Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

CLOSED SYSTEMS. Next consider an entropy balance for a process of a closed
system during which a chemical reaction occurs
S
P
2 S
R
5
#
a
dQ
T
b
b
1 s
(13.25)
S
R
and S
P
denote, respectively, the entropy of the reactants and the entropy of the
products.
When the reactants and products form ideal gas mixtures, the entropy balance can
be expressed on a per mole of fuel basis as
a
P
ns 2
a
R
ns 5
1
n
F
#
a
d
Q
T
b
b
1
s
n
F
(13.26)
where the coefficients n on the left are the coefficients of the reaction equation giv-
ing the moles of each reactant or product per mole of fuel. The entropy terms of Eq.
13.26 are evaluated from Eq. 13.23 using the temperature and partial pressures of the
reactants or products, as appropriate. In any such application, the fuel is mixed with
the oxidizer, so this must be taken into account when determining the partial pressures
of the reactants.
Example 13.10 provides an illustration of the evaluation of entropy change for
combustion at constant volume.
13.5 Absolute Entropy and the Third Law of Thermodynamics 813
s
CO
2
5 267.12 2 8.314 ln 0.033 5 295.481 kJ
/
kmol ? K
s
H
2
O
5 231.01 2 8.314 ln 0.0371 5 258.397 kJ
/
kmol ? K
s
O
2
5 242.12 2 8.314 ln 0.1546 5 257.642 kJ
/
kmol ? K
s
N
2
5 226.795 2 8.314 ln 0.7753 5 228.911 kJ
/
kmol ?
K
Inserting values into the expression for the rate of entropy production
s
#
cv
n
#
F
5 81295.48121 91258.39721 37.51257.64221 1881228.9112
2 360.79 2 50
1
218.01
2
2 188
1
193.46
2
5 9754 kJ
/
kmol
1
octane
2
? K
The use of IT to solve part (b) is left as an exercise.
➊ For several gases modeled as ideal gases, IT directly returns the absolute
entropies required by entropy balances for reacting systems. The entropy
data obtained from IT agree with values calculated from Eq. 13.23 using
table data.
➋ Although the rates of entropy production calculated in this example are
positive, as required by the second law, this does not mean that the proposed
reactions necessarily would occur, for the results are based on the assump-
tion of complete combustion. The possibility of achieving complete combus-
tion with specified reactants at a given temperature and pressure can be
investigated with the methods of Chap. 14, dealing with chemical equilib-
rium. For further discussion, see Sec. 14.4.1.
Ability to…
❑
apply the control volume
entropy balance to a
reacting system.
❑
evaluate entropy values
appropriately based on
absolute entropies.
✓
Skills Developed
How do combustion product temperature and rate of entropy
production vary, respectively, as percent excess air increases? Assume
complete combustion. Ans. Decrease, increase.
➋
c13ReactingMixturesandCombustio813 Page 813 7/13/10 11:41:34 AM user-s146 c13ReactingMixturesandCombustio813 Page 813 7/13/10 11:41:34 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
814 Chapter 13 Reacting Mixtures and Combustion
Determining Entropy Change for Combustion of Gaseous
Methane with Oxygen at Constant Volume
c c c c EXAMPLE 13.10 c
Determine the change in entropy of the system of Example 13.6 in kJ/K.
SOLUTION
Known:
A mixture of gaseous methane and oxygen, initially at 258C and 1 atm, burns completely within a closed
rigid container. The products are cooled to 900 K, 3.02 atm.
Find: Determine the change in entropy for the process in kJ/K.
Schematic and Given Data: See Fig. E13.6.
Engineering Model:
1.
The contents of the container are taken as the system.
2. The initial mixture can be modeled as an ideal gas mixture, as can the products of combustion.
3. Combustion is complete.
Analysis: The chemical equation for the complete combustion of methane with oxygen is
CH
4
1 2O
2
S
CO
2
1 2H
2
O
1
g
2
The change in entropy for the process of the closed system is ¢S 5 S
P
2 S
R
, where S
R
and S
P
denote, respec-
tively, the initial and final entropies of the system. Since the initial mixture forms an ideal gas mixture (assump-
tion 2), the entropy of the reactants can be expressed as the sum of the contributions of the components, each
evaluated at the mixture temperature and the partial pressure of the component. That is
S
R
5 1s
CH
4
1T
1
, y
CH
4
p
1
21 2s
O
2
1T
1
, y
O
2
p
1
2
where y
CH
4
5 1
/
3 and y
O
2
5 2
/
3 denote, respectively, the mole fractions of the methane and oxygen in the initial
mixture. Similarly, since the products of combustion form an ideal gas mixture (assumption 2)
S
P
5 1s
CO
2
1T
2
, y
CO
2
p
2
21 2s
H
2
O
1T
2
, y
H
2
O
p
2
2
where y
CO
2
5 1
/
3 and y
H
2
O
5 2
/
3 denote, respectively, the mole fractions of the carbon dioxide and water vapor
in the products of combustion. In these equations, p
1
and p
2
denote the pressure at the initial and final states,
respectively.
