Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

may include both CO
2
and CO, and there also may be unburned fuel in the products.
Unlike the complete combustion cases considered above, the products of combustion
of an actual combustion process and their relative amounts can be determined only
by measurement.
Among several devices for measuring the composition of products of combustion
are the Orsat analyzer, gas chromatograph, infrared analyzer, and flame ionization
detector. Data from these devices can be used to determine the mole fractions of the
gaseous products of combustion. The analyses are often reported on a “dry” basis. In
a dry product analysis, the mole fractions are given for all gaseous products except the
water vapor. In Examples 13.2 and 13.3, we show how analyses of the products of
combustion on a dry basis can be used to determine the balanced chemical reaction
equations.
Since water is formed when hydrocarbon fuels are burned, the mole fraction of
water vapor in the gaseous products of combustion can be significant. If the gaseous
products of combustion are cooled at constant mixture pressure, the dew point tem-
perature is reached when water vapor begins to condense. Since water deposited on
duct work, mufflers, and other metal parts can cause corrosion, knowledge of the dew
point temperature is important. Determination of the dew point temperature is illus-
trated in Example 13.2, which also features a dry product analysis.
dry product analysis
TAKE NOTE...
For cooling of combustion
products at constant
pressure, the dew point
temperature marks the
onset of condensation of
water vapor present in the
products. See Sec. 12.5.4
to review this concept.
Using a Dry Product Analysis for Combustion of Methane
c c c c EXAMPLE 13.2 c
Methane, CH
4
, is burned with dry air. The molar analysis of the products on a dry basis is CO
2
, 9.7%; CO, 0.5%;
O
2
, 2.95%; and N
2
, 86.85%. Determine (a) the air–fuel ratio on both a molar and a mass basis, (b) the percent
theoretical air, (c) the dew point temperature of the products, in 8F, if the mixture were cooled at 1 atm.
SOLUTION
Known:
Methane is burned with dry air. The molar analysis of the products on a dry basis is provided.
Find: Determine (a) the air–fuel ratio on both a molar and a mass basis, (b) the percent theoretical air, and (c) the
dew point temperature of the products, in 8F, if cooled at 1 atm.
Engineering Model:
1.
Each mole of oxygen in the combustion air is accompanied by 3.76 moles of nitrogen, which is inert.
2. The products form an ideal gas mixture and the dew point temperature of the mixture is conceptualized as
in Sec. 12.5.4.
Analysis:
➊ (a) The solution is conveniently conducted on the basis of 100 lbmol of dry products. The chemical equation
then reads
aCH
4
1 b1O
2
1 3.76N
2
2
S
9.7CO
2
1 0.5CO 1 2.95O
2
1 86.85N
2
1 cH
2
O
In addition to the assumed 100 lbmol of dry products, water must be included as a product.
Applying conservation of mass to carbon, hydrogen, and oxygen, respectively
C: 9.7 1 0.5 5 a
H: 2 c 5 4a
O: 19.721221 0.5 1 212.9521 c 5 2b
➋ Solving this set of equations gives a 5 10.2, b 5 23.1, c 5 20.4. The balanced chemical equation is
10.2CH
4
1 23.11O
2
1 3.76N
2
2S 9.7CO
2
1 0.5CO 1 2.95O
2
1 86.85N
2
1 20.4H
2
O
13.1 Introducing Combustion 783
c13ReactingMixturesandCombustion783 Page 783 7/14/10 8:34:02 AM user-s146c13ReactingMixturesandCombustion783 Page 783 7/14/10 8:34:02 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
784 Chapter 13 Reacting Mixtures and Combustion
In Example 13.3, a fuel mixture having a known molar analysis is burned with air,
giving products with a known dry analysis.
On a molar basis, the air–fuel ratio is
AF 5
23.114.76
2
10.2
5 10.78
lbmol 1air2
lbmol 1fuel2
On a mass basis
AF 5 110.782 a
28.9
7
16.04
b5 19.47
lb 1air
2
lb 1fuel2
(b) The balanced chemical equation for the complete combustion of methane with the theoretical amount of air is
CH
4
1 21O
2
1 3.76N
2
2
S
CO
2
1 2H
2
O 1 7.52N
2
The theoretical air–fuel ratio on a molar basis is
1AF2
theo
5
214.76
2
1
5 9.52
lbmol 1air2
lbmol 1fuel2
The percent theoretical air is then found from
% theoretical air 5
1A
F2
1A
F2
theo
5
10.78 lbmol 1air2
/
lbmol 1fuel2
9.52 lbmol 1air2
/
lbmol 1fuel2
5 1.13 1113%2
(c) To determine the dew point temperature requires the partial pressure of the water vapor p
v
. The partial pressure p
v
is found from p
v
5 y
v
p, where y
v
is the mole fraction of the water vapor in the combustion products and p is 1 atm.
Referring to the balanced chemical equation of part (a), the mole fraction of the water vapor is
y
v
5
20.4
100 1 20.4
5 0.169
➌ Thus, p
v
5 0.169 atm 5 2.484 lbf/in.
