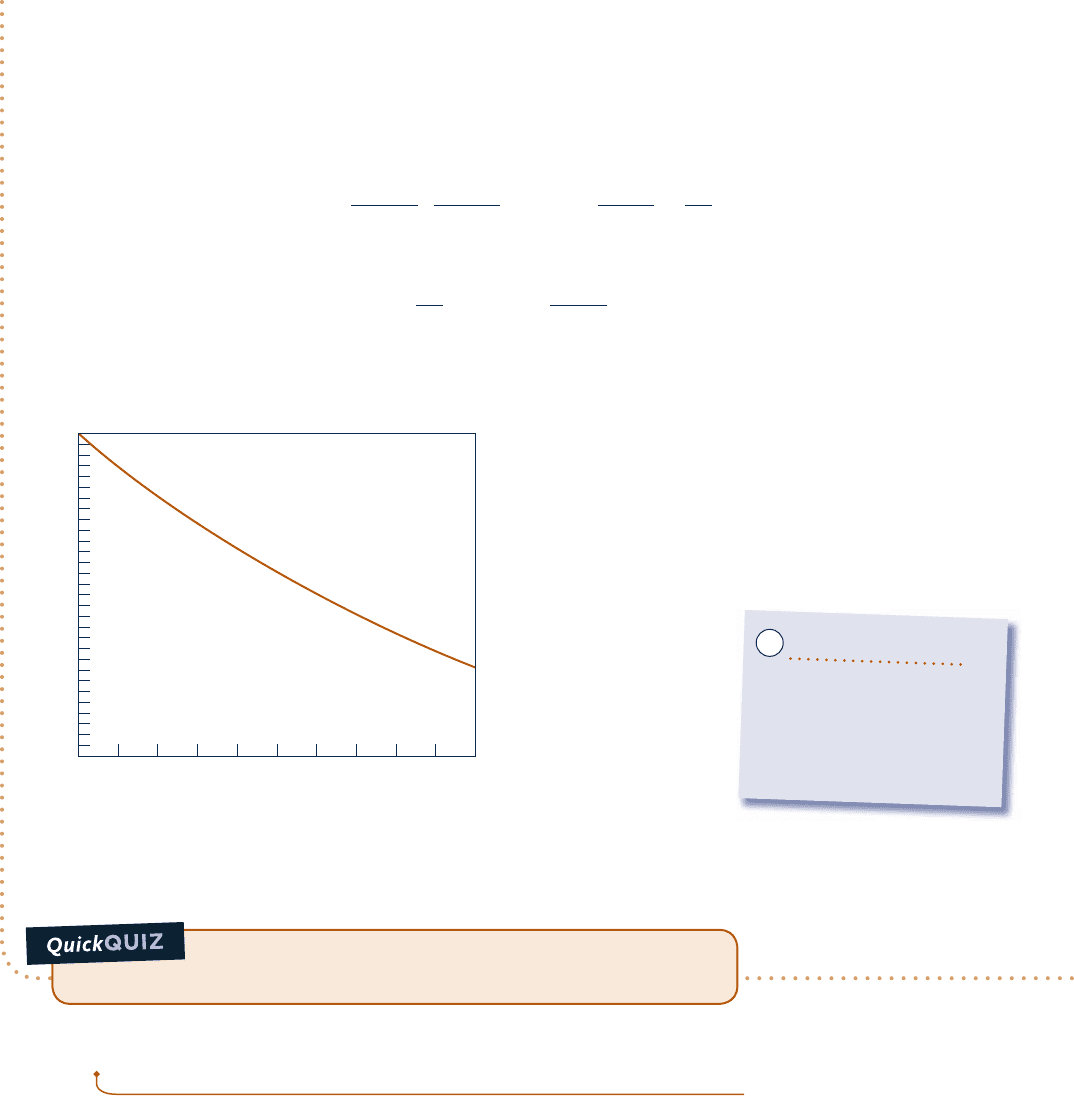
Figure E14.8 shows that ionization tends to occur to a lesser extent as pressure is raised. Ionization also tends
to occur to a greater extent as temperature is raised at fixed pressure.
