Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.


equilibrium mixture. Will lowering the pressure while keeping
the temperature fixed increase or decrease the amount of
CO
2
present? Explain.
14.19 An equimolar mixture of CO and H
2
O(g) reacts to form
an equilibrium mixture of CO
2
, CO, H
2
O, and H
2
at 1727°C,
1 atm.
(a) Will lowering the temperature increase or decrease the
amount of H
2
present? Explain.
(b) Will decreasing the pressure while keeping the temperature
constant increase or decrease the amount of H
2
present?
Explain.
14.20 Determine the temperature, in K, at which 9% of
diatomic hydrogen (H
2
) dissociates into monatomic hydrogen
(H) at a pressure of 10 atm. For a greater percentage of H
2
at the same pressure, would the temperature be higher or
lower? Explain.
14.21 Two kmol of CO
2
dissociate to form an equilibrium
mixture of CO
2
, CO, and O
2
in which 1.8 kmol of CO
2
is
present. Plot the temperature of the equilibrium mixture, in
K, versus the pressure p for 0.5 # p # 10 atm.
14.22 One kmol of H
2
O(g) dissociates to form an equilibrium
mixture of H
2
O(g), H
2
, and O
2
in which the amount of water
vapor present is 0.95 kmol. Plot the temperature of the
equilibrium mixture, in K, versus the pressure p for 1 # p #
10 atm.
14.23 A vessel initially containing 1 kmol of H
2
O(g) and x kmol
of N
2
forms an equilibrium mixture at 1 atm consisting of
H
2
O(g), H
2
, O
2
, and N
2
in which 0.5 kmol of H
2
O(g) is present.
Plot x versus the temperature T for 3000 # T # 3600 K.
14.24 A vessel initially containing 2 lbmol of N
2
and 1 lbmol of
O
2
forms an equilibrium mixture at 1 atm consisting of N
2
,
O
2
, and NO. Plot the amount of NO formed versus temperature
T for 3600 # T # 6300°R.
14.25 A vessel initially containing 1 kmol of CO and 4.76 kmol
of dry air forms an equilibrium mixture of CO
2
, CO, O
2
, and
N
2
at 3000 K, 1 atm. Determine the equilibrium composition.
14.26 A vessel initially containing 1 kmol of O
2
, 2 kmol of N
2
,
and 1 kmol of Ar forms an equilibrium mixture of O
2
, N
2
,
NO, and Ar at 2727°C, 1 atm. Determine the equilibrium
composition.
14.27 One kmol of CO and 0.5 kmol of O
2
react to form a
mixture at temperature T and pressure p consisting of CO
2
,
CO, and O
2
. If 0.35 kmol of CO is present in an equilibrium
mixture when the pressure is 1 atm, determine the amount
of CO present in an equilibrium mixture at the same
temperature if the pressure were 10 atm.
14.28 A vessel initially contains 1 kmol of H
2
and 4 kmol of
N
2
. An equilibrium mixture of H
2
, H, and N
2
forms at 3000 K,
1 atm. Determine the equilibrium composition. If the pressure
were increased while keeping the temperature fixed, would
the amount of monatomic hydrogen in the equilibrium
mixture increase or decrease? Explain.
14.29 Dry air enters a heat exchanger. An equilibrium mixture
of N
2
, O
2
, and NO exits at 3882°F, 1 atm. Determine the mole
fraction of NO in the exiting mixture. Will the amount of
Problems: Developing Engineering Skills 883
NO increase or decrease as temperature decreases at fixed
pressure? Explain.
14.30 A gaseous mixture with a molar analysis of 20% CO
2
,
40% CO, and 40% O
2
enters a heat exchanger and is heated
at constant pressure. An equilibrium mixture of CO
2
, CO,
and O
2
exits at 3000 K, 1.5 bar. Determine the molar analysis
of the exiting mixture.
14.31 An ideal gas mixture with the molar analysis 30% CO,
10% CO
2
, 40% H
2
O, 20% inert gas enters a reactor operating
at steady state. An equilibrium mixture of CO, CO
2
, H
2
O,
H
2
, and the inert gas exits at 1 atm.
(a) If the equilibrium mixture exits at 1200 K, determine on
a molar basis the ratio of the H
2
in the equilibrium mixture
to the H
2
O in the entering mixture.
(b) If the mole fraction of CO present in the equilibrium
mixture is 7.5%, determine the temperature of the equilibrium
mixture, in K.
14.32 A mixture of 1 kmol CO and 0.5 kmol O
2
in a closed
vessel, initially at 1 atm and 300 K, reacts to form an equilibrium
mixture of CO
2
, CO, and O
2
at 2500 K. Determine the final
pressure, in atm.
14.33 Methane burns with 90% of theoretical air to form an
equilibrium mixture of CO
2
, CO, H
2
O(g), H
2
, and N
2
at 1000 K,
1 atm. Determine the composition of the equilibrium mixture,
per kmol of mixture.
14.34 Octane (C
8
H
18
) burns with air to form an equilibrium
mixture of CO
2
, H
2
, CO, H
2
O(g), and N
2
at 1700 K, 1 atm.
Determine the composition of the products, in kmol per
kmol of fuel, for an equivalence ratio of 1.2.
14.35 Acetylene gas (C
2
H
2
) at 25°C, 1 atm enters a reactor
operating at steady state and burns with 40% excess air
entering at 25°C, 1 atm, 80% relative humidity. An equilibrium
mixture of CO
2
, H
2
O, O
2
, NO, and N
2
exits at 2200 K, 0.9 atm.
Determine, per kmol of C
2
H
2
entering, the composition of the
exiting mixture.
Chemical Equilibrium and the Energy Balance
14.36 Carbon dioxide gas at 25°C, 5.1 atm enters a heat exchanger
operating at steady state. An equilibrium mixture of CO
2
, CO,
and O
2
exits at 2527°C, 5 atm. Determine, per kmol of CO
2
entering,
(a) the composition of the exiting mixture.
(b) the heat transfer to the gas stream, in kJ.
Neglect kinetic and potential energy effects.
14.37 Saturated water vapor at 15 lbf/in.
2
enters a heat exchanger
operating at steady state. An equilibrium mixture of H
2
O(g),
H
2
, and O
2
exits at 4040°F, 1 atm. Determine, per kmol of steam
entering,
(a) the composition of the exiting mixture.
(b) the heat transfer to the flowing stream, in Btu.
Neglect kinetic and potential energy effects.
14.38 Carbon at 25°C, 1 atm enters a reactor operating at steady
state and burns with oxygen entering at 127°C, 1 atm. The
entering streams have equal molar flow rates. An equilibrium
c14ChemicalandPhaseEquilibrium.883 Page 883 8/12/10 8:43:12 AM user-s146 c14ChemicalandPhaseEquilibrium.883 Page 883 8/12/10 8:43:12 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

884 Chapter 14
Chemical and Phase Equilibrium
mixture of CO
2
, CO, and O
2
exits at 2727°C, 1 atm. Determine,
per kmol of carbon,
(a) the composition of the exiting mixture.
(b) the heat transfer between the reactor and its surroundings,
in kJ.
Neglect kinetic and potential energy effects.
14.39 An equimolar mixture of carbon monoxide and water
vapor at 200°F, 1 atm enters a reactor operating at steady
state. An equilibrium mixture of CO
2
, CO, H
2
O(g), and H
2
exits at 2240°F, 1 atm. Determine the heat transfer between
the reactor and its surroundings, in Btu per lbmol of CO
entering. Neglect kinetic and potential energy effects.
14.40 Carbon dioxide (CO
2
) and oxygen (O
2
) in a 1:2 molar
ratio enter a reactor operating at steady state in separate
streams at 1 atm, 127°C and 1 atm, 277°C, respectively. An
equilibrium mixture of CO
2
, CO, and O
2
exits at 1 atm. If the
mole fraction of CO in the exiting mixture is 0.1, determine
the rate of heat transfer from the reactor, in kJ per kmol of
CO
2
entering. Ignore kinetic and potential energy effects.
