Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

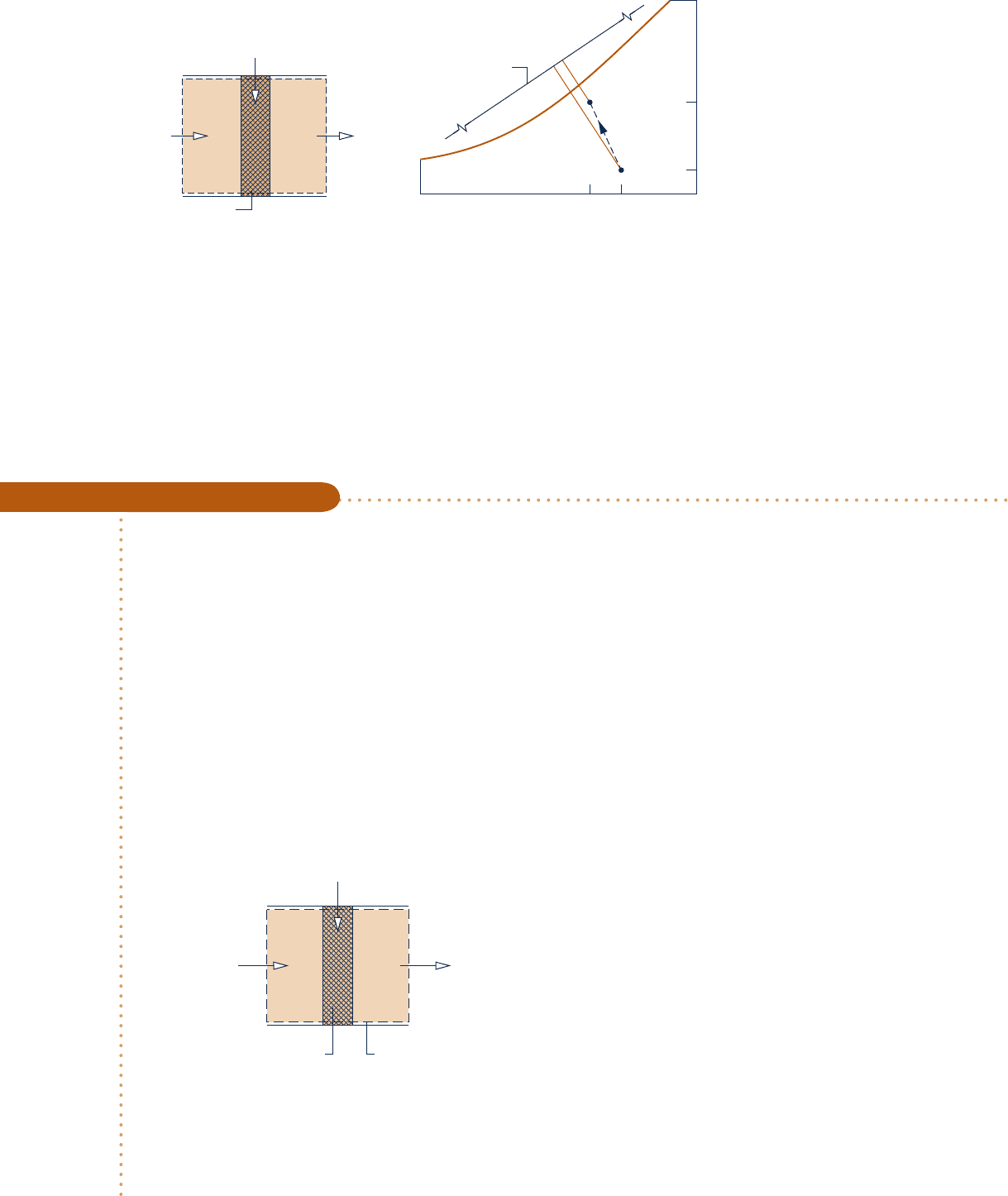
Moist air
m
a
, T
1
,
ω
1
T
2
< T
1
2
>
1
ωω
Water at T
w
Soaked
pad
(a)
(b)
Dry-bulb temperature
Mixture enthalpy
per unit mass
of dry air
T
2
T
1
ω
2
ω
1
1
2
ω
12
·
Fig. 12.13 Evaporative cooling. (a) Equipment schematic. (b) Psychrometric chart
representation.
mixture enthalpy are closely lines of constant wet-bulb temperature (Sec. 12.7), it
follows that evaporative cooling takes place at a nearly constant wet-bulb tem-
perature.
In the next example, we consider the analysis of an evaporative cooler.
12.8 Analyzing Air-Conditioning Processes 753
c c c c EXAMPLE 12.13 c
Water at 70°F
T
2
= 70°F
Boundar
y
21
Soaked pad
T
1
1
(AV)
1
= 100°F
= 10%
= 5000
φ
ft
3
___
min
Fig. E12.13
Engineering Model:
1.
The control volume shown in the accompanying figure oper-
ates at steady state. Changes in kinetic and potential energy
can be neglected and W
#
cv
5 0.
2. There is no heat transfer with the surroundings.
3. The water added to the soaked pad enters as a liquid and
evaporates fully into the moist air.
4. The pressure remains constant throughout at 1 atm.
5. The moist air streams are regarded as ideal gas mixtures
adhering to the Dalton model.
Considering an Evaporative Cooler
Air at 1008F and 10% relative humidity enters an evaporative cooler with a volumetric flow rate of 5000 ft
3
/min.
Moist air exits the cooler at 708F. Water is added to the soaked pad of the cooler as a liquid at 708F and evap-
orates fully into the moist air. There is no heat transfer with the surroundings and the pressure is constant
throughout at 1 atm. Determine (a) the mass flow rate of the water to the soaked pad, in lb/h, and (b) the
relative humidity of the moist air at the exit to the evaporative cooler.
SOLUTION
Known:
Air at 1008F and f 5 10% enters an evaporative cooler with a volumetric flow rate of 5000 ft
3
/min.
