Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

c KEY EQUATIONS
AF 5 AF a
M
air
M
fuel
b
(13.2) p. 780
Relation between air–fuel ratios
on mass and molar bases
c EXERCISES: THINGS ENGINEERS THINK ABOUT
1. If an engine burns rich, is the percent of theoretical air
greater than 100% or less than 100%?
2. When you burn wood in a fireplace, do you contribute to
global climate change? Explain.
3. Why are some high-efficiency, residential natural gas
furnaces equipped with drain tubes?
4. You read that for every gallon of gasoline burned by a car’s
engine, nearly 20 lb of carbon dioxide is produced. Is this
correct? Explain.
5. Can coal be converted to a liquid diesel-like fuel? Explain.
6. How does the octane rating of gasoline relate to the
combustion in a car’s engine?
7. In K, how close to absolute zero have experimenters reached?
8. How is the desired air–fuel ratio maintained in automotive
internal combustion engines?
9. Does complete combustion of natural gas in pure oxygen
produce a higher or lower adiabatic flame temperature than
complete combustion of natural gas in atmospheric air?
Explain.
10. What is the difference between octane rating and octane?
11. How do those instant hot and cold packs used by athletes
to treat injuries work? For what kinds of injury is each type
of pack best suited?
12. What is an advantage of using standard chemical exergies?
A disadvantage?
13. How might an exergetic efficiency be defined for the
hybrid power system of Fig. 13.5?
14. In the derivation of Eqs. 13.44, we assume the reactor of
Fig. 13.7 operates without internal irreversibilities. Is this
assumption necessary? Explain.
Exercises: Things Engineers Think About 833
h1T, p25 h8
f
1 3h1T, p22 h1T
ref
, p
ref
245 h8
f
1 ¢h (13.9) p. 789
Evaluating enthalpy at T, p in
terms of enthalpy of formation
Q
#
cv
n
#
F
2
W
#
cv
n
#
F
5
a
P
n
e
1h
o
f
1 ¢h2
e
2
a
R
n
i
1h
o
f
1 ¢h2
i
(13.15b) p. 791
Energy rate balance for a con-
trol volume at steady state
per mole of fuel entering
Q 2 W 5
a
P
n1h
o
f
1 ¢h2 2
a
R
n1h
o
f
1 ¢h22 RT
p
a
P
n 1 RT
R
a
R
n
(13.17b) p. 795
Closed system energy balance,
where reactants and products
are each ideal gas mixtures
s1T, p25 s81T22 R ln
p
p
ref
(13.22) p. 809
Absolute entropy of an ideal
gas (molar basis) at T, p,
where
–
s8(T) is from Tables A-23
s
i
1T, p
i
25 s8
i
1T22 R ln
y
i
p
p
ref
(13.23) p. 810
Absolute entropy for compo-
nent i of an ideal gas mixture
(molar basis) at T, p, where
–
s8
i
(T ) is from Tables A-23
g1T, p25 g8
f
1 3g1T, p22 g1T
ref
, p
ref
245 g8
f
1 ¢g
(13.28a) p. 815
Evaluating Gibbs function at
T, p in terms of Gibbs func-
tion of formation
where
¢g 5 3h1T, p22 h1T
ref
, p
ref
242 3Ts1T, p22 T
ref
s1T
ref
, p
ref
24 (13.28b) p. 815 (see Tables A-25 for
–
g
o
f
values)
e
f
5 h 2 h
0
2 T
0
1s 2 s
0
21
V
2
2
1 gz 1 e
ch
(13.47) p. 826
Total specific flow exergy
including thermomechanical
and chemical contributions
(see Secs. 13.6 and 13.7 for
chemical exergy expressions)
c13ReactingMixturesandCombusti833 Page 833 7/21/10 7:25:12 AM user-s146 c13ReactingMixturesandCombusti833 Page 833 7/21/10 7:25:12 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
834 Chapter 13
Reacting Mixtures and Combustion
c PROBLEMS: DEVELOPING ENGINEERING SKILLS
Working with Reaction Equations
13.1 A vessel contains a mixture of 60% O
2
and 40% CO on
a mass basis. Determine the percent excess or percent
deficiency of oxygen, as appropriate.
13.2 Ten grams of propane (C
3
H
8
) burns with just enough
oxygen (O
2
) for complete combustion. Determine the
amount of oxygen required and the amount of combustion
products formed, each in grams.
13.3 Ethane (C
2
H
6
) burns completely with the theoretical
amount of air. Determine the air–fuel ratio on a (a) molar
basis, (b) mass basis.
13.4 A gas turbine burns octane (C
8
H
18
) completely with
400% of theoretical air. Determine the amount of N
2
in the
products, in kmol per kmol of fuel.
13.5 One hundred kmol of butane (C
4
H
10
) together with
4000 kmol of air enter a furnace per unit of time. Carbon
dioxide, carbon monoxide, and unburned fuel appear in the
products of combustion exiting the furnace. Determine the
percent excess or percent deficiency of air, whichever is
appropriate.
13.6 Propane (C
3
H
8
) is burned with air. For each case, obtain
the balanced reaction equation for complete combustion
(a) with the theoretical amount of air.
(b) with 20% excess air.
(c) with 20% excess air, but only 90% of the propane being
consumed in the reaction.
13.7 Butane (C
4
H
10
) burns completely with air. The equivalence
ratio is 0.9. Determine
(a) the balanced reaction equation.
(b) the percent excess air.
13.8 A natural gas mixture having a molar analysis 60% CH
4
,
30% C
2
H
6
, 10% N
2
is supplied to a furnace like the one
shown in Fig. P13.8, where it burns completely with 20%
excess air. Determine
(a) the balanced reaction equation.
(b) the air–fuel ratio, both on a molar and a mass basis.
13.9 A fuel mixture with the molar analysis 40% CH
3
OH,
50% C
2
H
5
OH, and 10% N
2
burns completely with 33%
excess air. Determine
(a) the balanced reaction equation.
(b) the air–fuel ratio, both on a molar and mass basis.
13.10 A gas mixture with the molar analysis 25% H
2
, 25% CO,
50% O
2
reacts to form products consisting of CO
2
, H
2
O, and
O
2
only. Determine the amount of each product, in kg per
kg of mixture.
13.11 A natural gas with the molar analysis 78% CH
4
, 13%
C
2
H
6
, 6% C
3
H
8
, 1.7% C
4
H
10
, 1.3% N
2
burns completely with
40% excess air in a reactor operating at steady state. If the molar
flow rate of the fuel is 0.5 kmol/h, determine the molar flow
rate of the air, in kmol/h.
13.12 A natural gas fuel mixture has the molar analysis shown
below. Determine the molar analysis of the products for
complete combustion with 70% excess dry air.
Fuel CH
4
H
2
NH
3
y
i
25% 30% 45%
13.13 Coal with the mass analysis 77.54% C, 4.28% H, 1.46% S,
7.72% O, 1.34% N, 7.66% noncombustible ash burns completely
with 120% of theoretical air. Determine
(a) the balanced reaction equation.
(b) the amount of SO
2
produced, in kg per kg of coal.
13.14 A coal sample has a mass analysis of 80.4% carbon,
3.9% hydrogen (H), 5.0% oxygen (O), 1.1% nitrogen (N),
1.1% sulfur, and the rest is noncombustible ash. For complete
combustion with 120% of the theoretical amount of air,
determine the air–fuel ratio on a mass basis.