14.41 Methane gas at 25°C, 1 atm enters a reactor operating
at steady state and burns with 80% of theoretical air entering
at 227°C, 1 atm. An equilibrium mixture of CO
2
, CO, H
2
O(g),
H
2
, and N
2
exits at 1427°C, 1 atm. Determine, per kmol of
methane entering,
(a) the composition of the exiting mixture.
(b) the heat transfer between the reactor and its surroundings,
in kJ.
Neglect kinetic and potential energy effects.
14.42 Gaseous propane (C
3
H
8
) at 25°C, 1 atm enters a reactor
operating at steady state and burns with 80% of theoretical air
entering separately at 25°C, 1 atm. An equilibrium mixture of
CO
2
, CO, H
2
O(g), H
2
, and N
2
exits at 1227°C, 1 atm. Determine
the heat transfer between the reactor and its surroundings, in
kJ per kmol of propane entering. Neglect kinetic and potential
energy effects.
14.43 Gaseous propane (C
3
H
8
) at 77°F, 1 atm enters a reactor
operating at steady state and burns with the theoretical amount
of air entering separately at 240°F, 1 atm. An equilibrium
mixture of CO
2
, CO, H
2
O(g) O
2
, and N
2
exits at 3140°F, 1 atm.
Determine the heat transfer between the reactor and its
surroundings, in Btu per lbmol of propane entering. Neglect
kinetic and potential energy effects.
14.44 One kmol of CO
2
in a piston–cylinder assembly, initially
at temperature T and 1 atm, is heated at constant pressure
until a final state is attained consisting of an equilibrium
mixture of CO
2
, CO, and O
2
in which the amount of CO
2
present is 0.422 kmol. Determine the heat transfer and the
work, each in kJ, if T is (a) 298 K, (b) 400 K.
14.45 Hydrogen gas (H
2
) at 25°C, 1 atm enters an insulated reactor
operating at steady state and reacts with 250% excess oxygen
entering at 227°C, 1 atm. The products of combustion exit at
1 atm. Determine the temperature of the products, in K, if
(a) combustion is complete.
(b) an equilibrium mixture of H
2
O, H
2
, and O
2
exits.
Kinetic and potential energy effects are negligible.
14.46 For each case of Problem 14.45, determine the rate of
entropy production, in kJ/K per kmol of H
2
entering. What
can be concluded about the possibility of achieving complete
combustion?
14.47 Hydrogen (H
2
) at 25°C, 1 atm enters an insulated reactor
operating at steady state and reacts with 100% of theoretical
air entering at 25°C, 1 atm. The products of combustion exit
at temperature T and 1 atm. Determine T, in K, if
(a) combustion is complete.
(b) an equilibrium mixture of H
2
O, H
2
, O
2
, and N
2
exits.
14.48 Methane at 77°F, 1 atm enters an insulated reactor
operating at steady state and burns with 90% of theoretical
air entering separately at 77°F, 1 atm. The products exit at 1
atm as an equilibrium mixture of CO
2
, CO, H
2
O(g) H
2
and
N
2
. Determine the temperature of the exiting products, in
°R. Kinetic and potential energy effects are negligible.
14.49 Carbon monoxide at 77°F, 1 atm enters an insulated
reactor operating at steady state and burns with air entering at
77°F, 1 atm. The products exit at 1 atm as an equilibrium mixture
of CO
2
, CO, O
2
, and N
2
. Determine the temperature of the
equilibrium mixture, in °R, if the combustion occurs with
(a) 80% of theoretical air.
(b) 100% of theoretical air.
Kinetic and potential energy effects are negligible.
14.50 For each case of Problem 14.49, determine the rate of
exergy destruction, in kJ per kmol of CO entering the
reactor. Let T
0
5 537°R.
14.51 Carbon monoxide at 25°C, 1 atm enters an insulated
reactor operating at steady state and burns with excess
oxygen (O
2
) entering at 25°C, 1 atm. The products exit at
2950 K, 1 atm as an equilibrium mixture of CO
2
, CO, and O
2
.
Determine the percent excess oxygen. Kinetic and potential
energy effects are negligible.
14.52 A gaseous mixture of carbon monoxide and the theoretical
amount of air at 260°F, 1.5 atm enters an insulated reactor
operating at steady state. An equilibrium mixture of CO
2
,
CO, O
2
and N
2
exits at 1.5 atm. Determine the temperature
of the exiting mixture, in °R. Kinetic and potential energy
effects are negligible.
14.53 Methane at 25°C, 1 atm enters an insulated reactor
operating at steady state and burns with oxygen entering at
127°C, 1 atm. An equilibrium mixture of CO
2
, CO, O
2
, and
H
2
O(g) exits at 3250 K, 1 atm. Determine the rate at which
oxygen enters the reactor, in kmol per kmol of methane.
Kinetic and potential energy effects are negligible.
14.54 Methane at 77°F, 1 atm enters an insulated reactor
operating at steady state and burns with the theoretical
amount of air entering at 77°F, 1 atm. An equilibrium mixture
of CO
2
, CO, O
2
, H
2
O(g), and N
2
exits at 1 atm.
(a) Determine the temperature of the exiting products, in °R.
(b) Determine the rate of exergy destruction, in Btu per
lbmol of methane entering, for T
0
5 537°R.
Kinetic and potential energy effects are negligible.
14.55 Methane gas at 25°C, 1 atm enters an insulated reactor
operating at steady state, where it burns with x times the
c14ChemicalandPhaseEquilibrium.884 Page 884 8/12/10 8:43:19 AM user-s146 c14ChemicalandPhaseEquilibrium.884 Page 884 8/12/10 8:43:19 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

theoretical amount of air entering at 25°C, 1 atm. An equilibrium
mixture of CO
2
, CO, O
2
, H
2
O, and N
2
exits at 1 atm. For selected
values of x ranging from 1 to 4, determine the temperature of
the exiting equilibrium mixture, in K. Kinetic and potential
energy effects are negligible.
14.56 A mixture consisting of 1 kmol of carbon monoxide (CO),
0.5 kmol of oxygen (O
2
), and 1.88 kmol of nitrogen (N
2
),
initially at 227°C, 1 atm, reacts in a closed, rigid, insulated
vessel, forming an equilibrium mixture of CO
2
, CO, O
2
, and
N
2
. Determine the final equilibrium pressure, in atm.
14.57 A mixture consisting of 1 kmol of CO and the theoretical
amount of air, initially at 60°C, 1 atm, reacts in a closed, rigid,
insulated vessel to form an equilibrium mixture. An analysis of
the products shows that there are 0.808 kmol of CO
2
, 0.192 kmol
of CO, and 0.096 kmol of O
2
present. The temperature of the
final mixture is measured as 2465°C. Check the consistency
of these data.
Using the van’t Hoff Equation, Ionization
14.58 Estimate the enthalpy of reaction at 2000 K, in kJ/kmol,
for CO
2
S
d
CO 1
1
2
O
2
using the van’t Hoff equation and
equilibrium constant data. Compare with the value obtained
for the enthalpy of reaction using enthalpy data.
14.59 Estimate the enthalpy of reaction at 2000 K, in kJ/kmol,
for H
2
O
S
d
H
2
1
1
2
O
2
, using the van’t Hoff equation and
equilibrium constant data. Compare with the value obtained
for the enthalpy of reaction using enthalpy data.
14.60 Estimate the equilibrium constant at 2800 K for
CO
2
S
d
CO 1
1
2
O
2
using the equilibrium constant at 2000 K
from Table A-27, together with the van’t Hoff equation and
enthalpy data. Compare with the value for the equilibrium
constant obtained from Table A-27.