The specific entropies required to determine S
R
can be calculated from Eq. 13.23. Since T
1
5 T
ref
and p
1
5 p
ref
,
absolute entropy data from Table A-25 can be used as follows
s
CH
4
1T
1
, y
CH
4
p
1
25 s 8
CH
4
1T
ref
22 R ln
y
CH
4
p
ref
p
ref
5 186.16 2 8.314 ln
1
3
5 195.294 kJ
/
kmol ? K
Similarly
s
O
2
1T
1
, y
O
2
p
1
25 s8
O
2
1T
ref
22 R ln
y
O
2
p
ref
p
ref
5 205.03 2 8.314 ln
2
3
5 208.401 kJ
/
kmol ? K
At the final state, the products are at T
2
5 900 K and p
2
5 3.02 atm. With Eq. 13.23 and absolute entropy
data from Tables A-23
s
CO
2
1T
2
, y
CO
2
p
2
25 s8
CO
2
1T
2
22 R ln
y
CO
2
p
2
p
ref
5 263.559 2 8.314 ln
1
1
/
3
21
3.02
2
1
5 263.504 kJ
/
kmol ? K
c13ReactingMixturesandCombustio814 Page 814 7/13/10 11:41:40 AM user-s146 c13ReactingMixturesandCombustio814 Page 814 7/13/10 11:41:40 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
13.5.3
Evaluating Gibbs Function for Reacting Systems
The thermodynamic property known as the Gibbs function plays a role in the second
part of this chapter dealing with exergy analysis. The specific Gibbs function
g
, intro-
duced in Sec. 11.3, is
g
5 h 2 T s (13.27)
The procedure followed in setting a datum for the Gibbs function closely parallels
that used in defining the enthalpy of formation: To each stable element at the stan-
dard state is assigned a zero value of the Gibbs function. The Gibbs function of formation
of a compound,
g
8
f
, equals the change in the Gibbs function for the reaction in which
the compound is formed from its elements, the compound and the elements all being
at T
ref
5 258C (778F) and p
ref
5 1 atm. Tables A-25 and A-25E give the Gibbs function
of formation,
g
8
f
, for selected substances.
The Gibbs function at a state other than the standard state is found by adding to
the Gibbs function of formation the change in the specific Gibbs function ¢g between
the standard state and the state of interest
g
1T, p25
g
8
f
1 3
g
1T, p22
g
1T
ref
, p
ref
245
g
8
f
1 ¢
g
(13.28a)
With Eq. 13.27, ¢g can be written as
¢
g
5 3h1T, p22 h1T
ref
, p
ref
242 3T s 1T, p22 T
ref
s 1T
ref
, p
ref
24 (13.28b)
The Gibbs function of component i in an ideal gas mixture is evaluated at the partial
pressure of component i and the mixture temperature.
The procedure for determining the Gibbs function of formation is illustrated in
the next example.
s
H
2
O
1T
2
, y
H
2
O
p
2
25 s8
H
2
O
1T
2
22 R ln
y
H
2
O
p
2
p
ref
5 228.321 2 8.314 ln
1
2
/
3
21
3.02
2
1
5 222.503 kJ
/
kmol ? K
Finally, the entropy change for the process is
¢S 5 S
P
2 S
R
5 3263.504 1 21222.503242 3195.294 1 21208.40124
5 96.414 kJ
/
K
Ability to…
❑
apply the closed system
entropy balance to a
reacting system.
❑
evaluate entropy values
appropriately based on
absolute entropies.
✓Skills Developed
Applying the entropy balance, Eq. 13.25, is s greater than,
less than, or equal to DS? Ans. Greater than.
Gibbs function of
formation
TAKE NOTE...
Gibbs function is introduced
here because it contributes
to subsequent developments
of this chapter.
Gibbs function is a prop-
erty because it is defined
in terms of properties.
Like enthalpy, introduced
as a combination of proper-
ties in Sec. 3.6.1, Gibbs
function has no physical
significance—in general.
Determining the Gibbs Function of Formation for Methane
c c c c EXAMPLE 13.11 c
Determine the Gibbs function of formation of methane at the standard state, 258C and 1 atm, in kJ/kmol, and
compare with the value given in Table A-25.
13.5 Absolute Entropy and the Third Law of Thermodynamics 815
c13ReactingMixturesandCombustio815 Page 815 8/11/10 9:29:20 AM user-s146 c13ReactingMixturesandCombustio815 Page 815 8/11/10 9:29:20 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
816 Chapter 13 Reacting Mixtures and Combustion
Chemical Exergy
The objective of this part of the chapter is to extend the exergy concept introduced in
Chap. 7 to include chemical exergy. Several important exergy aspects are listed in Sec.
7.3.1. We suggest you review this material before continuing the current discussion.
A key aspect carried from Chap. 7 is that exergy is a measure of the departure of
the state of a system from that of a thermodynamic model of the Earth and its atmo-
sphere called the exergy reference environment, or simply the environment. In the
current discussion, the departure of the system state from the environment centers
on the respective temperature, pressure, and composition, for composition now plays
a key role. If one or more of system temperature, pressure, and composition differs
from that of the environment, the system has exergy.
Exergy is the maximum theoretical work obtainable from an overall system of sys-
tem plus environment as the system passes from a specified state to equilibrium with
the environment. Alternatively, exergy is the minimum theoretical work input required
to form the system from the environment and bring it to the specified state.
For conceptual and computational ease, we think of the system passing to equilib-
rium with the environment in two steps. With this approach, exergy is the sum of two
contributions: thermomechanical, developed in Chap. 7, and chemical, developed in
this chapter.
SOLUTION
Known:
The compound is methane.
Find: Determine the Gibbs function of formation at the standard state, in kJ/kmol, and compare with the Table
A-25 value.