Moist air exits the cooler at 708F. Water is added to the soaked pad of the cooler at 708F.
Find: Determine the mass flow rate of the water to the soaked pad, in lb/h, and the relative humidity of the
moist air at the exit of the cooler.
Schematic and Given Data:
c12IdealGasMixtureandPsychromet753 Page 753 6/29/10 11:58:01 AM user-s146 c12IdealGasMixtureandPsychromet753 Page 753 6/29/10 11:58:01 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
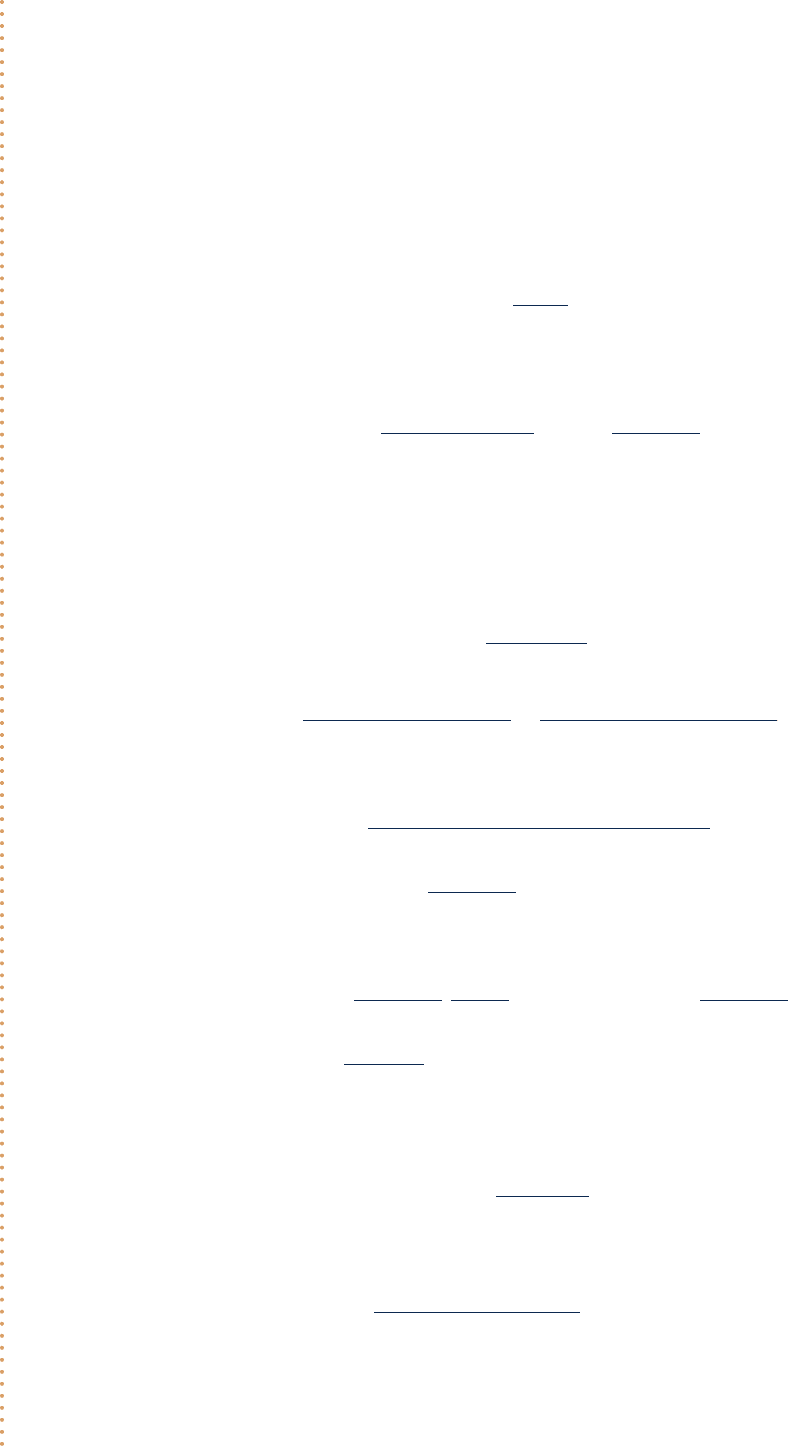
754 Chapter 12
Ideal Gas Mixture and Psychrometric Applications
Analysis:
(a)
Applying conservation of mass to the dry air and water individually as in previous examples gives
m
#
w
5 m
#
a
1
v
2
2 v
1
2
where m
#
w
is the mass flow rate of the water to the soaked pad. To find m
#
w
requires v
1
, m
#
a
, and v
2
. These will
now be evaluated in turn.
The humidity ratio v
1
can be found from Eq. 12.43, which requires p
v1
, the partial pressure of the moist
air entering the control volume. Using the given relative humidity f
1
and p
g
at T
1
from Table A-2E, we have
p
v1
5 f
1
p
g1
5 0.095 lbf/in.
2
With this, v
1
5 0.00405 lb(vapor)ylb(dry air).