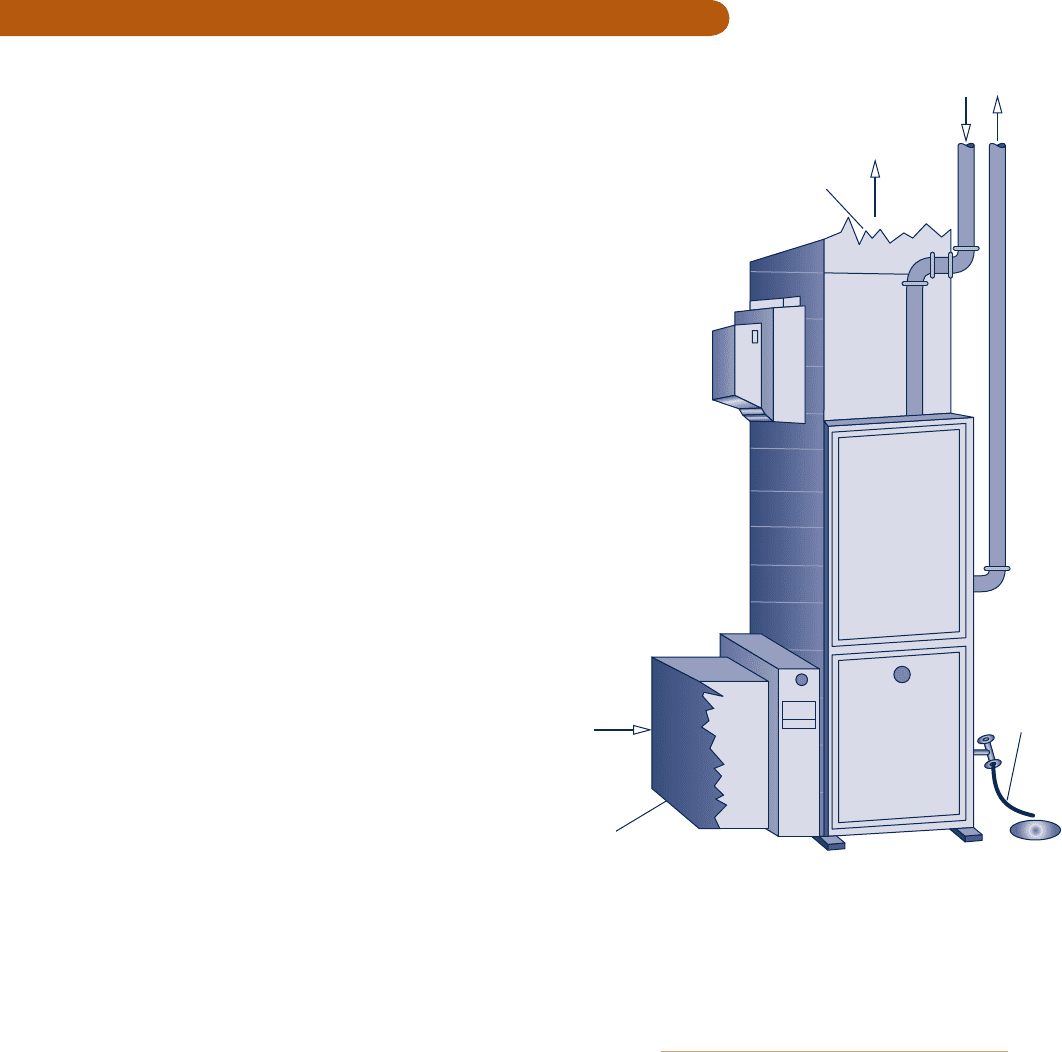
Combustion
air
Combustion
products
Warm air
supply duct
Condensate
drain
Cold air return duct
Fig. P13.8
c13ReactingMixturesandCombusti834 Page 834 7/14/10 10:30:18 AM user-s146 c13ReactingMixturesandCombusti834 Page 834 7/14/10 10:30:18 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
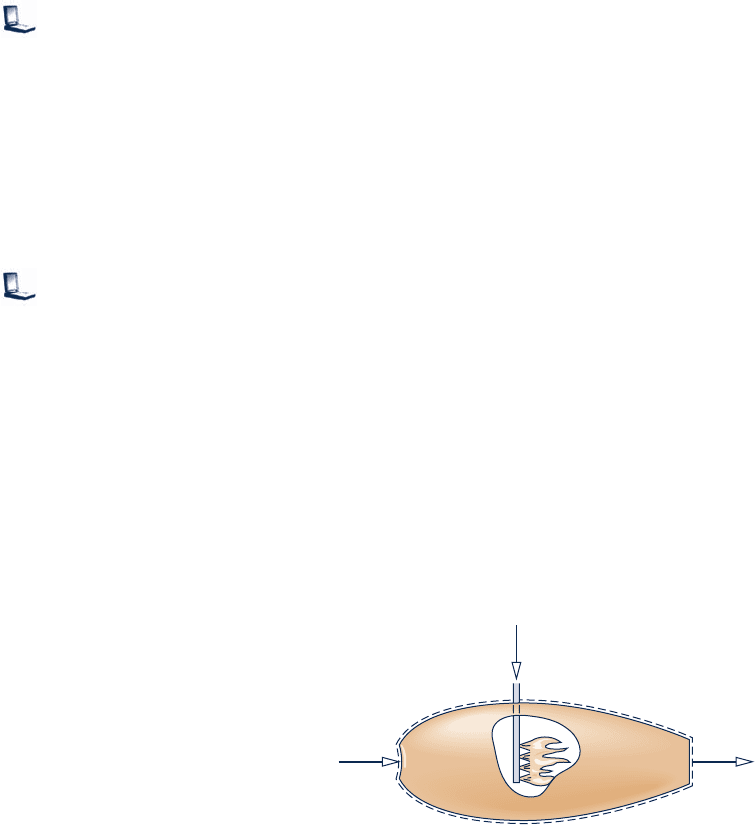
13.15 A sample of dried feedlot manure is being tested for use
as a fuel. The mass analysis of the sample is 42.7% carbon,
5.5% hydrogen (H), 31.3% oxygen (O), 2.4% nitrogen (N),
0.3% sulfur, and 17.8% noncombustible ash. The sample is
burned completely with 120% of theoretical air. Determine
(a) the balanced reaction equation.
(b) the air–fuel ratio on a mass basis.
13.16 A sample of dried Appanoose County coal has a mass
analysis of 71.1% carbon, 5.1% hydrogen (H), 9.0% oxygen (O),
1.4% nitrogen (N
2
), 5.8% sulfur, and the rest noncombustible
ash. For complete combustion with the theoretical amount
of air, determine
(a) the amount of SO
2
produced, in kg per kg of coal.
(b) the air–fuel ratio on a mass basis.
13.17 Octane (C
8
H
18
) burns completely with 120% of theoretical
air. Determine
(a) the air–fuel ratio on a molar and mass basis.
(b) the dew point temperature of the combustion products,
in
8C, when cooled at 1 atm.
13.18 Butane (C
4
H
10
) burns completely with 150% of
theoretical air. If the combustion products are cooled at 1
atm to temperature T, plot the amount of water vapor
condensed, in kmol per kmol of fuel, versus T ranging from
20 to 60
8C.
13.19 Ethylene (C
2
H
4
) burns completely with air and the
combustion products are cooled to temperature T at 1 atm.
The air–fuel ratio on a mass basis is AF.