2
Interpolating in Table A-2E, T 5 134°F.
➊
The solution could be obtained on the basis of any assumed amount of dry
products—for example, 1 lbmol. With some other assumed amount, the
values of the coefficients of the balanced chemical equation would differ
from those obtained in the solution, but the air–fuel ratio, the value for the
percent of theoretical air, and the dew point temperature would be
unchanged.
➋ The three unknown coefficients, a, b, and c, are evaluated here by applica-
tion of conservation of mass to carbon, hydrogen, and oxygen. As a check,
note that the nitrogen also balances
N: b(3.76) 5 86.85
This confirms the accuracy of both the given product analysis and the cal-
culations conducted to determine the unknown coefficients.
➌ If the products of combustion were cooled at constant pressure below the
dew point temperature of 1348F, some condensation of the water vapor
would occur.
Ability to…
❑
balance a chemical reaction
equation for incomplete
combustion given the
analysis of dry products
of combustion.
❑
apply definitions of air–fuel
ratio on mass and molar
bases as well as percent
theoretical air.
❑
determine the dew point
temperature of combustion
products.
✓
Skills Developed
Recalculate the dew point temperature as in part (c) if the
air supply were moist air, including 3.53 kmol of additional water vapor.
Ans. 139°F.
c13ReactingMixturesandCombustion784 Page 784 7/13/10 11:00:22 AM user-s146c13ReactingMixturesandCombustion784 Page 784 7/13/10 11:00:22 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

Burning Natural Gas with Excess Air
c c c c EXAMPLE 13.3 c
A natural gas has the following molar analysis: CH
4
, 80.62%; C
2
H
6
, 5.41%; C
3
H
8
, 1.87%; C
4
H
10
, 1.60%; N
2
, 10.50%.
The gas is burned with dry air, giving products having a molar analysis on a dry basis: CO
2
, 7.8%; CO, 0.2%; O
2
,
7%; N
2
, 85%. (a) Determine the air–fuel ratio on a molar basis. (b) Assuming ideal gas behavior for the fuel mixture,
determine the amount of products in kmol that would be formed from 100 m
3
of fuel mixture at 300 K and
1 bar. (c) Determine the percent of theoretical air.
SOLUTION
Known:
A natural gas with a specified molar analysis burns with dry air, giving products having a known molar
analysis on a dry basis.
Find: Determine the air–fuel ratio on a molar basis, the amount of products in kmol that would be formed from
100 m
3
of natural gas at 300 K and 1 bar, and the percent of theoretical air.
Engineering Model:
1.
Each mole of oxygen in the combustion air is accompanied by 3.76 moles of nitrogen, which is inert.
2. The fuel mixture can be modeled as an ideal gas.
Analysis:
(a)
The solution can be conducted on the basis of an assumed amount of fuel mixture or on the basis of an assumed
amount of dry products. Let us illustrate the first procedure, basing the solution on 1 kmol of fuel mixture. The
chemical equation then takes the form
10.8062CH
4
1 0.0541C
2
H
6
1 0.0187C
3
H
8
1 0.0160C
4
H
10
1 0.1050N
2
2
1 a1O
2
1 3.76N
2
2S b10.078CO
2
1 0.002CO 1 0.07O
2
1 0.85N
2
21 cH
2
O
The products consist of b kmol of dry products and c kmol of water vapor, each per kmol of fuel mixture.
Applying conservation of mass to carbon
b10.078 1 0.00225 0.8062 1 210.054121 310.018721 410.01602
Solving gives b 5 12.931. Conservation of mass for hydrogen results in
2c 5 410.806221 610.054121 810.018721 1010.01602
which gives c 5 1.93. The unknown coefficient a can be found from either an oxygen balance or a nitrogen bal-
ance. Applying conservation of mass to oxygen
12.9313210.07821 0.002 1 210.07241 1.93 5 2a
➊ giving a 5 2.892.
The balanced chemical equation is then
10.8062CH
4
1 0.0541C
2
H
6
1 0.0187C
3
H
8
1 0.0160C
4
H
10
1 0.1050N
2
2
1 2.8921O
2
1 3.76N
2
2
S
12.93110.078CO
2
1 0.002CO 1 0.07O
2
1 0.85N
2
2
1 1.93H
2
O
The air–fuel ratio on a molar basis is
AF 5
12.892214.76
2
1
5 13.77
kmol 1air2
kmol 1fuel2
(b) By inspection of the chemical reaction equation, the total amount of products is b 1 c 5 12.931 1 1.93 5
14.861 kmol of products per kmol of fuel. The amount of fuel in kmol, n
F
, present in 100 m
3
of fuel mixture
13.1 Introducing Combustion 785
c13ReactingMixturesandCombustion785 Page 785 7/13/10 11:00:24 AM user-s146c13ReactingMixturesandCombustion785 Page 785 7/13/10 11:00:24 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
786 Chapter 13 Reacting Mixtures and Combustion
13.1.4
Energy and Entropy Balances for Reacting Systems
Thus far our study of reacting systems has involved only the conservation of mass
principle. A more complete understanding of reacting systems requires application
of the first and second laws of thermodynamics. For these applications, energy
and entropy balances play important roles, respectively. Energy balances for react-
ing systems are developed and applied in Secs. 13.2 and 13.3; entropy balances
for reacting systems are the subject of Sec. 13.5. To apply such balances, it is
necessary to take special care in how internal energy, enthalpy, and entropy are
evaluated.