14.61 Estimate the equilibrium constant at 2800 K for the
reaction H
2
O
S
d
H
2
1
1
2
O
2
using the equilibrium constant
at 2500 K from Table A-27, together with the van’t Hoff
equation and enthalpy data. Compare with the value for the
equilibrium constant obtained from Table A-27.
14.62 At 25°C, log
10
K 5 8.9 for C 1 2H
2
S
d
CH
4
. Assuming
that the enthalpy of reaction does not vary much with
temperature, estimate the value of 1og
10
K at 500°C.
14.63 If the ionization-equilibrium constants for Cs
S
d
Cs
1
1 e
2
at 1600 and 2000 K are K 5 0.78 and K 5 15.63, respectively,
estimate the enthalpy of ionization, in kJ/kmol, at 1800 K
using the van’t Hoff equation.
14.64 An equilibrium mixture at 2000 K, 1 atm consists of Cs,
Cs
1
, and e
2
. Based on 1 kmol of Cs present initially, determine
the percent ionization of cesium. At 2000 K, the ionization-
equilibrium constant for Cs
S
d
Cs
1
1 e
2
is K 5 15.63.
14.65 An equilibrium mixture at 18,000°R and pressure p
consists of Ar, Ar
1
, and e
2
. Based on 1 lbmol of neutral
argon present initially, plot the percent ionization of argon
versus pressure for 0.01 # p # 0.05 atm. At 18,000°R, the
ionization-equilibrium constant for Ar
S
d
Ar
1
1 e
2
is K 5
4.2 3 10
24
.
14.66 At 2000 K and pressure p, 1 kmol of Na ionizes to form
an equilibrium mixture of Na, Na
1
, and e
2
in which the
Problems: Developing Engineering Skills 885
amount of Na present is x kmol. Plot the pressure, in atm,
versus x for 0.2 # x # 0.3 kmol. At 2000 K, the ionization-
equilibrium constant for Na
S
d
Na
1
1 e
2
is K 5 0.668.
14.67 At 12,000 K and 6 atm, 1 kmol of N ionizes to form an
equilibrium mixture of N, N
1
, and e
2
in which the amount
of N present is 0.95 kmol. Determine the ionization-
equilibrium constant at this temperature for N
S
d
N
1
1 e
2
.
Considering Simultaneous Reactions
14.68 Carbon dioxide (CO
2
), oxygen (O
2
), and nitrogen (N
2
)
enter a reactor operating at steady state with equal molar
flow rates. An equilibrium mixture of CO
2
, O
2
, N
2
, CO, and
NO exits at 3000 K, 5 atm. Determine the molar analysis of
the equilibrium mixture.
14.69 An equimolar mixture of carbon monoxide and water
vapor enters a heat exchanger operating at steady state. An
equilibrium mixture of CO, CO
2
, O
2
, H
2
O(g), and H
2
exits
at 2227°C, 1 atm. Determine the molar analysis of the exiting
equilibrium mixture.
14.70 A closed vessel initially contains a gaseous mixture consisting
of 3 lbmol of CO
2
, 6 lbmol of CO, and 1 lbmol of H
2
. An
equilibrium mixture at 4220°F, 1 atm is formed containing CO
2
,
CO, H
2
O, H
2
, and O
2
. Determine the composition of the
equilibrium mixture.
14.71 Butane (C
4
H
10
) burns with 100% excess air to form an
equilibrium mixture at 1400 K, 20 atm consisting of CO
2
, O
2
,
H
2
O(g), N
2
, NO, and NO
2
. Determine the balanced reaction
equation. For N
2
1 2O
2
S
d
2NO
2
at 1400 K, K 5 8.4 3 10
210
.
14.72 One lbmol of H
2
O(g) dissociates to form an equilibrium
mixture at 5000°R, 1 atm consisting of H
2
O(g), H
2
, O
2
, and
OH. Determine the equilibrium composition.
14.73 Steam enters a heat exchanger operating at steady state.
An equilibrium mixture of H
2
O, H
2
, O
2
, H, and OH exits at
temperature T, 1 atm. Determine the molar analysis of the
exiting equilibrium mixture for
(a) T 5 2800 K.
(b) T 5 3000 K.
Considering Phase Equilibrium
14.74 For a two-phase liquid–vapor mixture of water at 100°C,
use tabulated property data to show that the specific Gibbs
functions of the saturated liquid and saturated vapor are equal.
Repeat for a two-phase liquid–vapor mixture of Refrigerant
134a at 20°C.
14.75 Using the Clapeyron equation, solve the following
problems from Chap. 11: (a) 11.32, (b) 11.33, (c) 11.34, (d) 11.35,
(e) 11.40.
14.76 A closed system at 20°C, 1 bar consists of a pure liquid
water phase in equilibrium with a vapor phase composed of
water vapor and dry air. Determine the departure, in percent,
of the partial pressure of the water vapor from the saturation
pressure of pure water at 20°C.
14.77 Derive an expression for estimating the pressure at
which graphite and diamond exist in equilibrium at 25°C
in terms of the specific volume, specific Gibbs function, and
c14ChemicalandPhaseEquilibrium.885 Page 885 8/12/10 8:43:26 AM user-s146 c14ChemicalandPhaseEquilibrium.885 Page 885 8/12/10 8:43:26 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
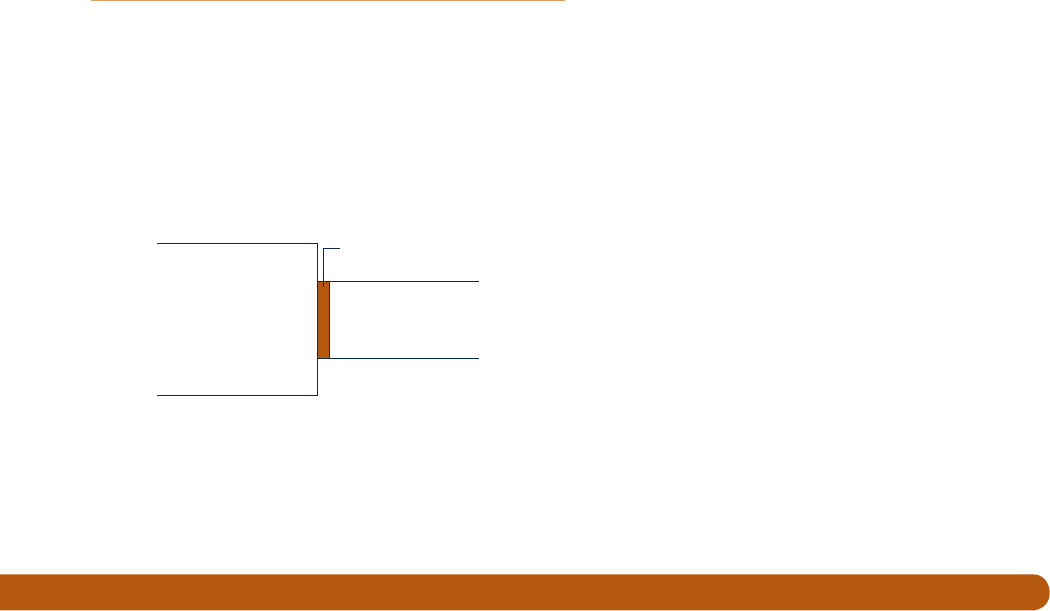
886 Chapter 14
Chemical and Phase Equilibrium
isothermal compressibility of each phase at 25°C, 1 atm.
Discuss.
14.78 An isolated system has two phases, denoted by A and B,
each of which consists of the same two substances, denoted by
1 and 2. Show that necessary conditions for equilibrium are
1. the temperature of each phase is the same, T
A
5 T
B
.
2. the pressure of each phase is the same, p
A
5 p
B
.
3. the chemical potential of each component has the same
value in each phase, m
A
1
5 m
B
1
, m
A
2
5 m
B
2
.