Assumptions: In the formation of methane from carbon and hydrogen (H
2
), the carbon and hydrogen are each
initially at 258C and 1 atm. The methane formed is also at 258C and 1 atm.
Analysis: Methane is formed from carbon and hydrogen according to C 1 2H
2
S CH
4
. The change in the Gibbs
function for this reaction is
g
P
2
g
R
5 1h 2 T s2
CH
4
2 1h 2 T s2
C
2 21h 2 T s2
H
2
5 1h
CH
4
2 h
C
2 2h
H
2
22 T1s
CH
4
2 s
C
2 2s
H
2
2 (1)
where g
P
and g
R
denote, respectively, the Gibbs functions of the products and reactants, each per kmol of methane.
In the present case, all substances are at the same temperature and pressure, 258C and 1 atm, which correspond
to the standard reference state values. At the standard reference state, the enthalpies and Gibbs functions for
carbon and hydrogen are zero by definition. Thus, in Eq. (1),
g
R
5 h
C
5 h
H
2
5 0. Also,
g
P
5 1
g
8
f
2
CH
4
. Eq. (1) then
reads
1
g
8
f
2
CH
4
5 1h8
f
2
CH
4
2 T
ref
1s8
CH
4
2 s8
C
2 2
s
8
H
2
2 (2)
where all properties are at T
ref
, p
ref
. With enthalpy of formation and absolute entropy data from Table A-25,
Eq. (2) gives
1g 8
f
2
CH
4
5274,850 2 298.153186.16 2 5.74 2 21130.57245250,783 kJ
/
kmol
The slight difference between the calculated value for the Gibbs function of
formation of methane and the value from Table A-25 can be attributed to
round-off.
Ability to…
❑
apply the definition of Gibbs
function of formation to
calculate
g8
f
.
✓
Skills Developed
Using the method applied in the example, calculate g8
f
for
monatomic oxygen at the standard state, in kJ/kmol. Begin by writing
1
2
O
2
S
O. Ans. 231,750 kJ/kmol, which agrees with Table A-25.
c13ReactingMixturesandCombustio816 Page 816 7/14/10 10:27:35 AM user-s146 c13ReactingMixturesandCombustio816 Page 816 7/14/10 10:27:35 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
Set of Substances Represented by C
a
H
b
O
c
C H
2
C
a
H
b
CO CO
2
H
2
O(liq.)
a 1 0 a 1 1 0
b 0 2 b 0 0 2
c 0 0 0 1 2 1
TABLE 13.3
Exergy Reference Environment Used in Sec. 13.6
Gas phase at T
0
5 298.15 K (258C), p
0
5 1 atm
Component y
e
(%)
N
2
75.67
O
2
20.35
H
2
O(g) 3.12
CO
2
0.03
Other 0.83
TABLE 13.4
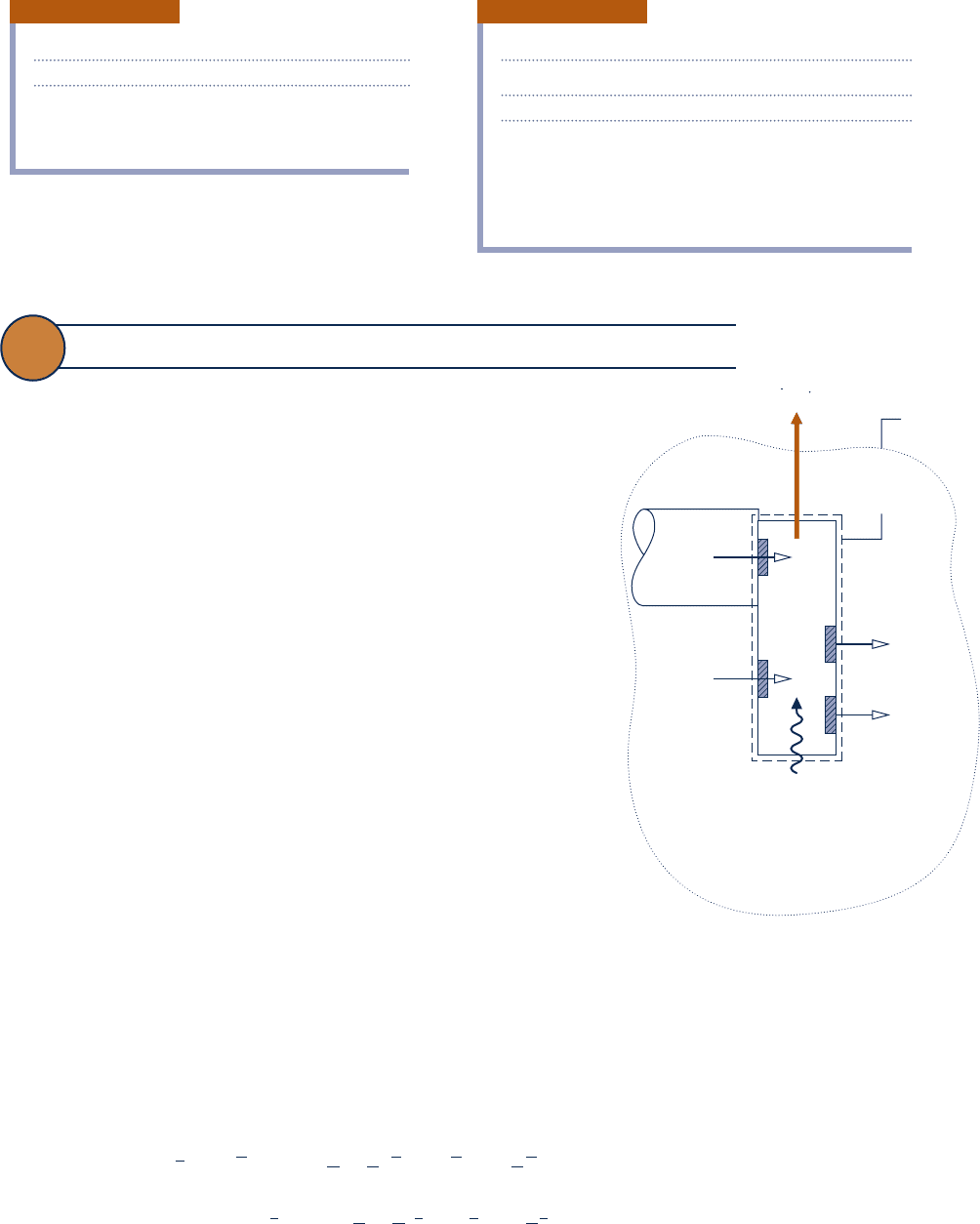
13.6 Conceptualizing Chemical Exergy
In this section, we consider a thought experiment to bring out impor-
tant aspects of chemical exergy. This involves
c a set of substances represented by C
a
H
b
O
c
(see Table 13.3),
c an environment modeling Earth’s atmosphere (see Table 13.4),
and
c an overall system including a control volume (see Fig. 13.6).