(a) Determine for AF
5 15 and T 5 708F, the percent excess
air and the amount of water vapor condensed, in lb per
lbmol of fuel.
(b) Plot the amount of water vapor condensed, in lb per lbmol
of fuel, versus T ranging from 70 to 100
8F, for AF 5 15, 20,
25, 30.
13.20 A gaseous fuel mixture with a specified molar analysis
burns completely with moist air to form gaseous products as
shown in Fig. P13.20. Determine the dew point temperature
of the products, in
8C.
13.21 The gas driven off when low-grade coal is burned with
insufficient air for complete combustion is known as producer
gas. A particular producer gas has the following volumetric
analysis: 3.8% CH
4
, 0.1% C
2
H
6
, 4.8% CO
2
, 11.7% H
2
, 0.6%
O
2
, 23.2% CO, and the remainder N
2
, Determine, for
complete combustion with the theoretical amount of air
(a) the molar analysis of the dry products of combustion.
(b) the amount of water vapor condensed, in lbmol/lbmol of
producer gas, if the products are cooled to 70
8F at a constant
pressure of 1 atm.
13.22 Acetylene (C
2
H
2
) enters a torch and burns completely with
110% of theoretical air entering at 74
8F, 1 atm, 50% relative
humidity. Obtain the balanced reaction equation, and determine
the dew point temperature of the products, in
8F, at 1 atm.
13.23 Butane (C
4
H
10
) burns completely with 160% of theoretical
air at 20
8C, 1 atm, and 90% relative humidity. Determine
(a) the balanced reaction equation.
(b) the dew point temperature, in
8C, of the products, when
cooled at 1 atm.
13.24 Ethane (C
2
H
6
) enters a furnace and burns completely
with 130% of theoretical air entering at 25
8C, 85 kPa, 50%
relative humidity. Determine
(a) the balanced reaction equation.
(b) the dew point temperature of the combustion products, in 8C,
at 85 kPa.
13.25 Propane (C
3
H
8
) burns completely with the theoretical
amount of air at 60
8F, 1 atm, 90% relative humidity. Determine
(a) the balanced reaction equation.
(b) the dew point temperature of the combustion products
at 1 atm.
(c) the amount of water condensed, in lbmol per lbmol of
fuel, if the combustion products are cooled to 75
8F, at 1 atm.
13.26 A liquid fuel mixture that is 40% octane (C
8
H
18
) and
60% decane (C
10
H
22
) by mass is burned completely with
10% excess air at 25
8C, 1 atm, 80% relative humidity.
(a) Determine the equivalent hydrocarbon composition,
C
a
H
b
, of a fuel that would have the same carbon–hydrogen
ratio on a mass basis as the fuel mixture.
(b) If the combustion products are cooled to 25
8C at a
pressure of 1 atm, determine the amount of water vapor that
condenses, in kg per kg of fuel mixture.
Fuel
70% CH
4
, 10% H
2
,
3% O
2
, 5% CO
2
, 12% N
2
Gaseous combustion products
CO
2
, H
2
O, N
2
at 1 atm
Moist air
= 0.01 kg H
2
O/kg dry air
ω
Fig. P13.20
Problems: Developing Engineering Skills 835
c13ReactingMixturesandCombusti835 Page 835 7/14/10 10:30:18 AM user-s146 c13ReactingMixturesandCombusti835 Page 835 7/14/10 10:30:18 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

836 Chapter 13
Reacting Mixtures and Combustion
13.27 Hydrogen (H
2
) enters a combustion chamber with a
mass flow rate of 5 lb/h and burns with air entering at 85
8F,
1 atm with a volumetric flow rate of 75 ft
3
/min. Determine
the percent of theoretical air used.
13.28 Methyl alcohol (CH
3
OH) burns with 200% theoretical
air, yielding CO
2
, H
2
O, O
2
, and N
2
. Determine the
(a) balanced reaction equation.
(b) air–fuel ratio on a mass basis.
(c) molar analysis of the products.
13.29 Octane (C
8
H
18
) is burned with 20% excess air, yielding
CO
2
, CO, O
2
, H
2
O, and N
2
only. If 5% of the dry products
(molar basis) is O
2
, determine
(a) the balanced reaction equation.
(b) the analysis of the products on a dry molar basis.
13.30 Hexane (C
6
H
14
) burns with dry air to give products with
the dry molar analysis 8.5% CO
2
, 5.2% CO, 3% O
2
, 83.3%
N
2
, Determine
(a) the balanced reaction equation.
(b) the percent of theoretical air.
(c) the dew point temperature, in
8C, of the products at 1 atm.
13.31 The components of the exhaust gas of a spark-ignition
engine using a fuel mixture represented as C
8
H
17
have a
dry molar analysis of 8.7% CO
2
, 8.9% CO, 0.3% O
2
, 3.7%
H
2
, 0.3% CH
4
, and 78.1% N
2
. Determine the equivalence
ratio.
13.32 The combustion of a hydrocarbon fuel, represented as
C
a
H
b
, results in products with the dry molar analysis 11% CO
2
,
0.5% CO, 2% CH
4
, 1.5% H
2
, 6% O
2
, and 79% N
2
. Determine
the air–fuel ratio on (a) a molar basis, (b) a mass basis.
13.33 Decane (C
10
H
22
) burns completely in dry air. The air–
fuel ratio on a mass basis is 33. Determine the
(a) analysis of the products on a dry molar basis.
(b) percent of theoretical air.
13.34 Butane (C
4
H
10
) burns with air, giving products having
the dry molar analysis 11.0% CO
2
, 1.0% CO, 3.5% O
2
, 84.5%
N
2
. Determine
(a) the percent theoretical air.
(b) the dew point temperature of the combustion products,
in
8C, at 1 bar.
13.35 A natural gas with the volumetric analysis 97.3% CH
4
,
2.3% CO
2
, 0.4% N
2
is burned with air in a furnace to give
products having a dry molar analysis of 9.20% CO
2
, 3.84%
O
2
, 0.64% CO, and the remainder N
2
. Determine
(a) the percent theoretical air.
(b) the dew point temperature, in
8F, of the combustion products
at 1 atm.
13.36 A fuel oil having an analysis on a mass basis of 85.7%
C, 14.2% H, 0.1% inert matter burns with air to give products
with a dry molar analysis of 12.29% CO
2
, 3.76% O
2
, 83.95%
N
2
. Determine the air–fuel ratio on a mass basis.
13.37 Ethyl alcohol (C
2
H
5
OH) burns with air. The product gas
is analyzed and the laboratory report gives only the following
percentages on a dry molar basis: 6.9% CO
2
, 1.4% CO, 0.5%
C
2
H
5
OH. Assuming the remaining components consist of O
2
and N
2
, determine
(a) the percentages of O
2
and N
2
in the dry molar analysis.
(b) the percent excess air.
13.38 A fuel oil with the mass analysis 87% C, 11% H, 1.4%
S, 0.6% inert matter burns with 120% of theoretical air. The
hydrogen and sulfur are completely oxidized, but 95% of the
carbon is oxidized to CO
2
and the remainder to CO.
(a) Determine the balanced reaction equation.
(b) For the CO and SO
2
, determine the amount, in kmol per
10
6
kmol of combustion products (that is, the amount in
parts per million).
13.39 Pentane (C
5
H
12
) burns with air so that a fraction x of
the carbon is converted to CO
2
. The remaining carbon
appears as CO. There is no free O
2
in the products. Develop
plots of the air–fuel ratio and the percent of theoretical air
versus x, for x ranging from zero to unity.