14.79 An isolated system has two phases, denoted by A and B,
each of which consists of the same two substances, denoted
by 1 and 2. The phases are separated by a freely moving thin
wall permeable only by substance 2. Determine the necessary
conditions for equilibrium.
14.80 Referring to Problem 14.79, let each phase be a binary
mixture of argon and helium and the wall be permeable only
to argon. If the phases initially are at the conditions tabulated
below, determine the final equilibrium temperature, pressure,
and composition in the two phases.
T(K) p(MPa) n(kmol) y
Ar
y
He
Phase A 300 0.2 6 0.5 0.5
Phase B 400 0.1 5 0.8 0.2
14.81 Figure P14.81 shows an ideal gas mixture at temperature
T and pressure p containing substance k, separated from a
gas phase of pure k at temperature T and pressure p9 by a
semipermeable membrane that allows only k to pass through.
Assuming the ideal gas model also applies to the pure gas
phase, determine the relationship between p and p9 for there
to be no net transfer of k through the membrane.
(a) one component?
(b) two components?
(c) three components?
14.83 Determine the number of degrees of freedom for systems
composed of
(a) ice and liquid water.
(b) ice, liquid water, and water vapor.
(c) liquid water and water vapor.
(d) water vapor only.
(e) water vapor and dry air.
(f) liquid water, water vapor, and dry air.
(g) ice, water vapor, and dry air.
(h) N
2
and O
2
at 20°C, 1 atm.
(i) a liquid phase and a vapor phase, each of which contains
ammonia and water.
(j) liquid mercury, liquid water, and a vapor phase of mercury
and water.
(k) liquid acetone and a vapor phase of acetone and N
2
.
14.84 Develop the phase rule for chemically reacting systems.
14.85 Apply the result of Problem 14.84 to determine the
number of degrees of freedom for the gas phase reaction:
CH
4
1 H
2
O
S
d
CO 1 3H
2
14.86 For a gas–liquid system in equilibrium at temperature T
and pressure p, Raoult’s law models the relation between the
partial pressure of substance i in the gas phase, p
i
, and the mole
fraction of substance i in the liquid phase, y
i
, as follows:
p
i
5 y
i
p
sat, i
1T2
where p
sat,i
(T) is the saturation pressure of pure i at
temperature T. The gas phase is assumed to form an ideal
gas mixture; thus, p
i
5 x
i
p where x
i
is the mole fraction of i in
the gas phase. Apply Raoult’s law to the following cases, which
are representative of conditions that might be encountered in
ammonia–water absorption systems (Sec. 10.5):
(a) Consider a two-phase, liquid–vapor ammonia–water
system in equilibrium at 20°C. The mole fraction of ammonia
in the liquid phase is 80%. Determine the pressure, in bar,
and the mole fraction of ammonia in the vapor phase.
(b) Determine the mole fractions of ammonia in the liquid
and vapor phases of a two-phase ammonia–water system in
equilibrium at 40°C, 12 bar.
14.82 What is the maximum number of homogeneous phases
that can exist at equilibrium for a system involving
c DESIGN & OPEN-ENDED PROBLEMS: EXPLORING ENGINEERING PRACTICE
14.1D Spark-ignition engine exhaust gases contain several
air pollutants including the oxides of nitrogen, NO and
NO
2
, collectively known as NO
x
. Additionally, the exhaust
gases may contain carbon monoxide (CO) and unburned
or partially burned hydrocarbons (HC). The pollutant
amounts actually present depend on engine design and
operating conditions, and they typically differ significantly
from values calculated on the basis of chemical equilibrium.
Discuss both the reasons for these discrepancies and
possible mechanisms by which such pollutants are formed in
an actual engine. In a memorandum, summarize your findings
and conclusions.
14.2D The Federal Clean Air Act of 1970 and succeeding Clean
Air Act Amendments target the oxides of nitrogen NO and
NO
2
, collectively known as NO
x
, as significant air pollutants.
NO
x
is formed in combustion via three primary mechanisms:
thermal NO
x
formation, prompt NO
x
formation, and fuel NO
x
Fig. P14.81
Ideal gas mixture
at T, p. Mole
fraction of substance
k is y
k
, partial
pressure p
k
= y
k
p
Membrane permeable
only to k
Ideal gas k
at T, p´
c14ChemicalandPhaseEquilibrium.886 Page 886 7/26/10 11:38:13 PM users-133 c14ChemicalandPhaseEquilibrium.886 Page 886 7/26/10 11:38:13 PM users-133 /Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New/Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New

formation. Discuss these formation mechanisms, including a
discussion of thermal NO
x
formation by the Zeldovich
mechanism. What is the role of NO
x
in the formation of
ozone? What are some NO
x
reduction strategies? Write a
report including at least three references.
14.3D Using appropriate software, develop plots giving the
variation with equivalence ratio of the equilibrium products
of octane–air mixtures at 30 atm and selected temperatures
ranging from 1700 to 2800 K. Consider equivalence ratios in
the interval from 0.2 to 1.4 and equilibrium products including,
but not necessarily limited to, CO
2
, CO, H
2
O, O
2
, O, H
2
, N
2
,
NO, OH. Under what conditions is the formation of nitric
oxide (NO) and carbon monoxide (CO) most significant?
Write a report including at least three references.
14.4D The amount of sulfur dioxide (SO
2
) present in off gases
from industrial processes can be reduced by oxidizing the
SO
2
to SO
3
at an elevated temperature in a catalytic reactor.
The SO
3
can be reacted in turn with water to form sulfuric
acid that has economic value. For an off gas at 1 atm having
the molar analysis of 12% SO
2
, 8% O
2
, 80% N
2
, estimate the
range of temperatures at which a substantial conversion of
SO
2
to SO
3
might be realized. Report your findings in a
PowerPoint presentation suitable for your class. Additionally,
in an accompanying memorandum, discuss your modeling
assumptions and provide sample calculations.
14.5D A gaseous mixture of hydrogen (H
2
) and carbon
monoxide (CO) enters a catalytic reactor and a gaseous
mixture of methanol (CH
3
OH), hydrogen, and carbon
monoxide exits. At the preliminary process design stage,
plausible estimates are required of the inlet hydrogen mole
fraction,
y
H
2
, the temperature of the exiting mixture, T
e
, and
the pressure of the exiting mixture, p
e
, subject to the following
four constraints: (1) 0.5 # y
H
2
# 0.75, (2) 300 # T
e
# 400 K,
(3) 1 # p
e
# 10 atm, and (4) the exiting mixture contains at
least 75% methanol on a molar basis. In a memorandum,
provide your estimates together with a discussion of the
modeling employed and sample calculations.
14.6D When systems in thermal, mechanical, and chemical
equilibrium are perturbed, changes within the systems can
occur, leading to a new equilibrium state. The effects of
perturbing the system considered in developing Eqs. 14.32
and 14.33 can be determined by study of these equations.
For example, at fixed pressure and temperature it can be
concluded that an increase in the amount of the inert
component E would lead to increases in n
C
and n
D
when
¢v 5 1n
C
1 n
D
2 n
A
2 n
B
2 is positive, to decreases in n
C
and
n
D
when Dv is negative, and no change when Dv 5 0.
(a) For a system consisting of NH
3
, N
2
, and H
2
at fixed pressure
and temperature, subject to the reaction
2NH
3
1g2
S
d
N
2
1g21 3H
2
1g2
investigate the effects, in turn, of additions in the amounts
present of NH
3
, H
2
, and N
2
.
(b) For the general case of Eqs. 14.32 and 14.33, investigate
the effects, in turn, of additions of A, B, C, and D.
Present your findings, together with the modeling assumptions
used, in a PowerPoint presentation suitable for your class.