The mass flow rate of the dry air m
#
a
can be found as in previous examples using the volumetric flow rate and
specific volume of the dry air. Thus
m
#
a
5
1AV2
1
y
a1
The specific volume of the dry air can be evaluated from the ideal gas equation of state. The result is y
a1
5 14.2 ft
3
/lb
(dry air). Inserting values, the mass flow rate of the dry air is
m
#
a
5
5000 ft
3
/
min
14.2 ft
3
/
lb1dry air2
5 352.1
lb1dry air
2
min
To find the humidity ratio v
2
, reduce the steady-state forms of the mass and energy rate balances using
assumption 1 to obtain
0 5
1
m
#
a
h
a1
1 m
#
v1
h
v1
2
1 m
#
w
h
w
2
1
m
#
a
h
a2
1 m
#
v2
h
v2
2
With the same reasoning as in previous examples, this can be expressed as the following special form of Eq. 12.55:
0 5 1h
a
1 vh
g
2
1
1 1v
2
2 v
1
2h
f
2 1h
a
1 vh
g
2
2
(a)
where h
f
denotes the specific enthalpy of the water entering the control volume at 708F. Solving for v
2
v
2
5
h
a1
2 h
a2
1 v
1
1
h
g1
2 h
f
2
h
g
2
2 h
f
5
c
pa
1
T
1
2 T
2
2
1 v
1
1
h
g1
2 h
f
2
h
g
2
2 h
f
where c
pa
5 0.24 Btu/lb ? 8R. With h
f
, h
g1
, and h
g2
from Table A-2E
v
2
5
0.24
1
100 2 70
2
1 0.00405
1
1105 2 38.1
2
1
1092 2 38.1
2
5 0.0109
lb
1
vapor
2
lb
1
dry air
2
Substituting values for m
#
a
, v
1
, and v
2
into the expression for m
#
w
m
#
w
5 c352.1
lb1dry a
ir2
min
`
60 m
in
1 h
`d 10.0109 2 0.004052
lb1water
2
lb
1
dry air
2
5 144.7
l
b
1
water
2
h
(b) The relative humidity of the moist air at the exit can be determined using Eq. 12.44. The partial pressure of
the water vapor required by this expression can be found by solving Eq. 12.43 to obtain
p
v2
5
v
2
p
v
2
1 0.622
Inserting values
p
v2
5
10.01092114.696 lbf
/
in.
2
2
1
0.0109 1 0.622
2
5 0.253 lbf
/
in.
2
➊
c12IdealGasMixtureandPsychromet754 Page 754 6/29/10 11:58:05 AM user-s146 c12IdealGasMixtureandPsychromet754 Page 754 6/29/10 11:58:05 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
At 708F, the saturation pressure is 0.3632 lbf/in.
2
Thus, the relative humidity at the exit is
f
2
5
0.253
0
.
363
2
5 0.697169.7%2
Alternative Psychrometric Chart Solution: Since the underlined term in Eq. (a)
is much smaller than either of the moist air enthalpies, the enthalpy of the moist
air remains nearly constant, and thus evaporative cooling takes place at a nearly
constant wet-bulb temperature. See Fig. 12.13b and the accompanying discus-
sion. Using this approach with the psychrometric chart, Fig. A-9E, determine
humidity ratio and relative humidity at the exit, and compare with the previ-
ously determined values. The details are left as an exercise.
➊
A constant value of the specific heat c
pa
has been used here to evaluate the
term (h
a1
2 h
a2
). As shown in previous examples, this term can be evaluated
alternatively using the ideal gas table for air.
Using steam table data, what is the dew point temperature at
the exit, in 8F? Ans. 59.68F.
Ability to…
❑
apply psychrometric termi-
nology and principles.
❑ apply mass and energy
balances for an evaporative
cooling process in a control
volume at steady state.
❑
retrieve property data for
dry air and water.
✓
Skills Developed
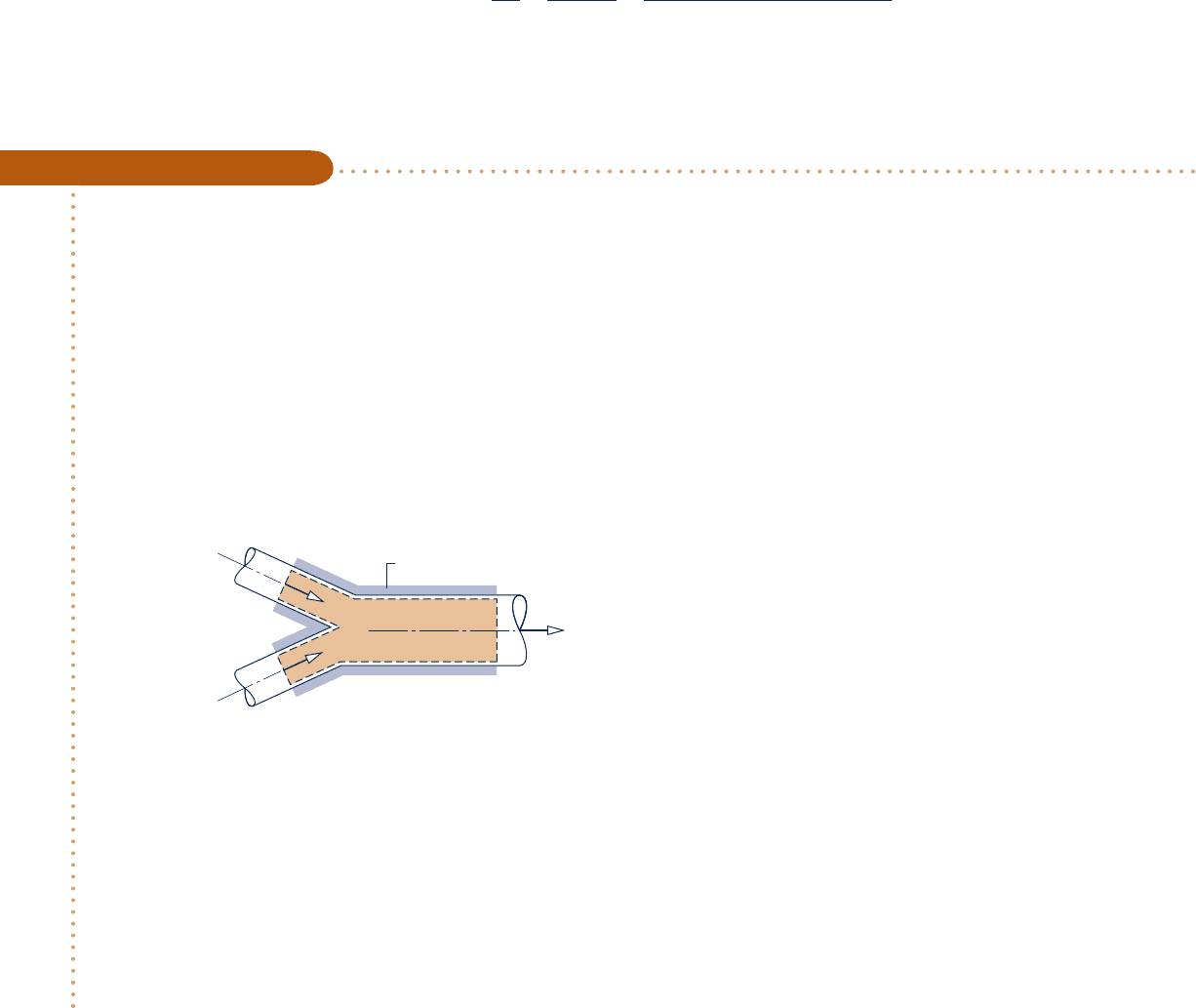
12.8.6 Adiabatic Mixing of Two Moist Air Streams
A common process in air-conditioning systems is the mixing of moist air streams, as
shown in Fig. 12.14. The objective of the thermodynamic analysis of such a process
is normally to fix the flow rate and state of the exiting stream for specified flow rates
and states of each of the two inlet streams. The case of adiabatic mixing is governed
by Eqs. 12.56 to follow.