Referring to Table 13.4, the exergy reference environment considered
in the present discussion is an ideal gas mixture modeling the Earth’s
atmosphere. T
0
and p
0
denote the temperature and pressure of the envi-
ronment, respectively. The composition of the environment is given in
terms of mole fractions denoted by y
e
, where superscript e is used to
signal the mole fraction of an environmental component. The values of
these mole fractions, and the values of T
0
and p
0
, are specified and
remain unchanged throughout the development to follow. The gas mix-
ture modeling the atmosphere adheres to the Dalton model (Sec. 12.2).
Considering Fig. 13.6, a substance represented by C
a
H
b
O
c
enters
the control volume at T
0
, p
0
. Depending on the particular substance,
compounds present in the environment enter (O
2
) and exit (CO
2
and
H
2
O(g)) at T
0
and their respective partial pressures in the environ-
ment. All substances enter and exit with negligible effects of motion
and gravity. Heat transfer between the control volume and environ-
ment occurs only at temperature T
0
. The control volume operates at
steady state, and the ideal gas model applies to all gases. Finally, for
the overall system whose boundary is denoted by the dotted line,
total volume is constant and there is no heat transfer across the
boundary.
Next, we apply conservation of mass, an energy balance, and an
entropy balance to the control volume of Fig. 13.6 with the objective of determin-
ing the maximum theoretical work per mole of substance C
a
H
b
O
c
entering—namely,
the maximum theoretical value of W
#
cv
/
n
#
F
. This value is the molar chemical exergy of
the substance. The chemical exergy is given by
e
ch
5 ch
F
1 aa 1
b
4
2
c
2
bh
O
2
2 ah
CO
2
2
b
2
h
H
2
O
d
2 T
0
cs
F
1 aa 1
b
4
2
c
2
bs
O
2
2 as
CO
2
2
b
2
s
H
2
O
d
(13.29)
where the superscript ch is used to distinguish this contribution to the exergy mag-
nitude from the thermomechanical exergy introduced in Chap. 7. The subscript F
Fig. 13.6
Illustration used to conceptualize
chemical exergy.
Boundary of
overall syste
m
Boundary of the
control volume
Heat transfer
with environment
Environment at T
0
, p
0
C
a
H
b
O
c
at
T
0
, p
0
T
0
CO
2
at T
0
, y
CO
2
p
0
e
C
a
H
b
O
c
+ [a + b/4 − c/2]O
2
→ aCO
2
+ b/2 H
2
O(g)
O
2
at T
0
, y
O
2
p
0
e
H
2
O at T
0
, y
H
2
O
p
0
e
W
cv
/n
F
13.6 Conceptualizing Chemical Exergy 817
c13ReactingMixturesandCombustio817 Page 817 7/14/10 10:27:37 AM user-s146 c13ReactingMixturesandCombustio817 Page 817 7/14/10 10:27:37 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

818 Chapter 13
Reacting Mixtures and Combustion
denotes the substance represented by C
a
H
b
O
c
. The other molar enthalpies and entro-
pies appearing in Eq. 13.29 refer to the substances entering and exiting the control
volume, each evaluated at the state at which it enters or exits. See the following box
for the derivation of Eq. 13.29.
TAKE NOTE...
Observe that the approach
used here to evaluate
chemical exergy parallels
those used in Secs. 7.3
and 7.5 to evaluate exergy
of a system and flow
exergy. In each case, energy
and entropy balances are
applied to evaluate maxi-
mum theoretical work in
the limit as entropy pro-
duction tends to zero.
Evaluating Chemical Exergy
Although chemical reaction does not occur in every case we will be considering, con-
servation of mass is accounted for generally by the following expression
C
a
H
b
O
c
1 3a 1 b
/
4 2 c
/
24O
2
S aCO
2
1 b
/
2
H
2
O1g2 (13.30)
which assumes that when reaction occurs, the reaction is complete.