For the energy and entropy balances of this chapter, combustion air and (normally)
products of combustion are modeled as ideal gas mixtures. Accordingly, ideal gas
mixture principles introduced in the first part of Chap. 12 play a role. For ease of
reference, Table 13.1 summarizes ideal gas mixture relations introduced in Chap. 12
that are used in this chapter.
at 300 K and 1 bar can be determined from the ideal gas equation of state as
n
F
5
p
V
RT
5
110
5
N
/
m
2
21100 m
3
2
18314 N ? m
/
kmol ? K21300 K2
5 4.01 kmol 1fuel2
Accordingly, the amount of product mixture that would be formed from 100 m
3
of fuel mixture is (14.861)(4.01) 5
59.59 kmol of product gas.
(c) The balanced chemical equation for the complete combustion of the fuel mixture with the theoretical amount
of air is
1
0.8062CH
4
1 0.0541C
2
H
6
1 0.0187C
3
H
8
1 0.0160C
4
H
10
1 0.1050N
2
2
1 2
1
O
2
1 3.76N
2
2
S
1.0345CO
2
1 1.93H
2
O 1 7.625N
2
The theoretical air–fuel ratio on a molar basis is
1AF2
theo
5
214.76
2
1
5 9.52
kmol 1air2
kmol 1fuel2
The percent theoretical air is then
% theoretical air 5
13.77 kmol 1air2
/
kmol 1fuel2
9.52 kmol 1air2
/
kmol 1fuel2
5 1.45 1145%2
➊
A check on both the accuracy of the given molar analyses and the calcula-
tions conducted to determine the unknown coefficients is obtained by apply-
ing conservation of mass to nitrogen. The amount of nitrogen in the reac-
tants is
0.105 1 13.76212.89225 10.98 kmol
/
kmol of fuel
The amount of nitrogen in the products is (0.85)(12.931) 5 10.99 kmol/kmol
of fuel. The difference can be attributed to round-off.
Ability to…
❑
balance a chemical reaction
equation for incomplete
combustion of a fuel mixture
given the analysis of dry
products of combustion.
❑
apply the definition of air–
fuel ratio on a molar basis as
well as percent theoretical air.
✓
Skills Developed
Determine the mole fractions of the products of combustion.
Ans.
y
CO
2
5 0.0679, y
CO
5 0.0017,
y
O
2
5 0.0609,
y
N
2
5 0.7396,
y
H
2
O
5
0.1299.
c13ReactingMixturesandCombustion786 Page 786 7/21/10 8:22:48 AM user-s146c13ReactingMixturesandCombustion786 Page 786 7/21/10 8:22:48 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
13.2 Conservation of Energy—
Reacting Systems
The objective of the present section is to illustrate the application of the conservation
of energy principle to reacting systems. The forms of the conservation of energy
principle introduced previously remain valid whether or not a chemical reaction
occurs within the system. However, the methods used for evaluating the properties
of reacting systems differ somewhat from the practices used to this point.
13.2.1
Evaluating Enthalpy for Reacting Systems
In most tables of thermodynamic properties used thus far, values for the specific
internal energy, enthalpy, and entropy are given relative to some arbitrary datum state
where the enthalpy (or alternatively the internal energy) and entropy are set to zero.
This approach is satisfactory for evaluations involving differences in property values
between states of the same composition, for then arbitrary datums cancel. However,
13.2 Conservation of Energy—Reacting Systems 787
TAKE NOTE...
When applying energy and
entropy balances to react-
ing systems, it is neces-
sary to take special care
in how internal energy,
enthalpy, and entropy are
evaluated.
Internal Energy, Enthalpy, and Entropy for Ideal Gas Mixtures
Notation: n
i
5 moles of gas i, y
i
5 mole fraction of gas i
T 5 mixture temperature, p 5 mixture pressure
p
i
5 y
i
p 5 partial pressure of gas i
u
i
5 specific internal energy of gas i, per mole of i
h
i
5 specific enthalpy of gas i, per mole of i
s
i
5 specific entropy of gas i, per mole of i
Mixture internal energy:
U 5 n
1
u
1
1 n
2
u
2
1
. . .
1 n
j
u
j
5
a
j
i5 1
n
i
u
i
1T2
(12.19)
Mixture enthalpy:
H 5 n
1
h
1
1n
2
h
2
1
. . .
1 n
j
h
j
5
a
j
i5 1
n
i
h
i
1T2
(12.20)
Mixture entropy:
S 5 n
1
s
1
1 n
2
s
2
1
. . .