The mass rate balances for the dry air and water vapor at steady state are, respec-
tively,
m
#
a1
1 m
#
a2
5 m
#
a3
1
dry air
2
m
#
v1
1 m
#
v2
5 m
#
v3
1
water vapor
2
(12.56a)
12.8 Analyzing Air-Conditioning Processes 755
(b)
T
2
T
3
T
1
w
1
w
3
w
2
1
3
Mixture enthalpy
per unit mass
of dry air
2
(h
a
+ w h
g
)
2
(h
a
+ w h
g
)
3
(h
a
+ w h
g
)
1
3
Insulation
1 m
·
a1
, T
1
,
1
ω
2 m
·
a2
, T
2
,
2
ω
m
·
a3
T
3
3
ω
(a)
Fig. 12.14 Adiabatic mixing of two moist air streams. (a) Equipment representation.
(b) Psychrometric chart representation.
c12IdealGasMixtureandPsychromet755 Page 755 6/29/10 11:58:08 AM user-s146 c12IdealGasMixtureandPsychromet755 Page 755 6/29/10 11:58:08 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
756 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
With m
#
v
5 vm
#
a
, the water vapor mass balance becomes
v
1
m
#
a1
1 v
2
m
#
a2
5 v
3
m
#
a3
1
water vapor
2
(12.56b)
Assuming
Q
#
cv
5 W
#
cv
5 0 and ignoring the effects of kinetic and potential energy,
the energy rate balance reduces at steady state to
m
#
a1
1
h
a1
1 v
1
h
g1
2
1 m
#
a2
1
h
a2
1 v
2
h
g2
2
5 m
#
a3
1
h
a3
1 v
3
h
g3
2
(12.56c)
where the enthalpies of the entering and exiting water vapor are evaluated as the
saturated vapor values at the respective dry-bulb temperatures.
If the inlet flow rates and states are known, Eqs. 12.56 are three equations in three
unknowns: m
#
a3
, v
3
, and
1
h
a3
1 v
3
h
g
3
2
. The solution of these equations is illustrated by
Example 12.14.
Let us also consider how Eqs. 12.56 can be solved geometrically with the psychro-
metric chart: Using Eq. 12.56a to eliminate m
#
a3
, the mass flow rate of dry air at 3,
from Eqs. 12.56b and 12.56c, we get
m
#
a1
m
#
a2
5
v
3
2 v
2
v
1
2 v
3
5
1
h
a3
1 v
3
h
g3
2
2
1
h
a2
1 v
2
h
g2
2
1h
a1
1 v
1
h
g1
22 1h
a3
1 v
3
h
g3
2
(12.57)
From the relations of Eqs. 12.57, we conclude that on a psychrometric chart state 3 of
the mixture lies on a straight line connecting states 1 and 2 of the two streams before
mixing (see end-of-chapter Prob. 12.93). This is shown in Fig. 12.14b.
Analyzing Adiabatic Mixing of Two Moist Air Streams
c c c c EXAMPLE 12.14 c
A stream consisting of 142 m
3
/min of moist air at a temperature of 58C and a humidity ratio of 0.002 kg(vapor)/
kg(dry air) is mixed adiabatically with a second stream consisting of 425 m
3
/min of moist air at 248C and 50%
relative humidity. The pressure is constant throughout at 1 bar. Determine (a) the humidity ratio and (b) the
temperature of the exiting mixed stream, in 8C.
SOLUTION
Known:
A moist air stream at 58C, v 5 0.002 kg(vapor)/kg(dry air), and a volumetric flow rate of 142 m
3
/min is
mixed adiabatically with a stream consisting of 425 m
3
/min of moist air at 248C and f 5 50%.
Find: Determine the humidity ratio and the temperature, in 8C, of the mixed stream exiting the control volume.
Schematic and Given Data:
3
1
2
kg (vapor)
__________
kg (dry air)
(AV)
1
T
1
1
= 142 m
3
/min
= 5°C
= 0.002
ω
(AV)
2
T
2
2
= 425 m
3
/min
= 24°C
= 50%
φ
T
3
= ?
3
= ?
ω
Insulation
Fig. E12.14
Engineering Model:
1.
The control volume shown in the accompanying
figure operates at steady state. Changes in kinetic
and potential energy can be neglected and W
#
cv
5 0.
2. There is no heat transfer with the surroundings.
3. The pressure remains constant throughout at 1 bar.
4. The moist air streams are regarded as ideal gas
mixtures adhering to the Dalton model.