Design & Open-Ended Problems: Exploring Engineering Practice 887
14.7D With reference to the equilibrium constant data of Table
A-27:
(a) For each of the tabulated reactions plot 1og
10
K versus
1/T and determine the slope of the line of best fit. What is
the thermodynamic significance of the slope? Check your
conclusion about the slope using data from the JANAF
tables.
1
(b) A text book states that the magnitude of the equilibrium
constant often signals the importance of a reaction, and
offers this rule of thumb: When K , 10
23
, the extent of the
reaction is usually not significant, whereas when K . 10
3
the
reaction generally proceeds closely to equilibrium. Confirm
or deny this rule.
Present your findings and conclusions in a report including
at least three references.
14.8D (a) For an equilibrium ideal gas mixture of N
2
, H
2
, and
NH
3
, evaluate the equilibrium constant from an expression
you derive from the van’t Hoff equation that requires only
standard state enthalpy of formation and Gibbs function of
formation data together with suitable analytical expressions
in terms of temperature for the ideal gas specific heats of N
2
,
H
2
, NH
3
.
(b) For the synthesis of ammonia by
1
2
N
2
1
3
2
H
2
S NH
3
provide a recommendation for the ranges of temperature and
pressure for which the mole fraction of ammonia in the mixture
is at least 0.5.
Write a report including your derivation, recommendations for
the ranges of temperature and pressure, sample calculations, and
at least three references.
14.9D U.S. Patent 5,298,233 describes a means for converting
industrial wastes to carbon dioxide and water vapor. Hydrogen-
and carbon-containing feed, such as organic or inorganic
sludge, low-grade fuel oil, or municipal garbage, is introduced
into a molten bath consisting of two immiscible molten metal
phases. The carbon and hydrogen of the feed are converted,
respectively, to dissolved carbon and dissolved hydrogen. The
dissolved carbon is oxidized in the first molten metal phase
to carbon dioxide, which is released from the bath. The
dissolved hydrogen migrates to the second molten metal
phase, where it is oxidized to form water vapor, which is also
released from the bath. Critically evaluate this technology for
waste disposal. Is the technology promising commercially?
Compare with alternative waste management practices such as
pyrolysis and incineration. Write a report including at least
three references.
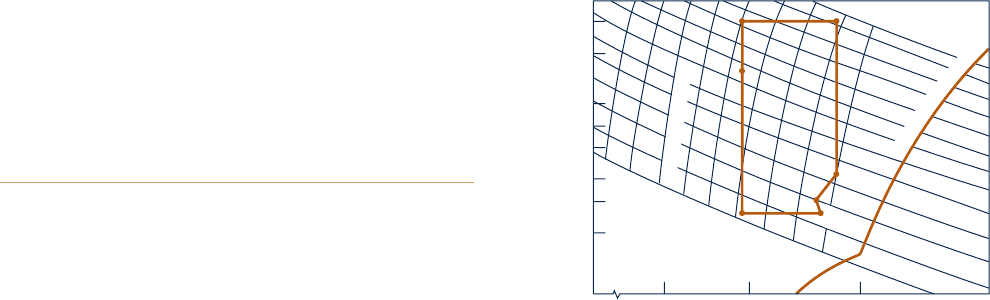
14.10D Figure P14.10D gives a table of data for a lithium
bromide–water absorption refrigeration cycle together with
the sketch of a property diagram showing the cycle. The
property diagram plots the vapor pressure versus the lithium
bromide concentration. Apply the phase rule to verify that the
numbered states are fixed by the property values provided.
What does the crystallization line on the equilibrium diagram
1
Stull, D. R., and H. Prophet, JANAF Thermochemical Tables, 2nd ed., NSRDS-
NBS 37, National Bureau of Standards, Washington, DC, June 1971.
c14ChemicalandPhaseEquilibrium.887 Page 887 7/26/10 11:38:15 PM users-133 c14ChemicalandPhaseEquilibrium.887 Page 887 7/26/10 11:38:15 PM users-133 /Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New/Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New
888 Chapter 14 Chemical and Phase Equilibrium
represent, and what is its significance for absorption cycle
operation? Locate the numbered states on an enthalpy-
concentration diagram for lithium bromide–water solutions
obtained from the literature. Finally, develop a sketch of the
equipment schematic for this refrigeration cycle. Present
your findings in a report including at least three references.
Temperature Pressure (mf)
LiBr
State (°F) (in. Hg) (%)
1 115 0.27 63.3
2 104 0.27 59.5
3 167 1.65 59.5
4 192 3.00 59.5
5 215 3.00 64.0
6 135 0.45 64.0
7 120 0.32 63.3
Fig. P14.10D
Specific gravity
Pressure, inches of mercury
0.4
0.6
0.8
1.0
2.0
0.3
4.0
3.0
0.2
0.1
2
3
4
5
6
7
Solution temperature, °F
200
220
180
160
140
120
100
Percent lithium bromide b
y
mass
55 60 65
C
r
y
s
t
a
l
l
i
z
a
t
i
o
n
l
i
n
e
1