1 n
j
s
j
5
a
j
i5 1
n
i
s
i
1T, p
i
2
(12.26)
c With Eq. 6.18:
s
i
1T, p
i
25 s
i
1T, p22 R ln
p
i
p
5
s
i
1T, p22 R ln y
i
(a)
c With Eq. 6.18 and p
ref
5 1 atm:
s
i
1T, p
i
25 s
i
1T, p
ref
22 R ln
p
i
p
ref
5 s8
i
1T 22 R ln
y
i
p
p
ref
(b)
1
where s8
i
is obtained from Table A-23 and A-23E, as appropriate.
1
Equation (b) corresponds to Eq. 13.23.
TABLE 13.1
c13ReactingMixturesandCombustion787 Page 787 7/21/10 8:22:58 AM user-s146c13ReactingMixturesandCombustion787 Page 787 7/21/10 8:22:58 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
788 Chapter 13
Reacting Mixtures and Combustion
when a chemical reaction occurs, reactants disappear and products are formed, so
differences cannot be calculated for all substances involved. For reacting systems, it
is necessary to evaluate h, u, and s in such a way that there are no subsequent ambi-
guities or inconsistencies in evaluating properties. In this section, we will consider
how this is accomplished for h and u. The case of entropy is handled differently and
is taken up in Sec. 13.5.
An enthalpy datum for the study of reacting systems can be established by assign-
ing arbitrarily a value of zero to the enthalpy of the stable elements at a state called
the standard reference state and defined by T
ref
5 298.15 K (258C) and p
ref
5 1 atm.
In English units the temperature at the standard reference state is closely 5378R
(778F). Note that only stable elements are assigned a value of zero enthalpy at the
standard state. The term stable simply means that the particular element is in a chem-
ically stable form. For example, at the standard state the stable forms of hydrogen,
oxygen, and nitrogen are H
2
, O
2
, and N
2
and not the monatomic H, O, and N. No
ambiguities or conflicts result with this choice of datum.
ENTHALPY OF FORMATION. Using the datum introduced above, enthalpy
values can be assigned to compounds for use in the study of reacting systems. The
enthalpy of a compound at the standard state equals its enthalpy of formation, sym-
bolized h8
f
. The enthalpy of formation is the energy released or absorbed when the
compound is formed from its elements, the compound and elements all being at T
ref
and p
ref
. The enthalpy of formation is usually determined by application of procedures
from statistical thermodynamics using observed spectroscopic data.
The enthalpy of formation also can be found in principle by measuring the heat
transfer in a reaction in which the compound is formed from the elements.
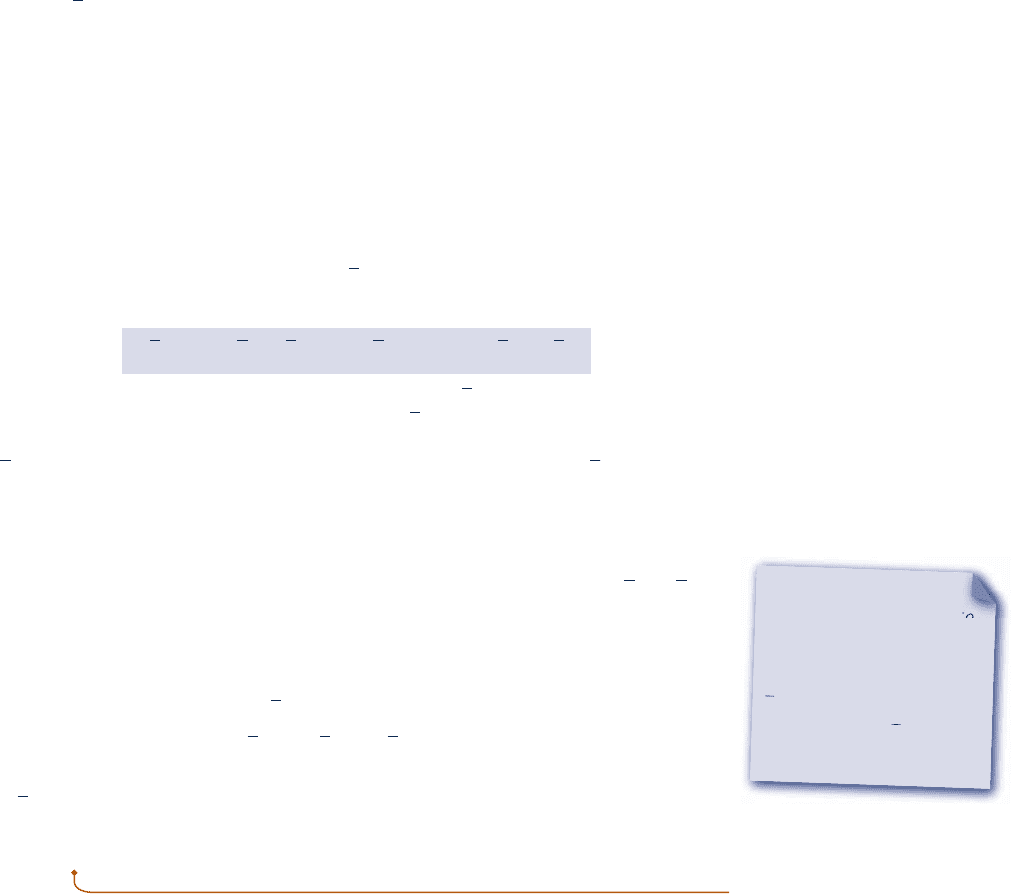
consider the simple reactor shown in Fig. 13.1, in which carbon
and oxygen each enter at T
ref
and p
ref
and react completely at steady state to form
carbon dioxide at the same temperature and pressure. Carbon dioxide is formed from
carbon and oxygen according to
C 1 O
2
S
CO
2
(13.6)
This reaction is exothermic, so for the carbon dioxide to exit at the same temperature
as the entering elements, there would be a heat transfer from the reactor to its sur-
roundings. The rate of heat transfer and the enthalpies of the incoming and exiting
streams are related by the energy rate balance
0 5 Q
#
cv
1 m
#
C
h
C
1 m
#
O
2
h
O
2
2 m
#
CO
2
h
CO
2
where m
#
and h denote, respectively, mass flow rate and specific enthalpy. In writing
this equation, we have assumed no work W
#
cv
and negligible effects of kinetic and
potential energy. For enthalpies on a molar basis, the energy rate balance appears as
0 5 Q
#
cv
1 n
#
C
h
C
1 n
#
O
2
h
O
2
2 n
#
CO
2
h
CO
2
where n
#
and h denote, respectively, the molar flow rate and specific enthalpy per mole.