Analysis:
(a)
The humidity ratio v
3
can be found by means of mass rate balances for the dry air and water vapor,
respectively
c12IdealGasMixtureandPsychromet756 Page 756 6/29/10 11:58:11 AM user-s146 c12IdealGasMixtureandPsychromet756 Page 756 6/29/10 11:58:11 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
m
#
a1
1 m
#
a2
5 m
#
a3
1
dry air
2
m
#
v1
1 m
#
v2
5 m
#
v3
1
water vapor
2
With m
#
v1
5 v
1
m
#
a1
, m
#
v2
5 v
2
m
#
a2
, and m
#
v3
5 v
3
m
#
a3
, the second of these balances becomes (Eq. 12.56b)
v
1
m
#
a1
1 v
2
m
#
a2
5 v
3
m
#
a3
Solving
v
3
5
v
1
m
#
a1
1 v
2
m
#
a2
m
#
a3
Since m
#
a3
5 m
#
a1
1 m
#
a2
, this can be expressed as
v
3
5
v
1
m
#
a1
1 v
2
m
#
a2
m
#
a1
1 m
#
a2
To determine
v
3
requires values for
v
2
, m
#
a
1
, and m
#
a
2
. The mass flow rates of the dry air, m
#
a
1
and m
#
a
2
, can be
found as in previous examples using the given volumetric flow rates
m
#
a1
5
1
AV
2
1
y
a
1
,
m
#
a2
5
1
AV
2
2
y
a
2
The values of y
a
1
, y
a
2
, and
v
2
are readily found from the psychrometric chart, Fig. A-9. Thus, at v
1
5 0.002 and
T
1
5 58C, y
a1
5 0.79 m
3
/
kg
1
dry air
2
. At f
2
5 50% and T
2
5 248C, y
a
2
5 0.855 m
3
/
k
g1
dr
y
air
2
and v
2
5
0
.
0094
. The
mass flow rates of the dry air are then m
#
a
1
5 180 k
g1
dr
y
air
2
/
min and m
#
a
2
5 497 k
g1
dr
y
air
2
/
min. Inserting values
into the expression for
v
3
v
3
5
1
0.002
21
180
2
1
1
0.0094
21
497
2
180 1 497
5 0.0074
kg
1
vapor
2
kg
1
dry air
2
(b) The temperature
T
3
of the exiting mixed stream can be found from an energy rate balance. Reduction of the
energy rate balance using assumptions 1 and 2 gives (Eq. 12.56c)
m
?
a1
1h
a
1 vh
g
2
1
1 m
?
a2
1h
a
1 vh
g
2
2
5 m
?
a3
1h
a
1 vh
g
2
3
(a)
Solving
1h
a
1 vh
g
2
3
5
m
#
a1
1
h
a
1 vh
g
2
1
1 m
#
a2
1
h
a
1 vh
g
2
2
m
#
a1
1 m
#
a2
(b)
With
1
h
a
1 vh
g
2
1
5 10 kJ
/
kg
1
dry air
2
and
1
h
a
1 vh
g
2
2
5 47.8 kJ
/
kg
1
dry air
2
from Fig. A-9 and other known values
1h
a
1 vh
g
2
3
5
180
1
10
2
1 497
1
47.8
2
180 1 497
5 37.7
kJ
kg
1
dry air
2
➊
This value for the enthalpy of the moist air at the exit, together with the previously determined value for
v
3
,
fixes the state of the exiting moist air. From inspection of Fig. A-9, T
3
5 198C.
Alternative Solutions:
The use of the psychrometric chart facilitates the solution for T
3
. Without the chart, an iterative solution of Eq. (b)
using data from Tables A-2 and A-22 could be used. Alternatively, T
3
can be determined using the following IT
program, where
f
2
is denoted as phi2, the volumetric flow rates at 1 and 2 are denoted as AV1
and AV2, respec-
tively, and so on.
// Given data
T1 5 5 // 8C
w1 5 0.002 // kg(vapor) / kg(dry air)
AV1 5 142 // m
3
/min
T2 5 24 // 8C
phi2 5 0.5
AV2 5 425 // m
3
/min
p 5 1 // bar
12.8 Analyzing Air-Conditioning Processes 757
c12IdealGasMixtureandPsychromet757 Page 757 6/30/10 8:20:42 PM user-s146 c12IdealGasMixtureandPsychromet757 Page 757 6/30/10 8:20:42 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
758 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
// Mass balances for water vapor and dry air:
w1 * mdota1 1 w2 * mdota2 5 w3 * mdota3
mdota1 1 mdota2 5 mdota3
// Evaluate mass flow rates of dry air
mdota1 5 AV1 / va1
➋
va1 5 va_Tw(T1, w1, p)
mdota2 5 AV2 / va2
va2 5 va_Tphi(T2, phi2, p)
// Determine w2
w2 5 w_Tphi(T2, phi2, p)
// The energy balance, Eq. (a), reads
mdota1 * h1 1 mdota2 * h2 5 mdota3 * h3
h1 5 ha_Tw(T1, w1)
h2 5 ha_Tphi(T2, phi2, p)
h3 5 ha_Tw(T3, w3)
Using the Solve button, the result is T
3
5 19.018C and v
3
5 0.00745 k
g
(vapor)/
kg (dry air), which agree with the psychrometric chart solution.
➊
A solution using the geometric approach based on Eqs. 12.57 is left as an
exercise.
➋
Note the use here of special Moist Air functions listed in the Properties
menu of IT.
Using the psychrometric chart, what is the relative humidity
at the exit? Ans. < 53%.
Ability to…
❑
apply psychrometric termi-
nology and principles.
❑
apply mass and energy
balances for an adiabatic
mixing process of two moist
air streams in a control
volume at steady state.
❑
retrieve property data
for moist air using the
psychrometric chart.
❑
apply IT for psychrometric
analysis.
✓
Skills Developed
12.9 Cooling Towers
Power plants invariably discharge considerable energy to their surroundings by heat
transfer (Chap. 8). Although water drawn from a nearby river or lake can be
employed to carry away this energy, cooling towers provide an alternative in loca-
tions where sufficient cooling water cannot be obtained from natural sources or
where concerns for the environment place a limit on the temperature at which
cooling water can be returned to the surroundings. Cooling towers also are fre-
quently employed to provide chilled water for applications other than those involv-
ing power plants.