Fig. E14.8
z (%)
100
95
90
85
80
75
70
0246810
p (atm)
c14ChemicalandPhaseEquilibrium.871 Page 871 7/26/10 11:37:48 PM users-133 c14ChemicalandPhaseEquilibrium.871 Page 871 7/26/10 11:37:48 PM users-133 /Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New/Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New

872 Chapter 14 Chemical and Phase Equilibrium
The stoichiometric coefficients of the above equations do not correspond to the
numbers of moles of the respective components present within the system, but changes
in the amounts of the components are related to the stoichiometric coefficients by
2dn
A
n
A
5
2dn
B
n
B
5
dn
C
n
C
5
dn
D
n
D
(14.25a)
following from Eq. 14.24, and
2dn
A
n
A¿
5
2dn
L
n
L
5
dn
M
n
M
5
dn
N
n
N
(14.47a)
following from Eq. 14.46. Introducing a proportionality factor de
1
, Eqs. 14.25a may
be represented by
dn
A
52n
A
de
1
,
dn
B
52n
B
de
1
dn
C
5 n
C
de
1
, dn
D
5 n
D
de
1
(14.25b)
Similarly, with the proportionality factor de
2
, Eqs. 14.47a may be represented by
dn
A
52n
A¿
de
2
,
dn
L
52n
L
de
2
dn
M
5 n
M
de
2
, dn
N
5 n
N
de
2
(14.47b)
Component A is involved in both reactions, so the total change in A is given by
dn
A
52n
A
de
1
2 n
A¿
de
2
(14.48)
Also, we have dn
E
5 0 because component E is inert.
For the system under present consideration, Eq. 14.10 is
dG4
T, p
5 m
A
dn
A
1 m
B
dn
B
1 m
C
dn
C
1 m
D
dn
D
1 m
E
dn
E
1 m
L
dn
L
1 m
M
dn
M
1 m
N
dn
N
(14.49)
Introducing the above expressions giving the changes in the n’s, this becomes
dG4
T, p
5 12n
A
m
A
2 n
B
m
B
1 n
C
m
C
1 n
D
m
D
2 de
1
1 12n
A¿
m
A
2 n
L
m
L
1 n
M
m
M
1 n
N
m
N
2 de
2
(14.50)
Since the two reactions are independent, de
1
and de
2
can be independently varied.
Accordingly, when dG4
T, p
5 0, the terms in parentheses must be zero and two equations
of reaction equilibrium result, one corresponding to each of the foregoing reactions:
n
A
m
A
1 n
B
m
B
5 n
C
m
C
1 n
D
m
D
(14.26b)
n
A¿
m
A
1 n
L
m
L
5 n
M
m
M
1 n
N
m
N
(14.51)
The first of these equations is exactly the same as that obtained in Sec. 14.2. For
the case of reacting ideal gas mixtures, this equation can be expressed as
2
a
¢G8
RT
b
1
5 ln c
y
n
C
C
y
n
D
D
y
n
A
A
y
n
B
B
a
p
p
ref
b
n
C
1n
D
2n
A
2n
B
d
(14.52)
Similarly, Eq. 14.51 can be expressed as
2a
¢G 8
RT
b
2
5 ln c
y
n
M
M
y
n
N
N
y
n
A¿
A
y
n
L
L
a
p
p
ref
b
n
M
1n
N
2 n
A¿
2n
L
d
(14.53)
In each of these equations, the DG8 term is evaluated as the change in Gibbs function
for the respective reaction, regarding each reactant and product as separate at tem-
perature T and a pressure of 1 atm.
From Eq. 14.52 follows the equilibrium constant
K
1
5
y
n
C
C
y
n
D
D
y
n
A
A
y
v
B
B
a
p
p
ref
b
n
C
1n
D
2n
A
2n
B
(14.54)
c14ChemicalandPhaseEquilibrium.872 Page 872 7/26/10 11:37:52 PM users-133 c14ChemicalandPhaseEquilibrium.872 Page 872 7/26/10 11:37:52 PM users-133 /Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New/Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New