For steady-state operation, the energy rate balance for the control volume of Fig. 13.6
reduces to give
W
#
cv
n
#
F
5
Q
#
cv
n
#
F
1 h
F
1 aa 1
b
4
2
c
2
bh
O
2
2 ah
CO
2
2
b
2
h
H
2
O
(13.31)
where the subscript F denotes a substance represented by C
a
H
b
O
c
(Table 13.3). Since the
control volume is at steady state, its volume does not change with time, so no portion
of W
#
cv
/
n
F
#
is required to displace the environment. Thus, in keeping with all specified
idealizations, Eq. 13.31 also gives the work developed by the overall system of control
volume plus environment whose boundary is denoted on Fig. 13.6 by a dotted line. The
potential for such work is the difference in composition between substance C
a
H
b
O
c
and
the environment.
Heat transfer is assumed to occur with environment only at temperature T
0
. An entropy
balance for the control volume takes the form
0 5
Q
#
cv
/
n
F
#
T
0
1 s
F
1 aa 1
b
4
2
c
2
b
s
O
2
2 as
CO
2
2
b
2
s
H
2
O
1
s
cv
#
n
#
F
(13.32)
Eliminating the heat transfer rate between Eqs. 13.31 and 13.32 gives
W
#
cv
n
#
F
5 ch
F
1 aa 1
b
4
2
c
2
bh
O
2
2 a
h
CO
2
2
b
2
h
H
2
O
d
2 T
0
cs
F
1 aa 1
b
4
2
c
2
bs
O
2
2 as
CO
2
2
b
2
s
H
2
O
d2 T
0
s
#
cv
n
#
F
(13.33)
In Eq. 13.33, the specific enthalpy h
F
and specific entropy s
F
are evaluated at T
0
and
p
0
. Since the ideal gas model applies to the environment (Table 13.4), the specific enthal-
pies of the first underlined term of Eq. 13.33 are determined knowing only the temperature
T
0
. Further, the specific entropy of each substance of the second underlined term is deter-
mined by temperature T
0
and the partial pressure in the environment of that substance.
Accordingly, since the environment is specified, all enthalpy and entropy terms of Eq.
13.33 are known and independent of the nature of the processes occurring within the
control volume.
The term T
0
s
#
cv
depends explicitly on the nature of such processes, however. In accor-
dance with the second law, T
0
s
#
cv
is positive whenever irreversibilities are present, van-
ishes in the limiting case of no irreversibilities, and is never negative. The maximum
theoretical value for the work developed is obtained when no irreversibilities are pres-
ent. Setting T
0
s
#
cv
to zero in Eq. 13.33, yields the expression for chemical exergy given
by Eq. 13.29.
c13ReactingMixturesandCombustio818 Page 818 7/13/10 11:41:51 AM user-s146 c13ReactingMixturesandCombustio818 Page 818 7/13/10 11:41:51 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
13.6.1
Working Equations for Chemical Exergy
For computational convenience, the chemical exergy given by Eq. 13.29 is written as
Eqs. 13.35 and 13.36. The first of these is obtained by recasting the specific entropies
of O
2
, CO
2
, and H
2
O using the following equation obtained by application of Eq. (a)
of Table 13.1:
s
i
1T
0
, y
e
i
p
0
25 s
i
1T
0
, p
0
22 R ln y
e
i
(13.34)
The first term on the right is the absolute entropy at T
0
and p
0
, and y
i
e
is the mole
fraction of component i in the environment.
Applying Eq. 13.34, Eq. 13.29 becomes
e
ch
5 ch
F
1 aa 1
b
4
2
c
2
b h
O
2
2 ah
CO
2
2
b
2
h
H
2
O1g2
d 1T
0
, p
0
2
2 T
0
cs
F
1 aa 1
b
4
2
c
2
b s
O
2
2 as
CO
2
2
b
2
s
H
2
O1g2
d 1T
0
, p
0
2
(13.35)
1 RT
0
ln c
1y
e
O
2
2
a1b
/
42c
/
2
1y
e
CO
2
2
a
1y
e
H
2
O
2
b
/
2
d
where the notation (T
0
, p
0
) signals that the specific enthalpy and entropy terms of
Eq. 13.35 are each evaluated at T
0
and p
0
, although T
0
suffices for the enthalpy of
substances modeled as ideal gases.
Recognizing the Gibbs function in Eq. 13.35—
g
F
5 h
F
2 T
0
s
F
, for instance—
Eq. 13.35 can be expressed alternatively in terms of the Gibbs functions of the several
substances as
e
ch
5 c
g
F
1 aa 1
b
4
2
c
2
b
g
O
2
2 a
g
CO
2
2
b
2
g
H
2
O1g2
d 1T
0
, p
0
2
1 RT
0
ln c
1y
e
O
2
2
a1b
/
42c
/
2
1y
e
CO
2
2
a
1y
e
H
2
O
2
b
/
2
d
(13.36)
The logarithmic term common to Eqs. 13.35 and 13.36 typically contributes only a
few percent to the chemical exergy magnitude. Other observations follow:
c The specific Gibbs functions of Eq. 13.36 are evaluated at the temperature T
0
and
pressure p
0
of the environment. These terms can be determined with Eq. 13.28a as
g
1T
0
, p
0
25
g
8
f
1 3
g
1T
0
, p
0
22
g
1T
ref
, p
ref
24 (13.37)
where g8
f
is the Gibbs function of formation and T
ref
5 258C (778F), p
ref
5 1 atm.
c For the special case where T
0
and p
0
are the same as T
ref
and p
ref
, respectively, the
second term on the right of Eq. 13.37 vanishes and the specific Gibbs function is
just the Gibbs function of formation. That is, the Gibbs function values of Eq. 13.36
can be simply read from Tables A-25 or similar compilations.
c Finally, note that the underlined term of Eq. 13.36 can be written more compactly
as 2¢G: the negative of the change in Gibbs function for the reaction, Eq. 13.30,
regarding each substance as separate at temperature T
0
and pressure p
0
.