Cooling towers can operate by natural or forced convection. Also they may be
counterflow, cross-flow, or a combination of these. A schematic diagram of a forced-
convection, counterflow cooling tower is shown in Fig. 12.15. The warm water to be
cooled enters at 1 and is sprayed from the top of the tower. The falling water usually
passes through a series of baffles intended to keep it broken up into fine drops to
promote evaporation. Atmospheric air drawn in at 3 by the fan flows upward, coun-
ter to the direction of the falling water droplets. As the two streams interact, a frac-
tion of the entering liquid water stream evaporates into the moist air, which exits at
4 with a greater humidity ratio than the incoming moist air at 3, while liquid water
exits at 2 with a lower temperature than the water entering at 1. Since some of the
incoming water is evaporated into the moist air stream, an equivalent amount of
c12IdealGasMixtureandPsychromet758 Page 758 6/30/10 8:21:23 PM user-s146 c12IdealGasMixtureandPsychromet758 Page 758 6/30/10 8:21:23 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
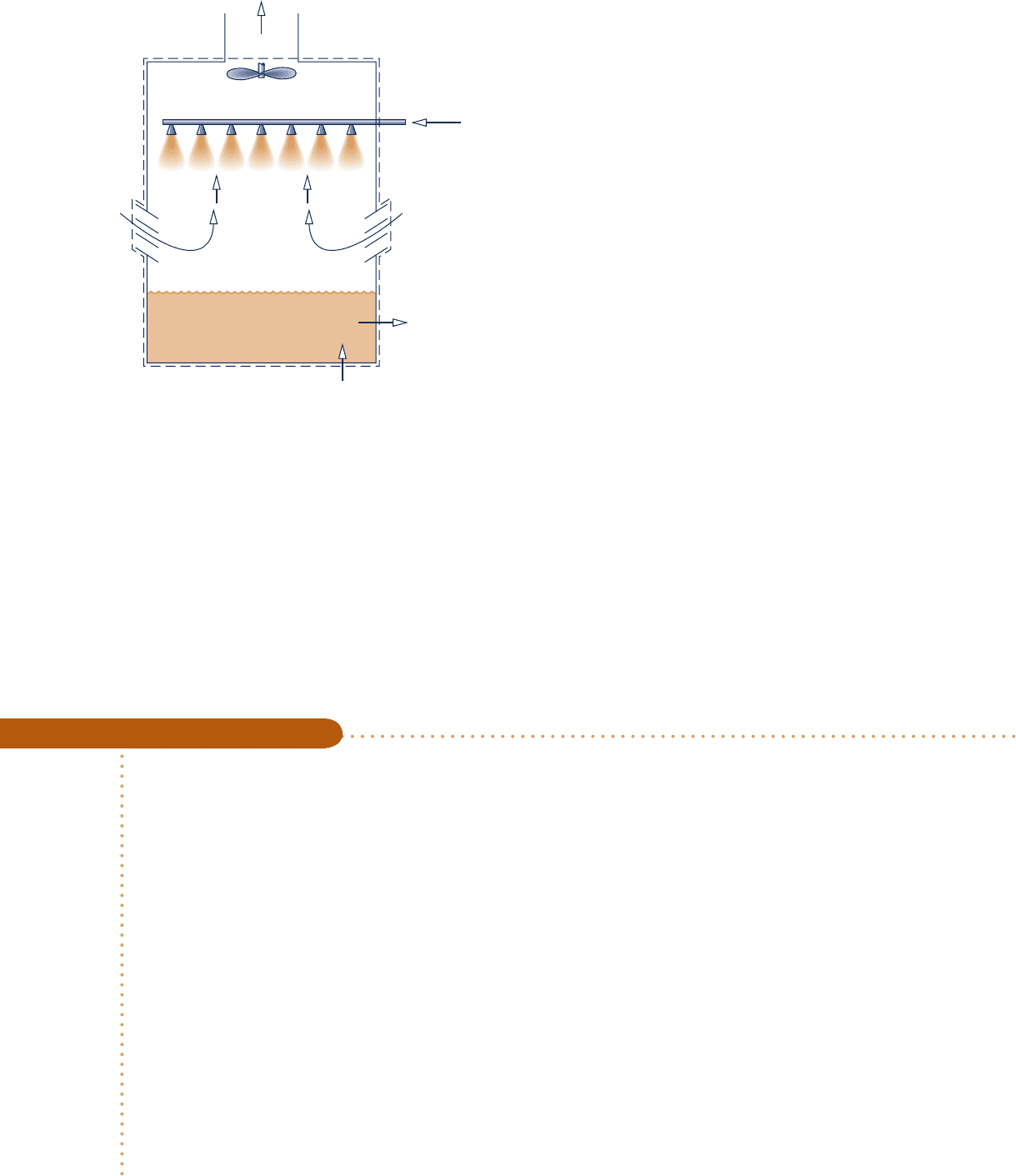
makeup water is added at 5 so that the return mass flow rate of the cool water equals
the mass flow rate of the warm water entering at 1.
For operation at steady state, mass balances for the dry air and water and an
energy balance on the overall cooling tower provide information about cooling tower
performance. In applying the energy balance, heat transfer with the surroundings is
usually neglected. The power input to the fan of forced-convection towers also may
be negligible relative to other energy rates involved. The example to follow illustrates
the analysis of a cooling tower using conservation of mass and energy together with
property data for the dry air and water.
Fan
Warm water inlet
T
1
, m
·
w
1
Return water
m
·
w
T
2
< T
1
2
4
5 Makeup
water
3
Liquid
Discharged moist air
m
·
a
, T
4
,
4
>
3
ωω
Atmospheric ai
r
m
·
a
, T
3
,
3
ω
Fig. 12.15 Schematic of a cooling
tower.
c c c c EXAMPLE 12.15 c
Determining Mass Flow Rates for a Power Plant Cooling Tower
Water exiting the condenser of a power plant at 388C enters a cooling tower with a mass flow rate of 4.5 3 10
7
kg/h.