13.40 For each of the following mixtures, determine the
equivalence ratio and indicate if the mixture is lean or rich:
(a) 1 kmol of butane (C
4
H
10
) and 32 kmol of air.
(b) 1 lb of propane (C
3
H
8
) and 14.5 lb of air.
13.41 Methyl alcohol (CH
3
OH) burns in dry air according to
the reaction
CH
3
OH 1 3.3
1
O
2
1 3.76N
2
2
S CO
2
1 2H
2
O
1 1.8O
2
1 12.408N
2
Determine the
(a) air–fuel ratio on a mass basis.
(b) equivalence ratio.
(c) percent excess air.
13.42 Ethyl alcohol (C
2
H
5
OH) burns in dry air according to
the reaction
C
2
H
5
OH 1 2.16
1
O
2
1 3.76N
2
2
S 0.32CO
2
1 1.68CO
1 3H
2
O 1 8.121
6
N
2
Determine the
(a) air–fuel ratio on a mass basis.
(b) equivalence ratio.
(c) percent theoretical air.
13.43 Octane (C
8
H
18
) enters an engine and burns with air to
give products with the dry molar analysis of CO
2
, 10.5%; CO,
5.8%; CH
4
, 0.9%; H
2
, 2.6%; O
2
, 0.3%; N
2
, 79.9%. Determine
the equivalence ratio.
13.44 Methane (CH
4
) burns with air to form products consisting
of CO
2
, CO, H
2
O, and N
2
only. If the equivalence ratio is
1.25, determine the balanced reaction equation.
Applying the First Law to Reacting Systems
13.45 Liquid octane (C
8
H
18
) at 778F, 1 atm enters a combustion
chamber operating at steady state and burns completely with
50% excess dry air entering at 120
8F, 1 atm. The products
exit at 1060
8F, 1 atm. Determine the rate of heat transfer
between the combustion chamber and its surroundings, in
c13ReactingMixturesandCombusti836 Page 836 7/13/10 11:43:20 AM user-s146 c13ReactingMixturesandCombusti836 Page 836 7/13/10 11:43:20 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
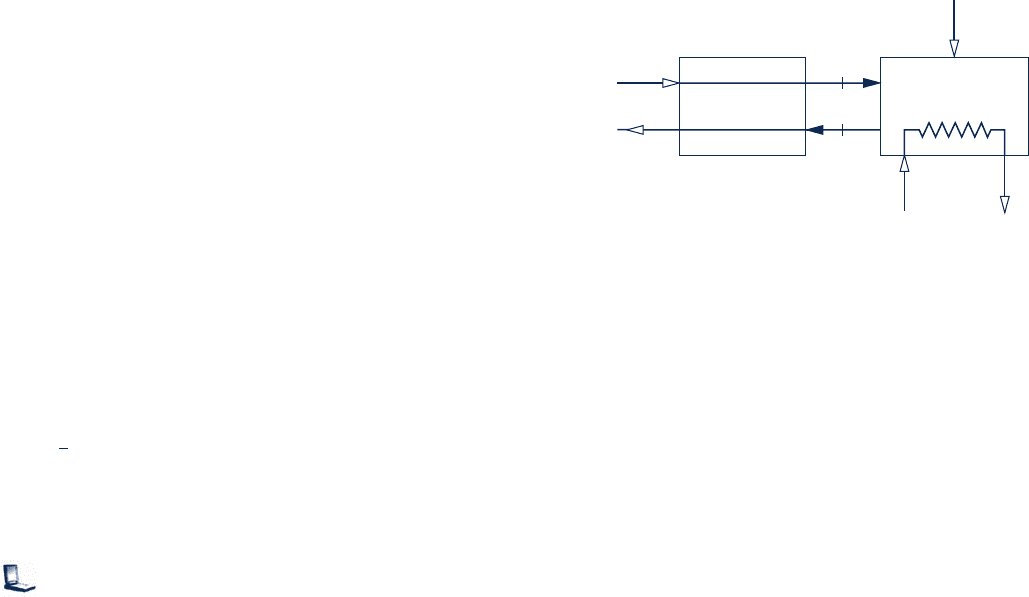
Btu per lbmol of fuel entering. Kinetic and potential energy
effects are negligible.
13.46 Propane (C
3
H
8
) at 298 K, 1 atm, enters a combustion
chamber operating at steady state with a molar flow rate of
0.7 kmol/s and burns completely with 200% of theoretical air
entering at 298 K, 1 atm. Kinetic and potential energy effects
are negligible. If the combustion products exit at 560 K, 1
atm, determine the rate of heat transfer for the combustion
chamber, in kW. Repeat for an exit temperature of 298 K.
13.47 Methane (CH
4
) at 258C, 1 atm enters a furnace operating
at steady state and burns completely with 140% of theoretical
air entering at 400 K, 1 atm. The products of combustion exit
at 700 K, 1 atm. Kinetic and potential energy effects are
negligible. If the rate of heat transfer from the furnace to
the surroundings is 400 kW, determine the mass flow rate of
methane, in kg/s.
13.48 Methane gas (CH
4
) at 258C, 1 atm enters a steam
generator operating at steady state. The methane burns
completely with 140% of theoretical air entering at 127
8C, 1
atm. Products of combustion exit at 427
8C, 1 atm. In a
separate stream, saturated liquid water enters at 8 MPa and
exits as superheated vapor at 480
8C with a negligible pressure
drop. If the vapor mass flow rate is 3.7 3 10
5
kg/h, determine
the volumetric flow rate of the methane, in m
3
/h.
13.49 Liquid ethanol (C
2
H
5
OH) at 778F, 1 atm enters a
combustion chamber operating at steady state and burns
completely with dry air entering at 340
8F, 1 atm. The fuel flow
rate is 50 lb/s, and the equivalence ratio is 0.8. Products of
combustion exit at 2000
8F, 1 atm. Ignoring kinetic and potential
energy effects, determine
(a) the air–fuel ratio on a mass basis.
(b) the rate of heat transfer, in Btu/s.
13.50 Octane gas (C
8
H
18
) at 258C, 1 atm enters a combustion
chamber operating at steady state and burns with 120%
theoretical air entering at 25
8C, 1 atm. The combustion
products exit at 1200 K and include only CO
2
, H
2
O, O
2
, and
N
2
. If the rate of heat transfer from the combustion chamber
to the surroundings is 2500 kW, determine the mass flow rate
of the fuel, in kg/s.
13.51 Liquid propane (C
3
H
8
) at 258C, 1 atm, enters a well-
insulated reactor operating at steady state. Air enters at
the same temperature and pressure. For liquid propane,
h8
f
52118,900 kJ
/
kmol. Determine the temperature of the
combustion products, in K, for complete combustion with
(a) the theoretical amount of air.
(b) 300% of theoretical air.
13.52 The energy required to vaporize the working fluid
passing through the boiler of a simple vapor power plant is
provided by the complete combustion of methane with
110% of theoretical air. The fuel and air enter in separate
streams at 25
8C, 1 atm. Products of combustion exit the stack
at 150
8C, 1 atm. Plot the mass flow rate of fuel required, in
kg/h per MW of power developed by the plant versus the
plant thermal efficiency, h. Consider h in the range 30–40%.
Kinetic and potential energy effects are negligible.