c14ChemicalandPhaseEquilibrium.888 Page 888 7/26/10 11:38:16 PM users-133 c14ChemicalandPhaseEquilibrium.888 Page 888 7/26/10 11:38:16 PM users-133 /Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New/Users/users-133/Desktop/Ramakant_04.05.09/WB00113_R1:JWCL170/New

889
Table A-1 Atomic or Molecular Weights and Critical Properties of Selected Elements and Compounds 890
Table A-2 Properties of Saturated Water (Liquid–Vapor): Temperature Table 891
Table A-3 Properties of Saturated Water (Liquid–Vapor): Pressure Table 893
Table A-4 Properties of Superheated Water Vapor 895
Table A-5 Properties of Compressed Liquid Water 899
Table A-6 Properties of Saturated Water (Solid–Vapor): Temperature Table 900
Table A-7 Properties of Saturated Refrigerant 22 (Liquid–Vapor): Temperature Table 901
Table A-8 Properties of Saturated Refrigerant 22 (Liquid–Vapor): Pressure Table 902
Table A-9 Properties of Superheated Refrigerant 22 Vapor 903
Table A-10 Properties of Saturated Refrigerant 134a (Liquid–Vapor): Temperature Table 907
Table A-11 Properties of Saturated Refrigerant 134a (Liquid–Vapor): Pressure Table 908
Table A-12 Properties of Superheated Refrigerant 134a Vapor 909
Table A-13 Properties of Saturated Ammonia (Liquid–Vapor): Temperature Table 912
Table A-14 Properties of Saturated Ammonia (Liquid–Vapor): Pressure Table 913
Table A-15 Properties of Superheated Ammonia Vapor 914
Table A-16 Properties of Saturated Propane (Liquid–Vapor): Temperature Table 918
Table A-17 Properties of Saturated Propane (Liquid–Vapor): Pressure Table 919
Table A-18 Properties of Superheated Propane Vapor 920
Table A-19 Properties of Selected Solids and Liquids: c
p
, r, and k. 924
Table A-20 Ideal Gas Specific Heats of Some Common Gases 925
Table A-21 Variation of c
p
with Temperature for Selected Ideal Gases 926
Table A-22 Ideal Gas Properties of Air 927
Table A-23 Ideal Gas Properties of Selected Gases 929
Table A-24 Constants for the van der Waals, Redlich–Kwong, and Benedict–Webb–Rubin
Equations of State 933
Table A-25 Thermochemical Properties of Selected Substances at 298 K and 1 atm 934
Table A-26 Standard Molar Chemical Exergy, e
ch
(kJ/kmol), of Selected Substances at 298 K and p
0
935
Table A-27 Logarithms to the Base 10 of the Equilibrium Constant K 936
Index to Tables in SI Units
BMIndextoTablesinSIUnits.indd Page 889 8/3/10 5:45:01 PM user-s146BMIndextoTablesinSIUnits.indd Page 889 8/3/10 5:45:01 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
890 Tables in SI Units
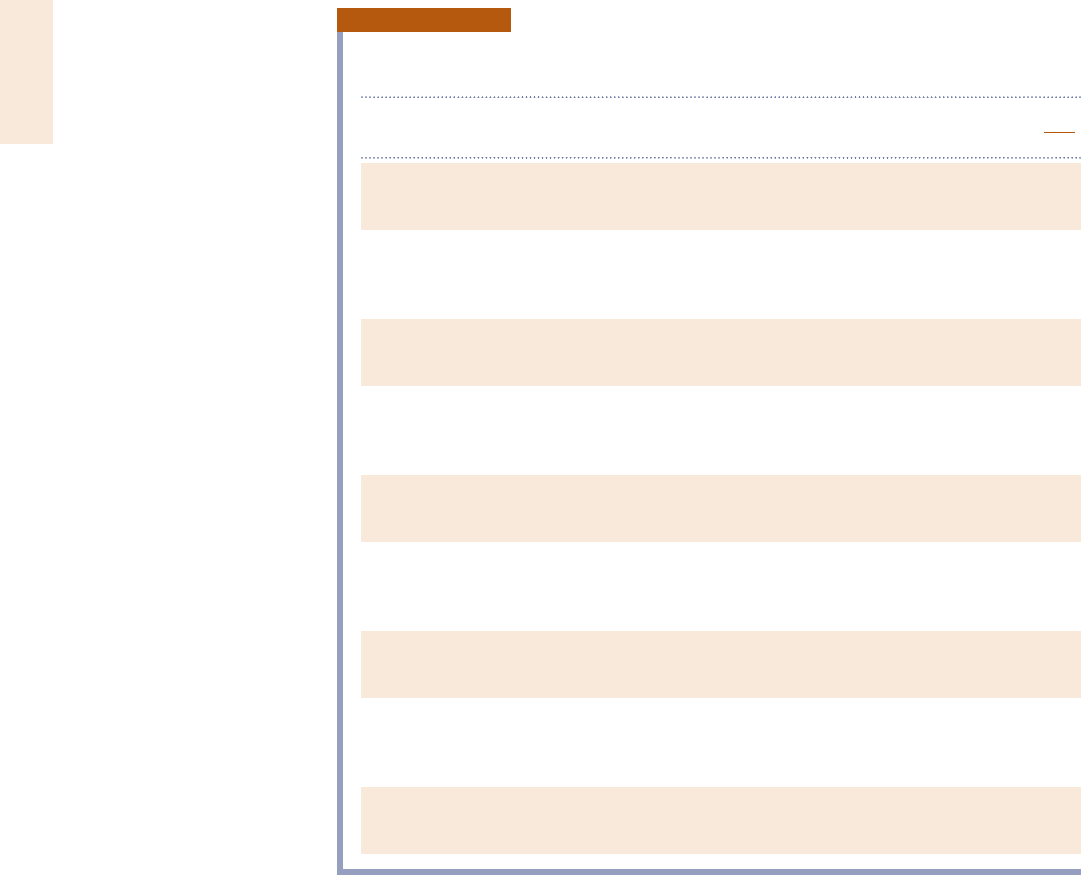
TABLE A-1
Atomic or Molecular Weights and Critical Properties of Selected
Elements and Compounds
Chemical
M T
c
p
c
Z
c
5
p
c
v
c
RT
c
Substance Formula (kg/kmol) (K) (bar)
Acetylene C
2
H
2
26.04 309 62.8 0.274
Air (equivalent) — 28.97 133 37.7 0.284
Ammonia NH
3
17.03 406 112.8 0.242
Argon Ar 39.94 151 48.6 0.290
Benzene C
6
H
6
78.11 563 49.3 0.274
Butane C
4
H
10
58.12 425 38.0 0.274
Carbon C 12.01 — — —
Carbon dioxide CO
2
44.01 304 73.9 0.276
Carbon monoxide CO 28.01 133 35.0 0.294
Copper Cu 63.54 — — —
Ethane C
2
H
6
30.07 305 48.8 0.285
Ethanol C
2
H
5
OH 46.07 516 63.8 0.249
Ethylene C
2
H
4
28.05 283 51.2 0.270
Helium He 4.003 5.2 2.3 0.300
Hydrogen H
2
2.016 33.2 13.0 0.304
Methane CH
4
16.04 191 46.4 0.290
Methanol CH
3
OH 32.04 513 79.5 0.220
Nitrogen N
2
28.01 126 33.9 0.291
Octane C
8
H
18
114.22 569 24.9 0.258
Oxygen O
2
32.00 154 50.5 0.290
Propane C
3
H
8
44.09 370 42.7 0.276
Propylene C
3
H
6
42.08 365 46.2 0.276
Refrigerant 12 CCl
2
F
2
120.92 385 41.2 0.278
Refrigerant 22 CHClF
2
86.48 369 49.8 0.267
Refrigerant 134a CF
3
CH
2
F 102.03 374 40.7 0.260
Sulfur dioxide SO
2
64.06 431 78.7 0.268
Water H
2
O 18.02 647.3 220.9 0.233
Sources: Adapted from International Critical Tables and L. C. Nelson and E. F. Obert, Generalized Compressibility
Charts, Chem. Eng., 61: 203 (1954).
Table A-1
BMIndextoTablesinSIUnits.indd Page 890 8/3/10 5:45:03 PM user-s146BMIndextoTablesinSIUnits.indd Page 890 8/3/10 5:45:03 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
Tables in SI Units 891
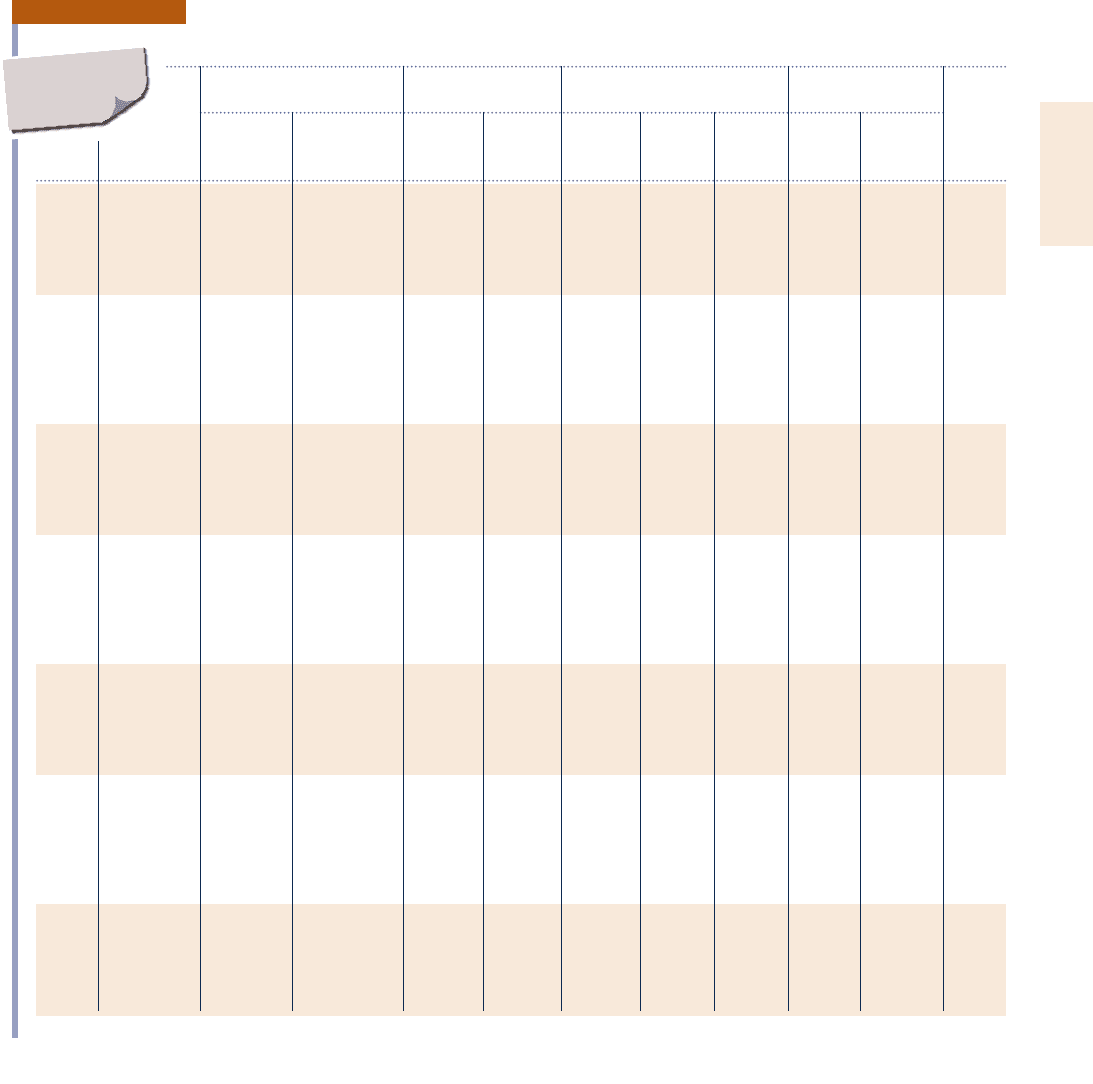
Properties of Saturated Water (Liquid–Vapor): Temperature Table
Specific Volume Internal Energy Enthalpy Entropy
m
3
/kg kJ/kg kJ/kg kJ/kg ? K
Sat. Sat. Sat. Sat. Sat. Sat. Sat. Sat.