Solving for the specific enthalpy of carbon dioxide and noting from Eq. 13.6 that all
molar flow rates are equal
h
CO
2
5
Q
#
cv
n
#
CO
2
1
n
#
C
n
#
CO
2
h
C
1
n
#
O
2
n
#
CO
2
h
O
2
5
Q
#
cv
n
#
CO
2
1 h
C
1 h
O
2
(13.7)
Since carbon and oxygen are stable elements at the standard state,
h
C
5 h
O
2
5 0, and Eq. 13.7 becomes
h
CO
2
5
Q
#
cv
n
#
CO
2
(13.8)
Accordingly, the value assigned to the specific enthalpy of carbon dioxide
at the standard state, the enthalpy of formation, equals the heat transfer,
standard reference state
enthalpy of formation
Fig. 13.1 Reactor used to discuss the
enthalpy of formation concept.
C
T
ref
, p
ref
O
2
T
ref
, p
ref
CO
2
T
ref
, p
ref
c13ReactingMixturesandCombustion788 Page 788 7/21/10 8:23:07 AM user-s146c13ReactingMixturesandCombustion788 Page 788 7/21/10 8:23:07 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
per mole of CO
2
, between the reactor and its surroundings. If the heat transfer could
be measured accurately, it would be found to equal 2393,520 kJ per kmol of carbon
dioxide formed (2169,300 Btu per lbmol of CO
2
formed). b b b b b
Tables A-25 and A-25E give values of the enthalpy of formation for several
compounds in units of kJ/kmol and Btu/lbmol, respectively. In this text, the super-
script 8 is used to denote properties at 1 atm. For the case of the enthalpy of
formation, the reference temperature T
ref
is also intended by this symbol. The
values of h8
f
listed in Tables A-25 and A-25E for CO
2
correspond to those given in
the previous example.
The sign associated with the enthalpy of formation values appearing in Tables A-25
corresponds to the sign convention for heat transfer. If there is heat transfer from a
reactor in which a compound is formed from its elements (an exothermic reaction as
in the previous example), the enthalpy of formation has a negative sign. If a heat
transfer to the reactor is required (an endothermic reaction), the enthalpy of formation
is positive.
Evaluating Enthalpy
The specific enthalpy of a compound at a state other than the standard state is found
by adding the specific enthalpy change ¢
h.
between the standard state and the state
of interest to the enthalpy of formation
h
1
T, p
2
5 h8
f
1
3
h
1
T, p
2
2 h
1
T
ref
, p
ref
24
5 h8
f
1 ¢h (13.9)
That is, the enthalpy of a compound is composed of h8
f
, associated with the forma-
tion of the compound from its elements, and ¢h, associated with a change of state
at constant composition. An arbitrary choice of datum can be used to determine
¢h, since it is a difference at constant composition. Accordingly, ¢
h
can be evalu-
ated from tabular sources such as the steam tables, the ideal gas tables when
appropriate, and so on. Note that as a consequence of the enthalpy datum adopted
for the stable elements, the specific enthalpy determined from Eq. 13.9 is often
negative.
Tables A-25 provide two values of the enthalpy of formation of water: h8
f
1l2, h8
f
1g2.
The first is for liquid water and the second is for water vapor. Under equilibrium
conditions, water exists only as a liquid at 258C (778F) and 1 atm. The vapor value
listed is for a hypothetical ideal gas state in which water is a vapor at 258C (778F) and
1 atm. The difference between the two enthalpy of formation values is given closely
by the enthalpy of vaporization h
f
g
at T
ref
. That is,
h8
f
1g22 h8
f
1l2< h
f
g
1258C2 (13.10)
Similar considerations apply to other substances for which liquid and vapor values
for h
o
f
are listed in Tables A-25.