13.6.2
Evaluating Chemical Exergy for Several Cases
Cases of practical interest corresponding to selected values of a, b, and c in the repre-
sentation C
a
H
b
O
c
can be obtained from Eq. 13.36. For example, a 5 8, b 5 18, c 5 0
13.6 Conceptualizing Chemical Exergy 819
c13ReactingMixturesandCombustio819 Page 819 7/14/10 10:27:37 AM user-s146 c13ReactingMixturesandCombustio819 Page 819 7/14/10 10:27:37 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
820 Chapter 13
Reacting Mixtures and Combustion
corresponds to octane, C
8
H
18
. An application of Eq. 13.36 to evaluate the chemical
exergy of octane is provided in Example 13.12. Further special cases follow:
c Consider the case of pure carbon monoxide at T
0
, p
0
. For CO we have a 5 1, b 5 0,
c 5 1. Accordingly, Eq. 13.30 reads CO 1
1
2
O
2
S CO
2
, and the chemical exergy
obtained from Eq. 13.36 is
e
ch
CO
5 3
g
CO
1
1
2
g
O
2
2
g
CO
2
41T
0
, p
0
21 RT
0
ln c
1y
e
O
2
2
1
/
2
y
e
CO
2
d
(13.38)
If carbon monoxide is not pure but a component of an ideal gas mixture at T
0
, p
0
,
each component i of the mixture enters the control volume of Fig. 13.6 at tem-
perature T
0
and the respective partial pressure y
i
p
0
. The contribution of carbon
monoxide to the chemical exergy of the mixture, per mole of CO, is then given by
Eq. 13.38, but with the mole fraction of carbon monoxide in the mixture,
y
CO
,
appearing in the numerator of the logarithmic term that then reads ln3y
CO
1y
e
O
2
2
1
/
2
/
y
e
CO
2
4.
This becomes important when evaluating the exergy of combustion products
involving carbon monoxide.
c Consider the case of pure water at T
0
and p
0
. Water is a liquid when at T
0
, p
0
, but
is a vapor within the environment of Table 13.4. Thus water enters the control
volume of Fig. 13.6 as a liquid and exits as a vapor at T
0
, y
e
H
2
O
p
0
, with no chemical
reaction required. In this case, a 5 0, b 5 2, and c 5 1. Equation 13.36 gives the
chemical exergy as
e
ch
H
2
O
5 3
g
H
2
O1l2
2
g
H
2
O1g2
41T
0
, p
0
21 RT
0
ln a
1
y
e
H
2
O
b
(13.39)
c Consider the case of pure carbon dioxide at T
0
, p
0
. Like water, carbon dioxide is
present within the environment and thus requires no chemical reaction to evaluate
its chemical exergy. With a 5 1, b 5 0, c 5 2, Eq. 13.36 gives the chemical exergy
simply in terms of a logarithmic expression of the form
e
ch
5 RT
0
ln a
1
y
e
CO
2
b
(13.40)
Provided the appropriate mole fraction y
e
is used, Eq. 13.40 also applies to
other substances that are gases in the environment, in particular to O
2
and N
2
.
Moreover, Eqs. 13.39 and 13.40 reveal that a chemical reaction does not always
play a part when conceptualizing chemical exergy. In the cases of liquid water,
CO
2
, O
2
, N
2
, and other gases present in the environment, we think of the work
that could be done as the particular substance passes by diffusion from the dead
state, where its pressure is p
0
, to the environment, where its pressure is the partial
pressure, y
e
p
0
.
c Finally, for an ideal gas mixture at T
0
, p
0
consisting only of substances present as
gases in the environment, the chemical exergy is obtained by summing the contri-
butions of each of the components. The result, per mole of mixture, is
e
ch
5 RT
0
a
j
i51
y
i
ln a
y
i
y
e
i
b
(13.41a)
where y
i
and y
e
i
denote, respectively, the mole fraction of component i in the
mixture at T
0
, p
0
and in the environment.
TAKE NOTE...
For liquid water, we think
only of the work that could
be developed as water
expands through a turbine,
or comparable device, from
pressure p
0
to the partial
pressure of the water
vapor in the environment:
H
2
O (l)
at
T
0
, p
0
H
2
O(g)
at
T
0
, y
H
2
O
p
0
e
T
0
CO
2
at
T
0
, p
0
CO
2
at
T
0
, y
CO
2
p
0
e
T
0
c13ReactingMixturesandCombustio820 Page 820 8/11/10 9:29:30 AM user-s146 c13ReactingMixturesandCombustio820 Page 820 8/11/10 9:29:30 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
Expressing the logarithmic term as 1ln11
/
y
i
e
21 ln y
i
2 and introducing a relation
like Eq. 13.40 for each gas i, Eq. 13.41a can be written alternatively as
e
ch
5
a
j
i51
y
i
e
ch
i
1 RT
0
a
j
i51
y
i
ln y
i
(13.41b)
The development of Eqs. 13.41a and 13.41b is left as an exercise.