Temp. Press. Liquid Vapor Liquid Vapor Liquid Evap. Vapor Liquid Vapor Temp.
8C bar v
f
3 10
3
v
g
u
f
u
g
h
f
h
fg
h
g
s
f
s
g
8C
.01 0.00611 1.0002 206.136 0.00 2375.3 0.01 2501.3 2501.4 0.0000 9.1562 .01
4 0.00813 1.0001 157.232 16.77 2380.9 16.78 2491.9 2508.7 0.0610 9.0514 4
5 0.00872 1.0001 147.120 20.97 2382.3 20.98 2489.6 2510.6 0.0761 9.0257 5
6 0.00935 1.0001 137.734 25.19 2383.6 25.20 2487.2 2512.4 0.0912 9.0003 6
8 0.01072 1.0002 120.917 33.59 2386.4 33.60 2482.5 2516.1 0.1212 8.9501 8
10 0.01228 1.0004 106.379 42.00 2389.2 42.01 2477.7 2519.8 0.1510 8.9008 10
11 0.01312 1.0004 99.857 46.20 2390.5 46.20 2475.4 2521.6 0.1658 8.8765 11
12 0.01402 1.0005 93.784 50.41 2391.9 50.41 2473.0 2523.4 0.1806 8.8524 12
13 0.01497 1.0007 88.124 54.60 2393.3 54.60 2470.7 2525.3 0.1953 8.8285 13
14 0.01598 1.0008 82.848 58.79 2394.7 58.80 2468.3 2527.1 0.2099 8.8048 14
15 0.01705 1.0009 77.926 62.99 2396.1 62.99 2465.9 2528.9 0.2245 8.7814 15
16 0.01818 1.0011 73.333 67.18 2397.4 67.19 2463.6 2530.8 0.2390 8.7582 16
17 0.01938 1.0012 69.044 71.38 2398.8 71.38 2461.2 2532.6 0.2535 8.7351 17
18 0.02064 1.0014 65.038 75.57 2400.2 75.58 2458.8 2534.4 0.2679 8.7123 18
19 0.02198 1.0016 61.293 79.76 2401.6 79.77 2456.5 2536.2 0.2823 8.6897 19
20 0.02339 1.0018 57.791 83.95 2402.9 83.96 2454.1 2538.1 0.2966 8.6672 20
21 0.02487 1.0020 54.514 88.14 2404.3 88.14 2451.8 2539.9 0.3109 8.6450 21
22 0.02645 1.0022 51.447 92.32 2405.7 92.33 2449.4 2541.7 0.3251 8.6229 22
23 0.02810 1.0024 48.574 96.51 2407.0 96.52 2447.0 2543.5 0.3393 8.6011 23
24 0.02985 1.0027 45.883 100.70 2408.4 100.70 2444.7 2545.4 0.3534 8.5794 24
25 0.03169 1.0029 43.360 104.88 2409.8 104.89 2442.3 2547.2 0.3674 8.5580 25
26 0.03363 1.0032 40.994 109.06 2411.1 109.07 2439.9 2549.0 0.3814 8.5367 26
27 0.03567 1.0035 38.774 113.25 2412.5 113.25 2437.6 2550.8 0.3954 8.5156 27
28 0.03782 1.0037 36.690 117.42 2413.9 117.43 2435.2 2552.6 0.4093 8.4946 28
29 0.04008 1.0040 34.733 121.60 2415.2 121.61 2432.8 2554.5 0.4231 8.4739 29
30 0.04246 1.0043 32.894 125.78 2416.6 125.79 2430.5 2556.3 0.4369 8.4533 30
31 0.04496 1.0046 31.165 129.96 2418.0 129.97 2428.1 2558.1 0.4507 8.4329 31
32 0.04759 1.0050 29.540 134.14 2419.3 134.15 2425.7 2559.9 0.4644 8.4127 32
33 0.05034 1.0053 28.011 138.32 2420.7 138.33 2423.4 2561.7 0.4781 8.3927 33
34 0.05324 1.0056 26.571 142.50 2422.0 142.50 2421.0 2563.5 0.4917 8.3728 34
35 0.05628 1.0060 25.216 146.67 2423.4 146.68 2418.6 2565.3 0.5053 8.3531 35
36 0.05947 1.0063 23.940 150.85 2424.7 150.86 2416.2 2567.1 0.5188 8.3336 36
38 0.06632 1.0071 21.602 159.20 2427.4 159.21 2411.5 2570.7 0.5458 8.2950 38
40 0.07384 1.0078 19.523 167.56 2430.1 167.57 2406.7 2574.3 0.5725 8.2570 40
45 0.09593 1.0099 15.258 188.44 2436.8 188.45 2394.8 2583.2 0.6387 8.1648 45
TABLE A-2
Pressure Conversions:
1 bar = 0.1 MPa
= 10
2
kPa
H
2
O
BMIndextoTablesinSIUnits.indd Page 891 8/3/10 5:45:03 PM user-s146BMIndextoTablesinSIUnits.indd Page 891 8/3/10 5:45:03 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
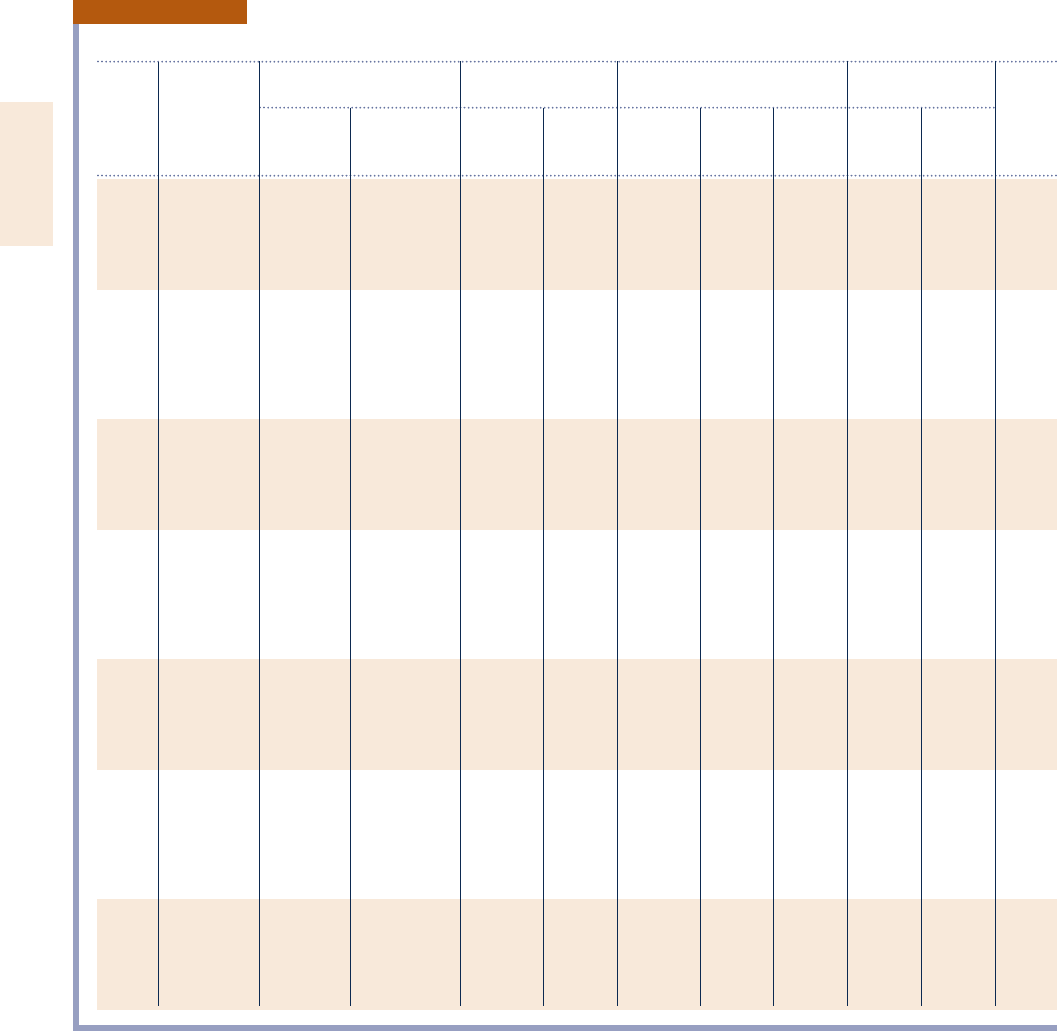
892 Tables in SI Units
(Continued)
Specific Volume Internal Energy Enthalpy Entropy
m
3
/kg kJ/kg kJ/kg kJ/kg ? K