13.2.2
Energy Balances for Reacting Systems
Several considerations enter when writing energy balances for systems involving
combustion. Some of these apply generally, without regard for whether combus-
tion takes place. For example, it is necessary to consider if significant work and
heat transfers take place and if the respective values are known or unknown.
Also, the effects of kinetic and potential energy must be assessed. Other consid-
erations are related directly to the occurrence of combustion. For example, it is
important to know the state of the fuel before combustion occurs. Whether the
fuel is a liquid, a gas, or a solid is important. It is also necessary to consider whether
the fuel is premixed with the combustion air or the fuel and air enter a reactor
separately.
TAKE NOTE...
When applying Eq. 13.9 to
water vapor, we use the
vapor value of the enthalpy
of formation of water,
h8
f
1g2,
from Tables A-25
together with
¢
h
for water
vapor from Tables A-23.
13.2 Conservation of Energy—Reacting Systems 789
c13ReactingMixturesandCombustion789 Page 789 7/14/10 8:34:12 AM user-s146c13ReactingMixturesandCombustion789 Page 789 7/14/10 8:34:12 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
790 Chapter 13 Reacting Mixtures and Combustion
The state of the combustion products also must be assessed. For instance, the pres-
ence of water vapor should be noted, for some of the water present will condense if
the products are cooled sufficiently. The energy balance must then be written to account
for the presence of water in the products as both a liquid and a vapor. For cooling of
combustion products at constant pressure, the dew point temperature method of Exam-
ple 13.2 is used to determine the temperature at the onset of condensation.
Analyzing Control Volumes at Steady State
To illustrate the many considerations involved when writing energy balances for
reacting systems, we consider special cases of broad interest, highlighting the under-
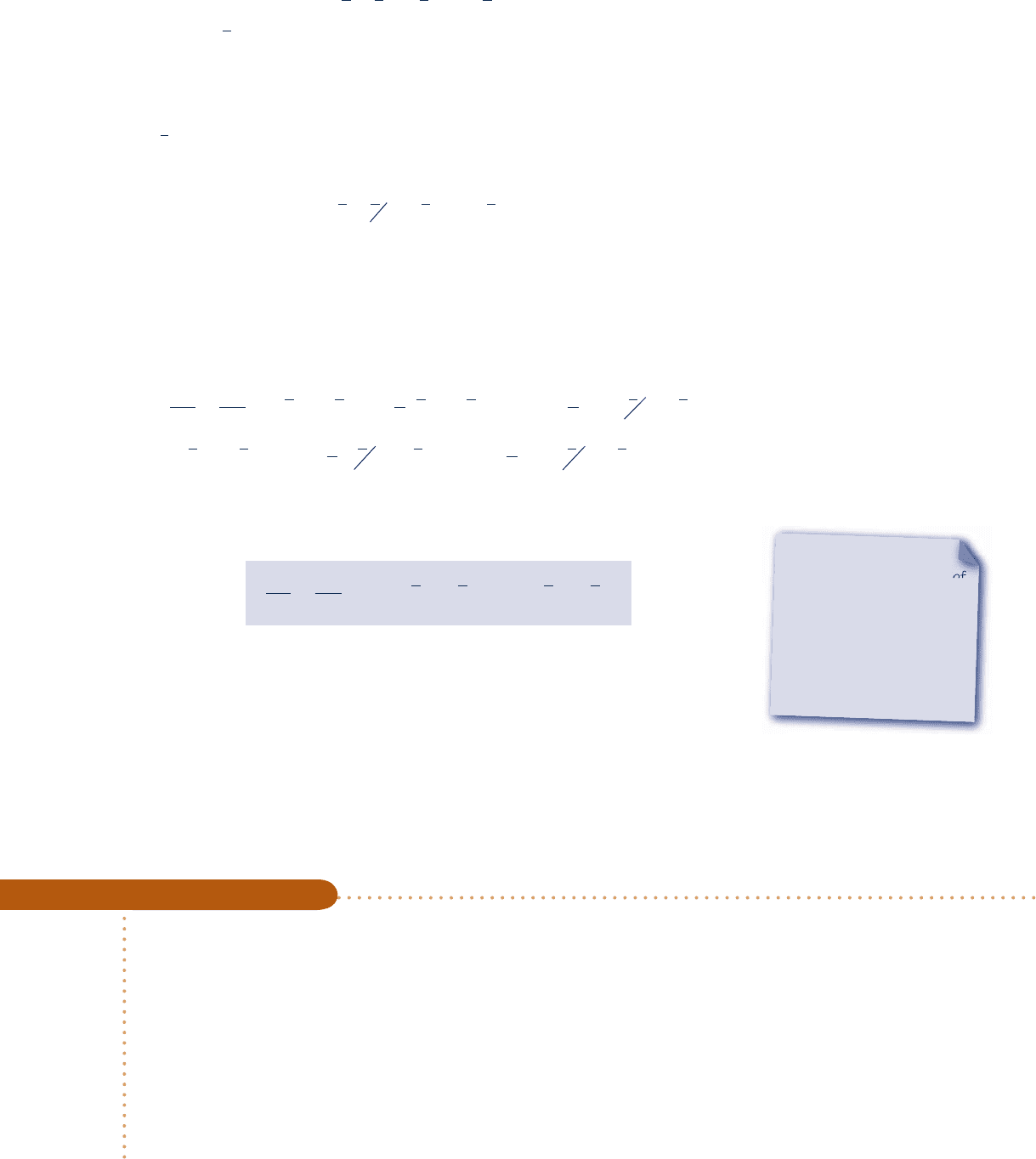
lying assumptions. Let us begin by considering the steady-state reactor shown in Fig.