13.53 Methane (CH
4
) at 258C, enters the combustor of a
simple open gas turbine power plant and burns completely
with 400% of theoretical air entering the compressor at
25
8C, 1 atm. Products of combustion exit the turbine at 5778C,
1 atm. The rate of heat transfer from the gas turbine is
estimated as 10% of the net power developed. Determine the
net power output, in MW, if the fuel mass flow rate is 1200 kg/h.
Kinetic and potential energy effects are negligible.
13.54 Octane gas C
8
H
18
at 258C enters a jet engine and burns
completely with 300% of theoretical air entering at 25
8C,
1 atm with a volumetric flow rate of 42 m
3
/s. Products of
combustion exit at 990 K, 1 atm. If the fuel and air enter
with negligible velocities, determine the thrust produced by
the engine in kN.
13.55 Figure P13.55 provides data for a boiler and air
preheater operating at steady state. Methane (CH
4
) entering
the boiler at 25
8C, 1 atm is burned completely with 170%
of theoretical air. Ignoring stray heat transfer and kinetic
and potential energy effects, determine the temperature, in
8C, of the combustion air entering the boiler from the
preheater.
Air
25°C, 1 atm
Combustion
gas at 662°C
Preheater
T = ?
797°
C
Boiler
Feedwater Steam
CH
4
25°C, 1 atm
Fig. P13.55
13.56 One lbmol of octane gas (C
8
H
18
) reacts with the
theoretical amount of air in a closed, rigid tank. Initially,
the reactants are at 77
8F, 1 atm. After complete combustion,
the pressure in the tank is 3.98 atm. Determine the heat transfer,
in Btu.
13.57 A closed, rigid vessel initially contains a gaseous mixture
at 25
8C, 1 atm with the molar analysis of 20% ethane (C
2
H
6
),
80% oxygen (O
2
). The initial mixture contains one kmol of
ethane. Complete combustion occurs, and the products are
cooled to 25
8C. Determine the heat transfer, in kJ, and the
final pressure, in atm.
13.58 A closed, rigid vessel initially contains a gaseous mixture
of 1 kmol of pentane (C
5
H
12
) and 150% of theoretical air at
25
8C, 1 atm. If the mixture burns completely, determine the
heat transfer from the vessel, in kJ, and the final pressure, in
atm, for a final temperature of 800 K.
13.59 Calculate the enthalpy of combustion of gaseous pentane
(C
5
H
12
), in kJ per kmol of fuel, at 258C with water vapor in
the products.
Problems: Developing Engineering Skills 837
c13ReactingMixturesandCombusti837 Page 837 8/11/10 9:26:48 AM user-s146 c13ReactingMixturesandCombusti837 Page 837 8/11/10 9:26:48 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

838 Chapter 13
Reacting Mixtures and Combustion
13.60 Plot the enthalpy of combustion for gaseous propane
(C
3
H
8
), in Btu per lbmol of fuel, at 1 atm versus temperature
in the interval 77 to 500
8F. Assume water vapor in the
products. For propane, let c
p
= 0.41 Btu/lb ? 8R.
13.61 Plot the enthalpy of combustion for gaseous methane
(CH
4
), in Btu per lbmol of fuel, at 1 atm versus temperature
in the interval from 537 to 1800
8R. Assume water vapor in
the products. For methane, let
c
p
5 4.52 1 7.371T
/
10002
Btu
/
lbmol ? 8R, where T is in 8R.
13.62 For the producer gas of Prob. 13.21, determine the
enthalpy of combustion, in Btu per lbmol of mixture, at 77
8F,
1 atm, assuming water vapor in the products.
13.63 Determine the lower heating value, in kJ per kmol of
fuel and in kJ per kg of fuel, at 25
8C, 1 atm for
(a) gaseous ethane (C
2
H
6
).
(b) liquid ethanol (C
2
H
5
OH).
(c) gaseous propane (C
3
H
8
).
(d) liquid octane (C
8
H
18
).
13.64 For a natural gas with a molar analysis of 86.5% CH
4
, 8%
C
2
H
6
, 2% C
3
H
8
, 3.5% N
2
, determine the lower heating value, in
kJ per kmol of fuel and in kJ per kg of fuel, at 25
8C, 1 atm.
13.65 Liquid octane (C
8
H
18
) at 258C, 1 atm enters an insulated
reactor operating at steady state and burns with 90% of
theoretical air at 25
8C, 1 atm to form products consisting of
CO
2
, CO, H
2
O, and N
2
only. Determine the temperature of the
exiting products, in K. Compare with the results of Example
13.8 and comment.
13.66 For each of the following fuels, plot the adiabatic flame
temperature, in K, versus percent excess air for complete
combustion in a combustor operating at steady state. The
reactants enter at 25
8C, 1 atm.
(a) carbon.
(b) hydrogen (H
2
).
(c) liquid octane (C
8
H
18
).
13.67 Propane gas (C
3
H
8
) at 258C, 1 atm enters an insulated
reactor operating at steady state and burns completely with
air entering at 25
8C, 1 atm. Plot the adiabatic flame temperature
versus percent of theoretical air ranging from 100 to 400%.
Why does the adiabatic flame temperature vary with increasing
combustion air?
13.68 Hydrogen (H
2
) at 778F, 1 atm enters an insulated reactor
operating at steady state and burns completely with x% of
theoretical air entering at 77
8F 1 atm. Plot the adiabatic
flame temperature for x ranging from 100 to 400%.
13.69 Methane gas (CH
4
) at 258C, 1 atm enters an insulated
reactor operating at steady state and burns completely with
x% of theoretical air entering at 25
8C, 1 atm. Plot the adiabatic
flame temperature for x ranging from 100 to 400%.
13.70 Methane (CH
4
) at 258C, 1 atm enters an insulated
reactor operating at steady state and burns with the
theoretical amount of air entering at 25
8C, 1 atm. The
products contain CO
2
, CO, H
2
O, O
2
, and N
2
, and exit at 2260 K.
Determine the fractions of the entering carbon in the fuel
that burn to CO
2
and CO, respectively.
13.71 Ethane (C
2
H
6
) gas at 778F, 1 atm enters a well-insulated
reactor operating at steady state and burns completely with
air entering at 240
8F, 1 atm. Determine the temperature of the
products, in
8F. Neglect kinetic and potential energy effects.
13.72 Liquid methanol (CH
3
OH) at 258C, 1 atm enters an
insulated reactor operating at steady state and burns
completely with air entering at 100
8C, 1 atm. If the combustion
products exit at 1256
8C, determine the percent excess air
used. Neglect kinetic and potential energy effects.
13.73 Methane (CH
4
) at 778F enters the combustor of a gas
turbine power plant operating at steady state and burns
completely with air entering at 400
8F. The temperature of the
products of combustion flowing from the combustor to the
turbine depends on the percent excess air for combustion. Plot
the percent excess air versus combustion product temperatures
ranging from 1400 to 1800
8F. There is no significant heat
transfer between the combustor and its surroundings, and
kinetic and potential energy effects can be ignored.
13.74 Air enters the compressor of a simple gas turbine power
plant at 70
8F, 1 atm, is compressed adiabatically to 40 lbf/in.
2
,
and then enters the combustion chamber where it burns
completely with propane gas (C
3
H
8
) entering at 778F, 40 lbf/in.
2
and a molar flow rate of 1.7 lbmol/h. The combustion products
at 1340
8F, 40 lbf/in.
2
enter the turbine and expand adiabatically
to a pressure of 1 atm. The isentropic compressor efficiency
is 83.3% and the isentropic turbine efficiency is 90%. Determine
at steady state
(a) the percent of theoretical air required.