A stream of cooled water is returned to the condenser from a cooling tower with a temperature of 308C and
the same flow rate. Makeup water is added in a separate stream at 208C. Atmospheric air enters the cooling
tower at 258C and 35% relative humidity. Moist air exits the tower at 358C and 90% relative humidity. Determine
the mass flow rates of the dry air and the makeup water, in kg/h. The cooling tower operates at steady state.
Heat transfer with the surroundings and the fan power can each be neglected, as can changes in kinetic and
potential energy. The pressure remains constant throughout at 1 atm.
SOLUTION
Known:
A liquid water stream enters a cooling tower from a condenser at 388C with a known mass flow rate. A
stream of cooled water is returned to the condenser at 308C and the same flow rate. Makeup water is added at
208C. Atmospheric air enters the tower at 258C and f 5 35%. Moist air exits the tower at 358C and f 5 90%.
Find: Determine the mass flow rates of the dry air and the makeup water, in kg/h.
12.9 Cooling Towers 759
c12IdealGasMixtureandPsychromet759 Page 759 6/29/10 11:58:21 AM user-s146 c12IdealGasMixtureandPsychromet759 Page 759 6/29/10 11:58:21 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
760 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
Schematic and Given Data:
Engineering Model:
1.
The control volume shown in the accompanying figure
operates at steady state. Heat transfer with the surround-
ings can be neglected, as can changes in kinetic and
potential energy; also W
#
cv
5 0.
2. To evaluate specific enthalpies, each liquid stream is
regarded as a saturated liquid at the corresponding speci-
fied temperature.
3. The moist air streams are regarded as ideal gas mixtures
adhering to the Dalton model.
4. The pressure is constant throughout at 1 atm.
Fig. E12.15
1
3
4
2
5
Makeup water
T
5
= 20°C
Atmospheric air
T
3
= 25°C,
3
= 35%
φ
Liquid water, T
1
= 38°C
m
·
1
= 4.5 × 10
7
kg/h
Liquid water, T
2
= 30°C
m
·
2
= 4.5 × 10
7
kg/h
Moist air
φ
T
4
= 35°C
4
= 90%
Analysis: The required mass flow rates can be found from mass and energy rate balances. Mass balances for the
dry air and water individually reduce at steady state to
m
#
a3
5 m
#
a4
1
dry air
2
m
#
1
1 m
#
5
1 m
#
v3
5 m
#
2
1 m
#
v4
1
water
2
The common mass flow rate of the dry air is denoted as m
#
a
. Since m
#
1
5 m
#
2
, the second of these equations
becomes
m
#
5
5 m
#
v4
2 m
#
v3
With m
#
v3
5 v
3
m
#
a
and m
#
v
4
5 v
4
m
#
a
m
#
5
5 m
#
a
1
v
4
2 v
3
2
Accordingly, the two required mass flow rates, m
#
a
and m
#
5
, are related by this equation. Another equation relating
the flow rates is provided by the energy rate balance.
Reducing the energy rate balance with assumption 1 results in
0 5 m
#
1
h
w1
1
1
m
#
a
h
a3
1 m
#
v3
h
v3
2
1 m
#
5
h
w5
2 m
#
2
h
w2
2
1
m
#
a
h
a4
1 m
#
v4
h
v4
2
Evaluating the enthalpies of the water vapor as the saturated vapor values at the respective temperatures and
the enthalpy of each liquid stream as the saturated liquid enthalpy at each respective temperature, the energy
rate equation becomes
0 5 m
#
1
h
f1
1
1
m
#
a
h
a3
1 m
#
v3
h
g
3
2
1 m
#
5
h
f5
2 m
#
2
h
f2
2
1
m
#
a
h
a4
1 m
#
v4
h
g
4
2
Introducing m
#
1
5 m
#
2
, m
#
5
5 m
#
a
1
v
4
2 v
3
2
, m
#
v3
5 v
3
m
#
a
, and m
#
v
4
5 v
4
m
#
a
and solving for m
#
a
m
#
a
5
m
#
1
1
h
f1
2 h
f2
2
h
a4
2 h
a3
1 v
4
h
g
4
2 v
3
h
g
3
2 1v
4
2 v
3
2h
f5
(a)
The humidity ratios
v
3
and
v
4
required by this expression can be determined from Eq. 12.43, using the partial
pressure of the water vapor obtained with the respective relative humidity. Thus, v
3
5 0.00688 kg(vapor)/kg(dry
air) and v
4
5 0.0327 kg(vapor)/kg(dry air).
With enthalpies from Tables A-2 and A-22, as appropriate, and the known values for v
3
,
v
4
, and m
#
1
, the expres-
sion for m
#
a
becomes
m
?
a
5
14.5 3 10
7
21159.21 2 125.792
1
308.2 2 298.2
2
1
1
0.0327
21
2565.3
2
2
1
0.00688
21
2547.2
2
2
1
0.0258
21
83.96
2
5 2.03 3 10
7
k
g
/
h
Finally, inserting known values into the expression for m
#
5
results in
m
#
5
5 12.03 3 10
7
210.0327 2 0.0068825 5.24 3 10
5
kg
/
h
c12IdealGasMixtureandPsychromet760 Page 760 6/29/10 11:58:24 AM user-s146 c12IdealGasMixtureandPsychromet760 Page 760 6/29/10 11:58:24 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
Alternative Psychrometric Chart Solution: Equation (a) can be rearranged to
read
m
#
a
5
m
#
1
1h
f1
2 h
f2
2
1h
a4
1 v
4
h
g4
22 1h
a3
1 v
3
h
g3
22 1v
4
2 v
3
2h
f5
The specific enthalpy terms h
f1
, h
f2
, and h
f5
are obtained from Table A-2, as
above. The underlined terms and v
3
and v
4
can be obtained by inspection of a
psychrometric chart from the engineering literature providing data at states
3 and 4. Figure A-9 does not suffice in this application at state 4. The details
are left as an exercise.