13.6.3
Closing Comments
The approach introduced in this section for conceptualizing the chemical exergy of
the set of substances represented by C
a
H
b
O
c
can also be applied, in principle, for
other substances. In any such application, the chemical exergy is the maximum theo-
retical work that could be developed by a control volume like that considered in Fig. 13.6
where the substance of interest enters the control volume at T
0
, p
0
and reacts com-
pletely with environmental components to produce environmental components. All
participating environmental components enter and exit the control volume at their
conditions within the environment. By describing the environment appropriately, this
approach can be applied to many substances of practical interest.
1
13.7 Standard Chemical Exergy
While the approach used in Sec. 13.6 for conceptualizing chemical exergy can be
applied to many substances of practical interest, complications are soon encountered.
For one thing, the environment generally must be extended; the simple environment
of Table 13.4 no longer suffices. In applications involving coal, for example, sulfur
dioxide or some other sulfur-bearing compound must appear among the environmen-
tal components. Furthermore, once the environment is determined, a series of calcu-
lations are required to obtain exergy values for the substances of interest. These
complexities can be sidestepped by using a table of standard chemical exergies.
Standard chemical exergy values are based on a standard exergy reference environ-
ment exhibiting standard values of the environmental temperature T
0
and pressure
p
0
such as 298.15 K (536.678R) and 1 atm, respectively. The exergy reference environ-
ment also consists of a set of reference substances with standard concentrations
reflecting as closely as possible the chemical makeup of the natural environment. To
exclude the possibility of developing work from interactions among parts of the envi-
ronment, these reference substances must be in equilibrium mutually.
The reference substances generally fall into three groups: gaseous components of
the atmosphere, solid substances from the Earth’s crust, and ionic and nonionic sub-
stances from the oceans. A common feature of standard exergy reference environ-
ments is a gas phase, intended to represent air, that includes N
2
, O
2
, CO
2
, H
2
O(g),
and other gases. The ith gas present in this gas phase is assumed to be at temperature
T
0
and the partial pressure p
e
i
5 y
e
i
p
0
.
Two standard exergy reference environments are considered in this book, called
Model I and Model II. For each of these models, Table A-26 gives values of the standard
chemical exergy for several substances, in units of kJ/kmol, together with a brief descrip-
tion of the underlying rationale. The methods employed to determine the tabulated
standard chemical exergy values are detailed in the references accompanying the tables.
Only one of the two models should be used in a particular analysis.
The use of a table of standard chemical exergies often simplifies the application
of exergy principles. However, the term “standard” is somewhat misleading, for there
is no one specification of the environment that suffices for all applications. Still,
chemical exergies calculated relative to alternative specifications of the environment
are generally in good agreement. For a broad range of engineering applications, the
1
For further discussion see M. J. Moran, Availability Analysis: A Guide to Efficient Energy Use, ASME Press, New
York, 1989, pp. 169–170.
standard chemical
exergy
TAKE NOTE...
Standard exergy Model II is
commonly used in practice.
Model I is provided to show
that other standard refer-
ence environments can at
least be imagined.
13.7 Standard Chemical Exergy 821
TAKE NOTE...
Equation 13.41b is also
applicable for mixtures
containing gases other
than those present in the
reference environment, for
example gaseous fuels.
Moreover, this equation can
be applied to mixtures that
do not adhere to the ideal
gas model. In all such appli-
cations, the terms
e
ch
i
may
be selected from a table of
standard chemical exergies,
introduced in Sec. 13.7 to
follow.
c13ReactingMixturesandCombustio821 Page 821 7/21/10 7:32:11 AM user-s146 c13ReactingMixturesandCombustio821 Page 821 7/21/10 7:32:11 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

822 Chapter 13 Reacting Mixtures and Combustion
convenience of using standard values generally outweighs the slight lack of accuracy
that might result. In particular, the effect of slight variations in the values of T
0
and
p
0
about their standard values normally can be neglected.
13.7.1
Standard Chemical Exergy of a Hydrocarbon: C
a
H
b
In principle, the standard chemical exergy of a substance not present in the environment
can be evaluated by considering a reaction of the substance with other substances for
which the chemical exergies are known.