(b) the net power developed, in horsepower.
13.75 A mixture of gaseous octane (C
8
H
18
) and 200% of
theoretical air, initially at 25
8C, 1 atm, reacts completely in a rigid
vessel.
(a) If the vessel were well-insulated, determine the temperature,
in
8C, and the pressure, in atm, of the combustion products.
(b) If the combustion products were cooled at constant volume
to 25
8C, determine the final pressure, in atm, and the heat
transfer, in kJ per kmol of fuel.
13.76 Methane gas (CH
4
) reacts completely with the theoretical
amount of oxygen (O
2
) in a piston-cylinder assembly. Initially,
the mixture is at 77
8F, 1 atm. If the process occurs at constant
pressure and the final volume is 1.9 times the initial volume,
determine the work and the heat transfer, each in Btu per
lbmol of fuel.
13.77 A 5 3 10
2
3
kg sample of liquid benzene (C
6
H
6
) together
with 20% excess air, initially at 25
8C and 1 atm, reacts
completely in a rigid, insulated vessel. Determine the
temperature, in
8C, and the pressure, in atm, of the combustion
products.
Applying the Second Law to Reacting Systems
13.78 Carbon monoxide (CO) at 258C, 1 atm enters an
insulated reactor operating at steady state and reacts
completely with the theoretical amount of air entering in a
separate stream at 25
8C, 1 atm. The products of combustion
exit as a mixture at 1 atm. For the reactor, determine the
c13ReactingMixturesandCombusti838 Page 838 10/9/10 11:39:42 AM user-s146 c13ReactingMixturesandCombusti838 Page 838 10/9/10 11:39:42 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

rate of entropy production, in kJ/K per kmol of CO entering.
Neglect kinetic and potential energy effects.
13.79 Methane (CH
4
) at 778F, 1 atm enters an insulated reactor
operating at steady state and burns completely with air entering
in a separate stream at 77
8F, 1 atm. The products of combustion
exit as a mixture at 1 atm. For the reactor, determine the rate
of entropy production, in Btu/
8R per lbmol of methane entering,
for combustion with
(a) the theoretical amount of air.
(b) 200% of theoretical air.
Neglect kinetic and potential energy effects.
13.80 Carbon monoxide (CO) reacts with water vapor in an
insulated reactor operating at steady state to form hydrogen
(H
2
) and carbon dioxide (CO
2
). The products exit as a
mixture at 1 atm. For the reactor, determine the rate of
entropy production, in kJ/K per kmol of carbon monoxide
entering. Neglect kinetic and potential energy effects. Consider
two cases:
(a) The carbon monoxide and water vapor enter the reactor
in separate streams, each at 400 K, 1 atm.
(b) The carbon monoxide and water vapor enter the reactor
as a mixture at 400 K, 1 atm.
Explain why the answers in parts (a) and (b) differ.
13.81 A gaseous mixture of butane (C
4
H
10
) and 80% excess
air at 25
8C, 3 atm enters a reactor. Complete combustion
occurs, and the products exit as a mixture at 1200 K, 3 atm.
Coolant enters an outer jacket as a saturated liquid and
saturated vapor exits at essentially the same pressure. No
significant heat transfer occurs from the outer surface of the
jacket, and kinetic and potential energy effects are negligible.
Determine for the jacketed reactor
(a) the mass flow rate of the coolant, in kg per kmol of fuel.
(b) the rate of entropy production, in kJ/K per kmol of fuel.
(c) the rate of exergy destruction, in kJ per kmol of fuel, for
T
0
5 258C.
Consider each of two coolants: water at 1 bar and ammonia
at 10 bar.
13.82 Liquid ethanol (C
2
H
5
OH) at 258C, 1 atm enters a
reactor operating at steady state and burns completely with
130% of theoretical air entering in a separate stream at
25
8C, 1 atm. Combustion products exit at 2278C, 1 atm. Heat
transfer from the reactor takes place at an average surface
temperature T
b
. For T
b
ranging from 25 to 2008C, determine
the rate of exergy destruction within the reactor, in kJ per
kmol of fuel. Kinetic and potential energy effects are
negligible. Let T
0
5 258C.
13.83 A gaseous mixture of ethane (C
2
H
6
) and the theoretical
amount of air at 25
8C, 1 atm enters a reactor operating at
steady state and burns completely. Combustion products exit
at 627
8C, 1 atm. Heat transfer from the reactor takes place
at an average surface temperature T
b
. For T
b
ranging from
25 to 600
8C, determine the rate of exergy destruction within
the reactor, in kJ per kmol of fuel. Kinetic and potential
energy effects are negligible. Let T
0
5 258C.
13.84 Determine the change in the Gibbs function, in kJ per kmol
of methane, at 25
8C, 1 atm for CH
4
1 2O
2
S CO
2
1 2H
2
O,
using
(a) Gibbs function of formation data.
(b) enthalpy of formation data, together with absolute
entropy data.
13.85 Determine the change in the Gibbs function, in Btu per
lbmol of hydrogen, at 77
8F, 1 atm for H
2
1
1
2
O
2
S H
2
O1g2,
using
(a) Gibbs function of formation data.
(b) enthalpy of formation data, together with absolute entropy
data.
13.86 Separate streams of hydrogen (H
2
) and oxygen (O
2
) at
25
8C, 1 atm enter a fuel cell operating at steady state, and
liquid water exits at 25
8C, 1 atm. The hydrogen flow rate is
2 3 10
2
4
kmol/s. If the fuel cell operates isothermally at
25
8C, determine the maximum theoretical power it can
develop and the accompanying rate of heat transfer, each in
kW. Kinetic and potential energy effects are negligible.
13.87 Streams of methane (CH
4
) and oxygen (O
2
), each at
25
8C, 1 atm, enter a fuel cell operating at steady state. Streams
of carbon dioxide and water exit separately at 25
8C, 1 atm.
If the fuel cell operates isothermally at 25
8C, 1 atm, determine
the maximum theoretical work that it can develop, in kJ per
kmol of methane. Ignore kinetic and potential energy effects.
13.88 Streams of hydrogen (H
2
) and oxygen (O
2
), each at 1 atm,
enter a fuel cell operating at steady state and water vapor
exits at 1 atm. If the cell operates isothermally at (a) 300 K,
(b) 400 K, and (c) 500 K, determine the maximum theoretical
work that can be developed by the cell in each case, in kJ per
kmol of hydrogen flowing, and comment. Heat transfer with
the surroundings takes place at the cell temperature, and
kinetic and potential energy effects can be ignored.
13.89 An inventor has developed a device that at steady state
takes in liquid water at 25
8C, 1 atm with a mass flow rate of
4 kg/h and produces separate streams of hydrogen (H
2
) and
oxygen (O
2
), each at 258C, 1 atm. The inventor claims that
the device requires an electrical power input of 14.6 kW
when operating isothermally at 25
8C. Heat transfer with the
surroundings occurs, but kinetic and potential energy effects
can be ignored. Evaluate the inventor’s claim.
13.90 Coal with a mass analysis of 88% C, 6% H, 4% O, 1%
N, 1% S burns completely with the theoretical amount of air.
Determine
(a) the amount of SO
2
produced, in kg per kg of coal.
(b) the air–fuel ratio on a mass basis.
(c) For environmental reasons, it is desired to separate the
SO
2
from the combustion products by supplying the products
at 340 K, 1 atm to a device operating isothermally at 340 K.