Using steam table data, determine the partial pressure of the
water vapor in the entering moist air stream, p
v3
, in bar. Ans. 0.0111 bar.
Ability to…
❑
apply psychrometric termi-
nology and principles.
❑
apply mass and energy
balances for a cooling tower
process in a control volume
at steady state.
❑
retrieve property data for
dry air and water.
✓Skills Developed
In this chapter we have applied the principles of thermodynamics
to systems involving ideal gas mixtures, including the special
case of psychrometric applications involving air–water vapor mix-
tures, possibly in the presence of liquid water. Both closed sys-
tem and control volume applications are presented.
The first part of the chapter deals with general ideal gas mix-
ture considerations and begins by describing mixture composi-
tion in terms of the mass fractions or mole fractions. The Dalton
model, which brings in the partial pressure concept, is then intro-
duced for the p–y–T relation of ideal gas mixtures.
Means are also introduced for evaluating the enthalpy, inter-
nal energy, and entropy of a mixture by adding the contribution
of each component at its condition in the mixture. Applications
are considered where ideal gas mixtures undergo processes at
constant composition as well as where ideal gas mixtures are
formed from their component gases.
In the second part of the chapter, we study psychrometrics.
Special terms commonly used in psychrometrics are intro-
duced, including moist air, humidity ratio, relative humidity,
mixture enthalpy, and the dew point, dry-bulb, and wet-bulb
temperatures. The psychrometric chart, which gives a graphi-
cal representation of important moist air properties, is intro-
duced. The principles of conservation of mass and energy are
formulated in terms of psychrometric quantities, and typical
air-conditioning applications are considered, including dehu-
midification and humidification, evaporative cooling, and mix-
ing of moist air streams. A discussion of cooling towers is also
provided.
The following list provides a study guide for this chapter.
When your study of the text and end-of-chapter exercises has
been completed, you should be able to
c
write out the meanings of the terms listed in the margin
throughout the chapter and understand each of the related
concepts. The subset of key concepts listed below is particu-
larly important.
c
describe mixture composition in terms of mass fractions or
mole fractions.
c
relate pressure, volume, and temperature of ideal gas mix-
tures using the Dalton model, and evaluate U, H, c
y
, c
p
, and
S of ideal gas mixtures in terms of the mixture composition
and the respective contribution of each component.
c
apply the conservation of mass and energy principles and the
second law of thermodynamics to systems involving ideal gas
mixtures.
For psychrometric applications, you should be able to
c
evaluate the humidity ratio, relative humidity, mixture enthalpy,
and dew point temperature.
c
use the psychrometric chart.
c
apply the conservation of mass and energy principles and the
second law of thermodynamics to analyze air-conditioning
processes and cooling towers.
c CHAPTER SUMMARY AND STUDY GUIDE
c KEY ENGINEERING CONCEPTS
mass fraction, p. 706
gravimetric analysis, p. 706
mole fraction, p. 706
molar (volumetric) analysis, p. 707
apparent molecular weight, p. 707
Dalton model, p. 710
partial pressure, p. 710
psychrometrics, p. 727
moist air, p. 727
humidity ratio, p. 728
relative humidity, p. 729
mixture enthalpy, p. 729
dew point temperature, p. 731
dry-bulb temperature, p. 738
wet-bulb temperature, p. 738
psychrometric chart, p. 740
Key Engineering Concepts 761
c12IdealGasMixtureandPsychromet761 Page 761 6/29/10 11:58:30 AM user-s146 c12IdealGasMixtureandPsychromet761 Page 761 6/29/10 11:58:30 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

762 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
c KEY EQUATIONS
Ideal Gas Mixtures: General Considerations
m
f
i
5 m
i
/
m (12.3) p. 706
Analysis in terms of mass fractions
1 5
a
j
i
51
mf
i
(12.4) p. 706
y
i
5 n
i
/
n (12.6) p. 706
1 5
a
j
i
51
y
i
(12.7) p. 707
Analysis in terms of mole fractions
M 5
a
j
i
51
y
i
M
i
(12.9) p. 707
Apparent molecular weight
p
i
5
y
i
p
(12.12) p. 710
p 5
a
j
i
51
p
i
(12.13) p. 710
u 5
a
j
i
51
y
i
u
i
(12.21) p. 712
h 5
a
j
i
51
y
i
h
i
(12.22) p. 712
s 5
a
j
i
51
y
i
s
i
(12.27) p. 713
c
y
5
a
j
i
51
y
i
c
y,i
(12.23) p. 712
Mixture specific heats on a molar basis
c
p
5
a
j
i
51
y
i
c
p,i
(12.24) p. 712
Psychrometric Applications
v 5
m
v
m
a
5 0.622
p
v
p
2
p
v
(12.42, 12.43) p. 729 Humidity ratio
f 5
p
v
p
g
b
T,
p
(12.44) p. 729 Relative humidity
H
m
a
5 h
a
1 vh
v
(12.46) p. 729 Mixture enthalpy per unit mass of dry air
Partial pressure of component i and relation to mixture
pressure p
Internal energy, enthalpy, and entropy per mole of mixture.
—
u
i
and
—
h
i
evaluated at mixture temperature T.
—
s
i
evaluated at T and
partial pressure p
i
.
c EXERCISES: THINGS ENGINEERS THINK ABOUT
1. In an equimolar mixture of O
2
and N
2
, are the mass fractions
equal? Explain.
2. If two different ideal gases mix spontaneously, is the process
irreversible? Explain.
3. Which component of the fuel–air mixture in a cylinder of an
automobile engine would have the greater mass fraction?
4. A rigid, insulated container is divided into two compartments
by a partition, and each compartment contains air at the
c12IdealGasMixtureandPsychrome762 Page 762 7/29/10 5:03:44 PM user-s146 c12IdealGasMixtureandPsychrome762 Page 762 7/29/10 5:03:44 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