Sat. Sat. Sat. Sat. Sat. Sat. Sat. Sat.
Temp. Press. Liquid Vapor Liquid Vapor Liquid Evap. Vapor Liquid Vapor Temp.
8C bar v
f
3 10
3
v
g
u
f
u
g
h
f
h
fg
h
g
s
f
s
g
8C
50 .1235 1.0121 12.032 209.32 2443.5 209.33 2382.7 2592.1 .7038 8.0763 50
55 .1576 1.0146 9.568 230.21 2450.1 230.23 2370.7 2600.9 .7679 7.9913 55
60 .1994 1.0172 7.671 251.11 2456.6 251.13 2358.5 2609.6 .8312 7.9096 60
65 .2503 1.0199 6.197 272.02 2463.1 272.06 2346.2 2618.3 .8935 7.8310 65
70 .3119 1.0228 5.042 292.95 2469.6 292.98 2333.8 2626.8 .9549 7.7553 70
75 .3858 1.0259 4.131 313.90 2475.9 313.93 2321.4 2635.3 1.0155 7.6824 75
80 .4739 1.0291 3.407 334.86 2482.2 334.91 2308.8 2643.7 1.0753 7.6122 80
85 .5783 1.0325 2.828 355.84 2488.4 355.90 2296.0 2651.9 1.1343 7.5445 85
90 .7014 1.0360 2.361 376.85 2494.5 376.92 2283.2 2660.1 1.1925 7.4791 90
95 .8455 1.0397 1.982 397.88 2500.6 397.96 2270.2 2668.1 1.2500 7.4159 95
100 1.014 1.0435 1.673 418.94 2506.5 419.04 2257.0 2676.1 1.3069 7.3549 100
110 1.433 1.0516 1.210 461.14 2518.1 461.30 2230.2 2691.5 1.4185 7.2387 110
120 1.985 1.0603 0.8919 503.50 2529.3 503.71 2202.6 2706.3 1.5276 7.1296 120
130 2.701 1.0697 0.6685 546.02 2539.9 546.31 2174.2 2720.5 1.6344 7.0269 130
140 3.613 1.0797 0.5089 588.74 2550.0 589.13 2144.7 2733.9 1.7391 6.9299 140
150 4.758 1.0905 0.3928 631.68 2559.5 632.20 2114.3 2746.5 1.8418 6.8379 150
160 6.178 1.1020 0.3071 674.86 2568.4 675.55 2082.6 2758.1 1.9427 6.7502 160
170 7.917 1.1143 0.2428 718.33 2576.5 719.21 2049.5 2768.7 2.0419 6.6663 170
180 10.02 1.1274 0.1941 762.09 2583.7 763.22 2015.0 2778.2 2.1396 6.5857 180
190 12.54 1.1414 0.1565 806.19 2590.0 807.62 1978.8 2786.4 2.2359 6.5079 190
200 15.54 1.1565 0.1274 850.65 2595.3 852.45 1940.7 2793.2 2.3309 6.4323 200
210 19.06 1.1726 0.1044 895.53 2599.5 897.76 1900.7 2798.5 2.4248 6.3585 210
220 23.18 1.1900 0.08619 940.87 2602.4 943.62 1858.5 2802.1 2.5178 6.2861 220
230 27.95 1.2088 0.07158 986.74 2603.9 990.12 1813.8 2804.0 2.6099 6.2146 230
240 33.44 1.2291 0.05976 1033.2 2604.0 1037.3 1766.5 2803.8 2.7015 6.1437 240
250 39.73 1.2512 0.05013 1080.4 2602.4 1085.4 1716.2 2801.5 2.7927 6.0730 250
260 46.88 1.2755 0.04221 1128.4 2599.0 1134.4 1662.5 2796.6 2.8838 6.0019 260
270 54.99 1.3023 0.03564 1177.4 2593.7 1184.5 1605.2 2789.7 2.9751 5.9301 270
280 64.12 1.3321 0.03017 1227.5 2586.1 1236.0 1543.6 2779.6 3.0668 5.8571 280
290 74.36 1.3656 0.02557 1278.9 2576.0 1289.1 1477.1 2766.2 3.1594 5.7821 290
300 85.81 1.4036 0.02167 1332.0 2563.0 1344.0 1404.9 2749.0 3.2534 5.7045 300
320 112.7 1.4988 0.01549 1444.6 2525.5 1461.5 1238.6 2700.1 3.4480 5.5362 320
340 145.9 1.6379 0.01080 1570.3 2464.6 1594.2 1027.9 2622.0 3.6594 5.3357 340
360 186.5 1.8925 0.006945 1725.2 2351.5 1760.5 720.5 2481.0 3.9147 5.0526 360
374.14 220.9 3.155 0.003155 2029.6 2029.6 2099.3 0 2099.3 4.4298 4.4298 374.14
Source: Tables A-2 through A-5 are extracted from J. H. Keenan, F. G. Keyes, P. G. Hill, and J. G. Moore, Steam Tables, Wiley, New York, 1969.
TABLE A-2
H
2
O
BMIndextoTablesinSIUnits.indd Page 892 8/3/10 5:45:15 PM user-s146BMIndextoTablesinSIUnits.indd Page 892 8/3/10 5:45:15 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