At steady state, a stream of SO
2
and a stream of the remaining
gases exit, each at 340 K, 1 atm. If the coal is burned at a
rate of 10 kg/s, determine the minimum theoretical power
input required by the device, in kW. Heat transfer with the
surroundings occurs, but kinetic and potential energy effects
can be ignored.
Problems: Developing Engineering Skills 839
c13ReactingMixturesandCombusti839 Page 839 7/13/10 11:43:25 AM user-s146 c13ReactingMixturesandCombusti839 Page 839 7/13/10 11:43:25 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

840 Chapter 13
Reacting Mixtures and Combustion
Using Chemical Exergy
13.91 Applying Eq. 13.36 for (a) carbon, (b) hydrogen (H
2
),
(c) methane, (d) carbon monoxide, (e) nitrogen (N
2
), (f)
oxygen (O
2
), and (g) carbon dioxide, determine the chemical
exergy, in kJ/kg, relative to the following environment in which
the gas phase obeys the ideal gas model:
Environment
T
0
5 298.15 K (258C), p
0
5 1 atm
Gas Phase: Component y
e
(%)
N
2
75.67
O
2
20.35
H
2
O(g) 3.12
CO
2
0.03
Other 0.83
13.92 The accompanying table shows an environment consisting
of a gas phase and a condensed water phase. The gas phase
forms an ideal gas mixture.
Environment
T
0
5 298.15 K (258C), p
0
5 1 atm
Condensed Phase: H
2
O(l) at T
0
, p
0
Gas Phase: Component y
e
(%)
N
2
75.67
O
2
20.35
H
2
O(g) 3.12
CO
2
0.03
Other 0.83
(a) Show that the chemical exergy of the hydrocarbon C
a
H
b
can be determined as
e
ch
5 c
g
F
1 aa 1
b
4
b
g
O
2
2 a
g
CO
2
2
b
2
g
H
2
O1l2
d1 R T
0
ln c
1y
e
O
2
2
a1 b
/
4
1y
e
CO
2
2
a
d
(b) Using the result of part (a), repeat parts (a) through (c)
of Problem 13.91.
13.93 Justify the use of Eq. 13.36 for liquid methanol, CH
3
OH,
and liquid ethanol, C
2
H
5
OH, and apply it to evaluate the
chemical exergy, in kJ/kmol, of each substance relative to the
environment of Prob. 13.91. Compare with the respective standard
chemical exergy values from Table A-26 (Model II).
13.94 Showing all important steps, derive (a) Eqs. 13.41a, b
(b) Eqs. 13.44 a, b.
13.95 Using data from Tables A-25 and A-26, together with Eq.
13.44b, determine the standard molar chemical exergy, in
kJ/kmol, of propane C
3
H
8
(g). Compare this value with the
standard chemical exergy from Table A-26 (Model II).
13.96 Evaluate the total specific flow exergy of nitrogen (N
2
),
in Btu/lb, at 200
8F, 4 atm. Neglect the effects of motion and
gravity. Perform calculations
(a) relative to the environment of Problem 13.91.
(b) using data from Table A-26 (Model II).
13.97 Evaluate the total specific flow exergy of water vapor,
in kJ/kg, at 200
8C, 1 bar. Neglect the effects of motion and
gravity. Perform calculations
(a) relative to the environment of Problem 13.91.
(b) using data from Table A-26 for each of the models.
13.98 Evaluate the total specific flow exergy of an equimolar
mixture of oxygen (O
2
) and nitrogen (N
2
), in kJ/kg, at 2278C,
1 atm. Neglect the effects of motion and gravity. Perform
calculations
(a) relative to the environment of Problem 13.91.
(b) using data from Table A-26 (Model II).
13.99 A mixture of methane gas (CH
4
) and 150% of theoretical
air enters a combustion chamber at 77
8F, 1 atm. Determine
the total specific flow exergy of the entering mixture, in Btu
per lbmol of methane. Ignore the effects of motion and
gravity. Perform calculations
(a) relative to the environment of Problem 13.91.
(b) using data from Table A-26 (Model II).
13.100 A mixture having an analysis on a molar basis of 85%
dry air, 15% CO enters a device at 125
8C, 2.1 atm, and a
velocity of 250 m/s. If the mass flow rate is 1.0 kg/s, determine
the rate exergy enters, in MW. Neglect the effect of gravity.
Perform calculations
(a) relative to the environment of Problem 13.91.
(b) using data from Table A-26 (Model II).
13.101 The following flow rates in lb/h are reported for the exiting
syngas (synthesis gas) stream in a certain process for producing
syngas from bituminous coal:
C
H
4
429,684 lb/h
CO
2
9,093 lb/h
N
2
3,741 lb/h
H
2
576 lb/h
CO 204 lb/h
H
2
O
60 lb/h
If the syngas stream is at 77°F, 1 atm, determine the rate at
which exergy exits, in Btu/h. Perform calculations relative to
the environment of Problem 13.91. Neglect the effects of
motion and gravity.
Exergy Analysis of Reacting and Psychrometric Systems
13.102 Carbon at 258C, 1 atm enters an insulated reactor
operating at steady state and reacts completely with the
theoretical amount of air entering separately at 25
8C, 1 atm.
For the reactor, (a) determine the rate of exergy destruction,
in kJ per kmol of carbon, and (b) evaluate an exergetic
efficiency. Perform calculations relative to the environment
of Problem 13.91. Neglect the effects of motion and gravity.
13.103 Propane gas (C
3
H
8
) at 258C, 1 atm and a volumetric
flow rate of 0.03 m
3
/min enters a furnace operating at steady
state and burns completely with 200% of theoretical air
entering at 25
8C, 1 atm. The furnace provides energy by heat
transfer at 227
8C for an industrial process and combustion
products at 277
8C, 1 atm for cogeneration of hot water. For
the furnace, perform a full exergy accounting, in kJ/min, of
the exergy supplied by the fuel. Use standard chemical
exergies from Table A-26 (Model II), as required, and ignore
the effects of motion and gravity.
c13ReactingMixturesandCombusti840 Page 840 7/13/10 11:43:26 AM user-s146 c13ReactingMixturesandCombusti840 Page 840 7/13/10 11:43:26 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
13.104 Figure P13.104 shows a coal gasification reactor making
use of the carbon–steam process. The energy required for the
endothermic reaction is supplied by an electrical resistor.
The reactor operates at steady state, with no stray heat
transfers and negligible effects of motion and gravity. Evaluate
in Btu per lbmol of carbon entering
(a) the required electrical input.
(b) the exergy entering with the carbon.
(c) the exergy entering with the steam.
(d) the exergy exiting with the product gas.
(e) the exergy destruction within the reactor.
Perform calculations relative to the environment of Problem
13.91.
Also, evaluate an exergetic efficiency for the reactor. Perform
calculations relative to the environment of Problem 13.91.
Neglect the effects of motion and gravity.
13.106 Acetylene gas (C
2
H
2
) at 778F, 1 atm enters an insulated
reactor operating at steady state and burns completely with
180% of theoretical air, entering in a separate stream at 77
8F,
1 atm. The products exit as a mixture at 1 atm. Determine
in Btu per lbmol of fuel
(a) the exergy of the fuel entering the reactor.
(b) the exergy exiting with the products.
(c) the rate of exergy destruction.
Also, evaluate an exergetic efficiency for the reactor. Perform
calculations relative to the environment of Problem 13.91.
Neglect the effects of motion and gravity.