13.2, in which a hydrocarbon fuel C
a
H
b
burns completely with the theoretical amount
of air according to
C
a
H
b
1
a
a 1
b
4
b
1O
2
1 3.76N
2
2S
aCO
2
1
b
2
H
2
O 1
a
a 1
b
4
b
3.76N
2
(13.11)
The fuel enters the reactor in a stream separate from the combustion air, which is
regarded as an ideal gas mixture. The products of combustion also are assumed to
form an ideal gas mixture. Kinetic and potential energy effects are ignored.
With the foregoing idealizations, the mass and energy rate balances for the two-
inlet, single-exit reactor can be used to obtain the following equation on a per mole
of fuel basis:
Q
#
cv
n
#
F
2
W
#
cv
n
#
F
5 cah
CO
2
1
b
2
h
H
2
O
1 aa 1
b
4
b 3.76 h
N
2
d
2 h
F
2
ca
a 1
b
4
b
h
O
2
1
a
a 1
b
4
b
3.76 h
N
2
d
(13.12a)
where n
#
F
denotes the molar flow rate of the fuel. Note that each coefficient on the
right side of this equation is the same as the coefficient of the corresponding sub-
stance in the reaction equation.
The first underlined term on the right side of Eq. 13.12a is the enthalpy of the
exiting gaseous products of combustion per mole of fuel. The second underlined term
on the right side is the enthalpy of the combustion air per mole of fuel. In accord
with Table 13.1, the enthalpies of the combustion products and the air have been
evaluated by adding the contribution of each component present in the respective
ideal gas mixtures. The symbol h
F
denotes the molar enthalpy of the fuel.
Equation 13.12a can be expressed more concisely as
Q
#
cv
n
#
F
2
W
#
cv
n
#
F
5 h
P
2 h
R
(13.12b)
where h
P
and h
R
denote, respectively, the enthalpies of the products and reactants per
mole of fuel.
EVALUATING ENTHALPY TERMS. Once the energy balance has been written,
the next step is to evaluate the individual enthalpy terms. Since each component of
Fig. 13.2 Reactor at steady
state.
Air at
T
A
Combustion products
at T
P
W
cv
·
Q
cv
·
C
a
H
b
at
T
F
c13ReactingMixturesandCombustion790 Page 790 7/21/10 8:23:18 AM user-s146c13ReactingMixturesandCombustion790 Page 790 7/21/10 8:23:18 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
the combustion products is assumed to behave as an ideal gas, its contribution to the
enthalpy of the products depends solely on the temperature of the products, T
P
.
Accordingly, for each component of the products, Eq. 13.9 takes the form
h 5 h8
f
1 3h1T
P
22 h1T
ref
24 (13.13)
In Eq. 13.13, h8
f
is the enthalpy of formation from Table A-25 or A-25E, as appropri-
ate. The second term accounts for the change in enthalpy from the temperature T
ref
to the temperature T
P
. For several common gases, this term can be evaluated from
tabulated values of enthalpy versus temperature in Tables A-23 and A-23E, as appro-
priate. Alternatively, the term can be obtained by integration of the ideal gas specific
heat c
p
obtained from Tables A-21 or some other source of data.
A similar approach is employed to evaluate the enthalpies of the oxygen and
nitrogen in the combustion air. For these
h 5 h8
f
0
1
3
h
1
T
A
2
2 h
1
T
ref
24
(13.14)
where T
A
is the temperature of the air entering the reactor. Note that the enthalpy of
formation for oxygen and nitrogen is zero by definition and thus drops out of Eq. 13.14
as indicated.
The evaluation of the enthalpy of the fuel is also based on Eq. 13.9. If the fuel can
be modeled as an ideal gas, the fuel enthalpy is obtained using an expression of the
same form as Eq. 13.13, with the temperature of the incoming fuel replacing T
P
.
With the foregoing considerations, Eq. 13.12a takes the form
Q
#
cv
n
#
F
2
W
#
cv
n
#
F
5 a1h8
f
1 ¢h2
CO
2
1
b
2
1h8
f
1 ¢h2
H
2
O
1 aa 1
b
4
b 3.761h8
f
0
1 ¢h2
N
2
21h8
f
1 ¢h2
F
2
a
a 1
b
4
b
1h8
f
0
1 ¢h2
O
2
2
a
a 1
b
4
b
3.761h8
f
0
1 ¢h2
N
2
(13.15a)
The terms set to zero in this expression are the enthalpies of formation of oxygen
and nitrogen.