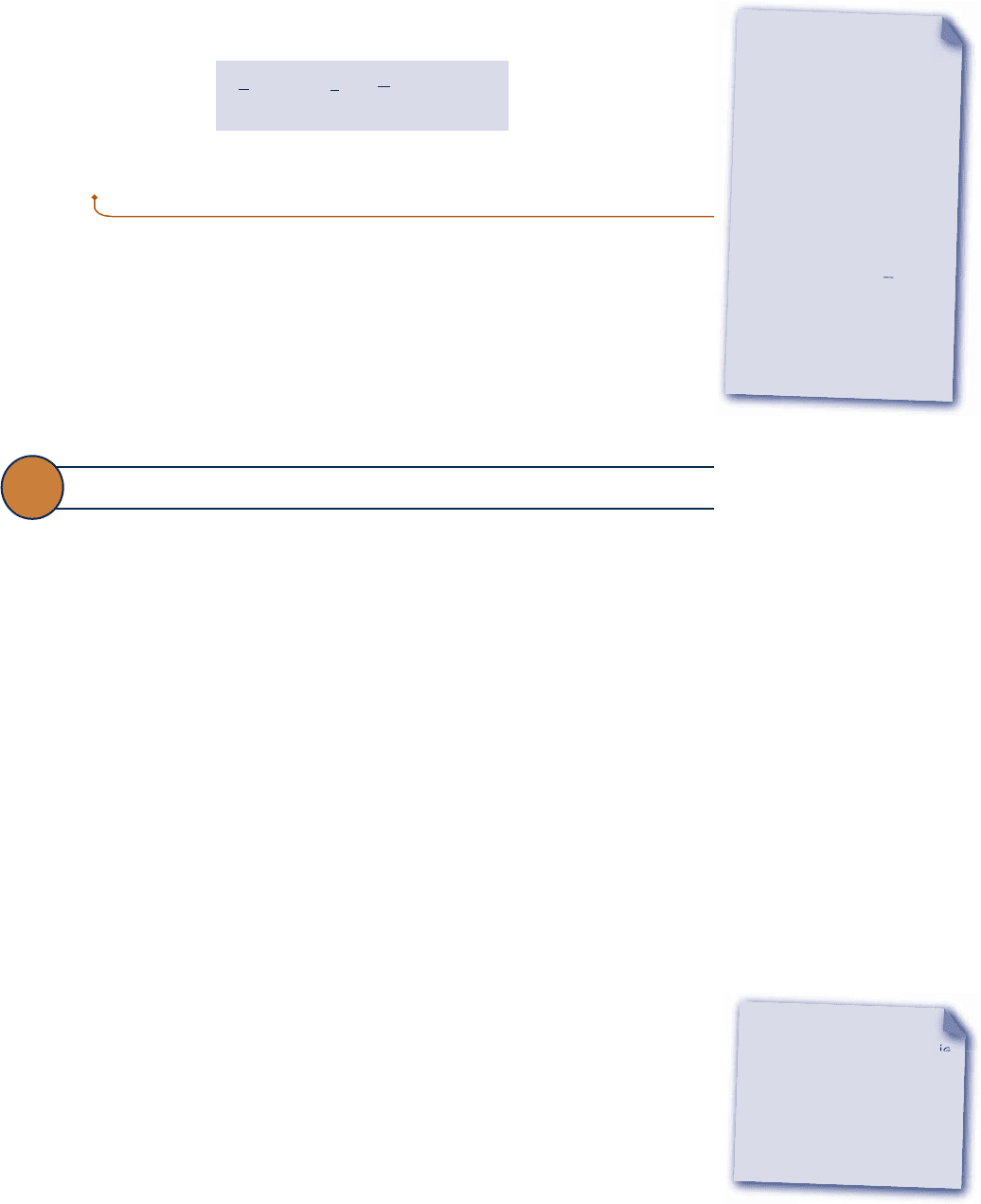
To illustrate this for the case of a pure hydrocarbon fuel
C
a
H
b
at T
0
, p
0
, refer to
the control volume at steady state shown in Fig. 13.7 where the fuel reacts completely
with oxygen to form carbon dioxide and liquid water. All substances are assumed to
enter and exit at T
0
, p
0
and heat transfer occurs only at temperature T
0
.
Assuming no irreversibilities, an exergy rate balance for the control volume reads
0 5
a
j
c1 2
T
0
T
j
d
0
a
Q
#
j
n
#
F
b2 a
W
#
cv
n
#
F
b
int
rev
1 e
F
ch
1 aa 1
b
4
b
e
ch
O
2
2 ae
ch
CO
2
2 a
b
2
be
ch
H
2
O1l2
2 E
#
0
d
where the subscript F denotes C
a
H
b
. Solving for the chemical exergy e
c
h
F
, we get
e
ch
F
5 a
W
#
cv
n
#
F
b
int
rev
1 ae
ch
CO
2
1 a
b
2
b e
ch
H
2
O1l2
2 aa 1
b
4
b e
ch
O
2
(13.42)
Applying energy and entropy balances to the control volume, as in the development
in the box on p. 818, we get
a
W
#
cv
n
#
F
b
int
rev
5 ch
F
1 aa 1
b
4
b h
O
2
2 ah
CO
2
2
b
2
h
H
2
O1l2
d1T
0
, p
0
2
2 T
0
cs
F
1 aa 1
b
4
b s
O
2
2 as
CO
2
2
b
2
s
H
2
O1l2
d1T
0
, p
0
2
(13.43)
The underlined term in Eq. 13.43 is recognized from Sec. 13.2.3 as the molar higher
heating value HH
V
(T
0
, p
0
). Substituting Eq. 13.43 into Eq. 13.42, we obtain
e
ch
F
5 HHV1T
0
, p
0
22 T
0
cs
F
1 aa 1
b
4
b
s
O
2
2 as
CO
2
2
b
2
s
H
2
O1l2
d1T
0
, p
0
2
1 ae
ch
CO
2
1 a
b
2
b e
ch
H
2
O1l2
2 aa 1
b
4
b e
ch
O
2
(13.44a)
C
a
H
b
at T
0
, p
0
O
2
at T
0
, p
0
CO
2
at T
0
, p
0
H
2
O (l)
at T
0
, p
0
T
0
Q
cv
·
W
cv
·
C
a
H
b
+
(
a +
)
b
4
O
2
→ aCO
2
+ H
2
O(l)
b
2
Fig. 13.7 Reactor used to introduce the standard chemical exergy of C
a
H
b
.
c13ReactingMixturesandCombustio822 Page 822 8/11/10 9:29:41 AM user-s146 c13ReactingMixturesandCombustio822 Page 822 8/11/10 9:29:41 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