13.107 Liquid octane (C
8
H
18
) at 258C, 1 atm and a mass flow
rate of 0.57 kg/h enters an internal combustion engine
operating at steady state. The fuel burns with air entering
the engine in a separate stream at 25
8C, 1 atm. Combustion
products exit at 670 K, 1 atm with a dry molar analysis of
11.4% CO
2
, 2.9% CO, 1.6% O
2
, and 84.1% N
2
. If the engine
develops power at the rate of 3 kW, determine
(a) the rate of heat transfer from the engine, in kW.
(b) an exergetic efficiency for the engine.
Use the environment of Problem 13.91 and neglect the effects
of motion and gravity.
13.108 Figure P13.108 shows a simple vapor power plant. The
fuel is methane that enters at 77
8F, 1 atm and burns
completely with 200% theoretical air entering at 77
8F, 1 atm.
Steam exits the steam generator at 900
8F, 500 lbf/in.
2
The
vapor expands through the turbine and exits at 1 lbf/in.
2
, and
Carbon (T
0
, p
0
)
Water vapor at
600°F, 1 atm
Electrical
input
Product gas at
1700°F, 1 atm
C + 1.25H
2
O(g) → CO + H
2
+ 0.25H
2
O(g)
Fig. P13.104
13.105 Carbon monoxide (CO) at 258C, 1 atm enters an insulated
reactor operating at steady state and reacts completely with
the theoretical amount of air entering in a separate stream
at 25
8C, 1 atm. The products exit as a mixture at 1 atm. Determine
in kJ per kmol of CO
(a) the exergy entering with the carbon monoxide.
(b) the exergy exiting with the products.
(c) the rate of exergy destruction.
4
32
1
1 lbf/in.
2
900°F
500 lbf/in.
2
x
2
= 97%
T
s
Pump
Steam
generator
Condenser
14
32
W
t
·
Methane (T
0
, p
0
)
Air (T
0
, p
0
)
Cooling water
out at 90°F
1 atm
Cooling water
in at T
0
1 atm
Products at
500°F, 1 atm
Turbine
Fig. P13.108
Problems: Developing Engineering Skills 841
c13ReactingMixturesandCombusti841 Page 841 7/13/10 11:43:28 AM user-s146 c13ReactingMixturesandCombusti841 Page 841 7/13/10 11:43:28 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
842 Chapter 13 Reacting Mixtures and Combustion
a quality of 97%. At the condenser exit, the pressure is
1 lbf/in.
2
and the water is a saturated liquid. The plant operates
at steady state with no stray heat transfers from any plant
component. Pump work and the effects of motion and gravity
are negligible. Determine
(a) the balanced reaction equation.
(b) the vapor mass flow rate, in lb per lbmol of fuel.
(c) the cooling water mass flow rate, in lb per lbmol of fuel.
(d) each of the following, expressed as a percent of the exergy
entering the steam generator with the fuel, (i) the exergy
exiting with the stack gases, (ii) the exergy destroyed in the
steam generator, (iii) the power developed by the turbine,
(iv) the exergy destroyed in the turbine, (v) the exergy
exiting with the cooling water, (vi) the exergy destroyed in
the condenser.
Base exergy values on the environment of Problem 13.91.
13.109 Consider a furnace operating at steady state idealized
as shown in Fig. P13.109. The fuel is methane, which enters
at 25
8C, 1 atm and burns completely with 200% theoretical
air entering at the same temperature and pressure. The furnace
delivers energy by heat transfer at an average temperature of
227
8C. Combustion products at 600 K, 1 atm are provided to
the surroundings for cogeneration of steam. There are no
stray heat transfers, and the effects of motion and gravity
can be ignored. Determine in kJ per kmol of fuel
(a) the exergy entering the furnace with the fuel.
(b) the exergy exiting with the products.
(c) the rate of exergy destruction.
Also, evaluate an exergetic efficiency for the furnace and
comment. Perform calculations relative to the environment of
Problem 13.91.
where h/c, o/c, and n/c denote, respectively, the mass ratio of
hydrogen to carbon, oxygen to carbon, and nitrogen to
carbon, and s is the mass fraction of sulfur in kg per kg of
fuel.
3
The environment is closely the same as in Problem
13.92, but extended appropriately to account for the presence
of sulfur in the coal.
(a) Using the above expression, calculate the chemical exergy
of the coal, in kJ/kg.
(b) Compare the answer of part (a) with the values that would
result by approximating the chemical exergy with each of
the measured heating values.
(c) What data would be required to determine the chemical
exergy in this case using the methodology of Sec. 13.6?
Discuss.
13.111 For psychrometric applications such as those considered
in Chap. 12, the environment often can be modeled simply
as an ideal gas mixture of water vapor and dry air at
temperature T
0
and pressure p
0
. The composition of the
environment is defined by the dry air and water vapor mole
fractions y
e
a
, y
e
v
, respectively.
(a) Show that relative to such an environment the total
specific flow exergy of a moist air stream at temperature T and
pressure p with dry air and water vapor mole fractions y
a
and y
v
, respectively, can be expressed on a molar basis as
e
f
5 T
0
e1y
a
c
pa
1 y
v
c
pv
2ca
T
T
0
b
2 1
2 ln a
T
T
0
bd1 R ln a
p
p
0
bf
1
RT
0
cy
a
lna
y
a
y
e
a
b1 y
v
ln a
y
v
y
e
v
bd
where
c
pa
and c
pv
denote the molar specific heats of dry air
and water vapor, respectively. Neglect the effects of motion
and gravity.
(b) Express the result of part (a) on a per unit mass of dry
air basis as
e
f
5 T
0
e1c
pa
1 vc
pv
2c
T
T
0
2 1 2 lna
T
T
0
bd1 11 1 v
|
2R
a
ln 1p
y
p
0
2f
1 R
a
T
0
e11 1 v
|
2 ln a
1 1 v
e
|
1 1
v
|
b1 v
|
ln a
v
|
v
|
e
bf
where R
a
5 R
/
M
a
and v
|
5 vM
a
/
M
v
5 y
v
y
y
a
.
13.112 For each of the following, use the result of Problem
13.111(a) to determine the total specific flow exergy, in kJ/kg,
relative to an environment consisting of moist air at 20
8C,
1 atm, f
5 100%
(a) moist air at 208C, 1 atm, f 5 90%.
(b) moist air at 20
8C, 1 atm, f 5 50%.
(c) moist air at 20
8C, 1 atm, f 5 10%.
Combustion products
at 600 K, 1 atm
Furnace
Heat transfe
r
Temperature = 227°C
Methane
(T
0
, p
0
)
Air
(T
0
, p
0
)
Fig. P13.109
13.110 Coal enters the combustor of a power plant with a mass
analysis of 49.8% C, 3.5% H, 6.8% O, 6.4% S, 14.1% H
2
O,
and 19.4% noncombustible ash. The higher heating value of
the coal is measured as 21,220 kJ/kg, and the lower heating
value on a dry basis, (LHV)
d
, is 20,450 kJ/kg. The following
expression can be used to estimate the chemical exergy of
the coal, in kJ/kg:
e
ch
5 1LHV2
d
a1.0438 1 0.0013
h
c
1 0.1083
o
c
1 0.0549
n
c
b1 6740s
3
Moran, Availability Analysis, pp. 192–193.
c13ReactingMixturesandCombusti842 Page 842 7/21/10 7:25:31 AM user-s146 c13ReactingMixturesandCombusti842 Page 842 7/21/10 7:25:31 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