Equation 13.15a can be written more concisely as
Q
#
cv
n
#
F
2
W
#
cv
n
#
F
5
a
P
n
e
1h8
f
1 ¢h2
e
2
a
R
n
i
1h8
f
1 ¢h2
i
(13.15b)
where i denotes the incoming fuel and air streams and e the exiting combustion
products.
Although Eqs. 13.15 have been developed with reference to the reaction of Eq. 13.11,
equations having the same general forms would be obtained for other combustion
reactions.
In Examples 13.4 and 13.5, the energy balance is applied together with tabular
property data to analyze control volumes at steady state involving combustion. Exam-
ple 13.4 involves a reciprocating internal combustion engine while Example 13.5
involves a simple gas turbine power plant.
TAKE NOTE...
The coefficients n
i
and n
e
of
Eq. 13.15b correspond to
the respective coefficients
of the reaction equation
giving the moles of reac-
tants and products per
mole of fuel, respectively.
Analyzing an Internal Combustion Engine Fueled with Liquid Octane
c c c c EXAMPLE 13.4 c
Liquid octane enters an internal combustion engine operating at steady state with a mass flow rate of 0.004 lb/s
and is mixed with the theoretical amount of air. The fuel and air enter the engine at 778F and 1 atm. The mixture
burns completely and combustion products leave the engine at 11408F. The engine develops a power output of
50 horsepower. Determine the rate of heat transfer from the engine, in Btu/s, neglecting kinetic and potential
energy effects.
13.2 Conservation of Energy—Reacting Systems 791
c13ReactingMixturesandCombustion791 Page 791 7/13/10 11:00:40 AM user-s146c13ReactingMixturesandCombustion791 Page 791 7/13/10 11:00:40 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
792 Chapter 13 Reacting Mixtures and Combustion
SOLUTION
Known:
Liquid octane and the theoretical amount of air enter an internal combustion engine operating at steady
state in separate streams at 778F, 1 atm. Combustion is complete and the products exit at 11408F. The power
developed by the engine and fuel mass flow rate are specified.
Find: Determine the rate of heat transfer from the engine, in Btu/s.
Schematic and Given Data:
Analysis:
The balanced chemical equation for complete combustion with the theoretical amount of air is obtained
from the solution to Example 13.1 as
C
8
H
18
1 12.5O
2
1 47N
2
S
8CO
2
1 9H
2
O
1
g
2
1 47N
2
The energy rate balance reduces, with assumptions 1–3, to give
Q
#
cv
n
#
F
5
W
#
cv
n
#
F
1 h
P
2 h
R
➊
5
W
#
cv
n
#
F
1 583h8
f
1 ¢h4
CO
2
1 93h8
f
1 ¢h4
H
2
O1g2
1 473h8
f
0
1 ¢h4
N
2
6
2 53h8
f
1 ¢h4
0
C
8
H
1
8
1
l
2
1 12.53h8
f
0
1 ¢h4
0
O
2
1 473h8
f
0
1 ¢h4
0
N
2
6
where each coefficient is the same as the corresponding term of the balanced chemical equation and Eq. 13.9
has been used to evaluate enthalpy terms. The enthalpy of formation terms for oxygen and nitrogen are zero;
and
¢
h
5
0
for each of the reactants because the fuel and combustion air enter at 778F.
With the enthalpy of formation for C
8
H
18
(l) from Table A-25E
h
R
5 1h8
f
2
C
8
H
1
8
1
l
2
52107,530 Btu
/
lbmol1fuel2
With enthalpy of formation values for CO
2
and H
2
O(g) from Table A-25E, and enthalpy values for N
2
, H
2
O,
and CO
2
from Table A-23E
h
P
5 8
3
2169,300 1
1
15,829 2 4027.5
24
1 9
3
2104,040 1
1
13,494.4 2 4258
24
1 47
3
11,409.7 2 3729.5
4
521,752,251 Btu
/
lbmol
1
fuel
2
Using the molecular weight of the fuel from Table A-1E, the molar flow rate of the fuel is
n
#
F
5
0.004 lb
1
fuel
2
/
s
114.22 lb
1
fuel
2
/
lbmol
1
fuel
2
5 3.5 3 10
25
lbmol1fuel2
/
s
Engineering Model:
1.
The control volume identified by a dashed line on
the accompanying figure operates at steady state.
2. Kinetic and potential energy effects can be ignored.
3. The combustion air and the products of combus-
tion each form ideal gas mixtures.
4. Each mole of oxygen in the combustion air is
accompanied by 3.76 moles of nitrogen. The nitro-
gen is inert and combustion is complete.
Fig. E13.4
Drive
shaft
Combustion products
at 1140°F
50 hp
Fuel at 77°
F, 1 atm
Air at 77°F, 1 atm
c13ReactingMixturesandCombustion792 Page 792 7/13/10 11:00:42 AM user-s146c13ReactingMixturesandCombustion792 Page 792 7/13/10 11:00:42 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
